Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Universidad y ciencia
versión impresa ISSN 0186-2979
Universidad y ciencia vol.27 no.2 Villahermosa ago. 2011
Nota científica
Arthroscopic treatment of equine full–thickness chondral defects with constructs of collagen encapsulated cells
Tratamiento artroscópico de defectos condrales equinos de espesor total con células encapsuladas en colágena
G Lombardero1*, C Velasquillo2, E Aburto1, H Villegas2, E Morales1, M Masri1, V Martínez2, R Gómez2, T Chávez2, C Ibarra2
1 Facultad de Medicina Veterinaria y Zootecnia Universidad Nacional Autónoma de México (UNAM) 04510, México, DF Fax: (55) 56225920 y 95 Celular: (044-55) 54149772 (GL)(EA)(EM)(MM) *Correo electrónico: sgtododo@hotmail.com
2 Instituto Nacional de Rehabilitación (INR) Secretaría de Salud, 14389 México, D.F. (CV)(HV)(VM)(RG)(TCH)(CI)
Recibido: 25 de mayo de 2009,
Aceptado: 26 de junio de 2011
ABSTRACT
A full-thickness chondral defect in the stifle joint of eight mares was treated by perforation of the subchondral bone. In addition, the experimental group received an arthroscopic implant of a tissue-engineered construct. The objective was the arthroscopic evaluation of the quality of the repair tissue, six months after the treatments. The experimental group presented a statistically significant (p = 0.001) improvement in the repair tissue.
Key words: Chondral defect, arthroscopic implant, construct, tissue engineering.
RESUMEN
Un defecto condral de espesor total en la babilla de ocho yeguas fue tratado con una perforación en el hueso subcondral. Adicionalmente, en el grupo experimental fue implantado artroscópicamente un constructo creado por ingeniería de tejidos. El objetivo fue la evaluación artroscópica de la calidad del tejido de reparación, seis meses después de los tratamientos. El grupo experimental mostró mejoría estadísticamente significativa (p = 0.001) en el tejido de reparación.
Palabras clave: Defecto condral, implante artroscópico, constructo, ingeniería de tejidos.
INTRODUCTION
Damage to the equine articular cartilage caused by trauma or joint disease has a devastating effect on the function of a joint. Some lesions (defects) of the cartilage have clinical consequences, such as chronic articular pain, disability and the development of osteoarthritis. The prognosis for a horse affected with this type of lesion to return to sporting activity depends greatly on the location, as well as the type, thickness and size, of the lesion (van der Harst MR et al. 2004. Osteoarthritis and Cartilage 12 (9): 752-761; Scopp JM, Mandelbaum BR 2005. Orthopedic Clinics of North America 36 (4): 419-426; Strauss EJ et al. 2005. American Journal of Sports Medicine 33 (11): 1647-1653).
The ability of the articular cartilage of the adult to repair itself is very limited, and its potential regeneration is practically nill. However, defects repair themselves with fibrotic or fibrocartilage extrinsic tissue, which is biomechanically inferior and rapidly deteriorates when subjected to continuous exercise (Otto WR, Rao J 2004. Cell Proliferation 37: 97-110). Full-thickness chondral defects have poor potential for self-repair (Lubiatowski P et al. 2006. Transplantation Proceedings 38 (1): 320-322; Bos PK et al. 2008. Osteoarthritis and Cartilage 16 (2): 204-211).
The available surgical alternatives for treatment of chondral defects offer insufficient and unpredictable clinical results. The use of chondral repair techniques that are based only on the liberation of cells from the bone marrow (curettage, subchondral perforations, abrasion arthroplasty and microfracture), has shown that the repair tissue formed is imperfectly integrated to the defect, when the subchondral bone is destroyed in order to stimulate bleeding towards the defect and in this way achieve the formation of fibrocartilage in the region of the lesion. This in turn predisposes the tissue to be released and the lesion to recur (Frisbie DD et al. 2003. Clinical Orthopaedics and Related Research (407): 215-227; Frisbie DD et al. 2006. American Journal of Sports Medicine 34 (11): 1824-1831; Mienaltowski MJ et al. 2009. BMC Med Genomics 4 (2): 60). Reconstructive and regenerative procedures attempt to fill chondral defects with repair tissue that has the same mechanical properties as the normal cartilage, and can thus consolidate and integrate with the native surrounding cartilage (Beris AE et al. 2005. Injury 36 (4): S14-S23; Slynarski K, Deszczynski J 2006. Transplantation Proceedings 38 (1): 316-317).
Despite progress in orthopedic surgery, the lack of efficient modalities of treatment for large chondral defects has prompted research on tissue engineering, combining cells (chondrocytes and multipotent mesenchymal stromal cells), scaffold materials and environmental factors. This entails the manufacture in vitro of constructs (polymers seeded with cells) that may be surgically implanted over a chondral defect (Barnewitz D et al. 2006. Biomaterials 27 (14): 2882-2889; Vinatier C et al. 2009. Current Stem Cell Research Therapy 4 (4): 318-329).
Animals commonly used in cartilage repair studies include murine, lapine, canine, caprine, porcine and equine models. Small rodent and lapine models are cost effective, easy to house, and useful for pilot and proof of concept studies.
Large animal models (equine) with thicker articular cartilage allow the study of both partial-thickness and full-thickness chondral repair, as well as of osteochondral repair (Hangody L et al. 2008. Injury 39 Supplement 1: 32-39). The arthroscopic evaluation of chondral repair tissue is a fundamental part of the assessment of treatment effectiveness (Smith GD et al. 2005. Arthroscopy: The Journal of Arthroscopic and Related Surgery 21 (12): 14621467). The objective of this study was to compare the arthroscopic quality of the repair tissue in an equine full-thickness chondral defect treated with two techniques: perforation of the subchondral bone versus perforation of the subchondral bone plus implantation of a tissue-engineered construct.
MATERIALS AND METHODS
Animals
The protocol of this study was revised and approved by the Scientific Surveillance Committee for the care and welfare of experimental animals, of the School of Veterinary Medicine, National Autonomous University of Mexico (UNAM).
A follow up study was performed in eight clinically healthy creole mares between three and five years of age, which were allocated to two groups of four animals each (experimental and control groups). Each animal was randomly assigned a letter for its identification. Mares A, B, D and E comprised the control group, and mares C, F, H and J the experimental group.
Manufacturing of the constructs
An osteochondral plug was obtained from each mare of the experimental group by arthros-copy, using a harvester (Osteochondral transplantation system COR™ Mitek, MA, USA). The sample was 8 millimeters (mm) in diameter and 15 mm in length, and was taken from the medial femoral trochlea of the left stifle joint (cartilage donor).
The cartilage from the osteochondral sample was fragmented manually with a No. 11 surgical blade into portions of 1 to 2 mm3, and was digested using type II collagenase at a concentration of 0.3 % (Worthington, NJ, USA) to separate the chondrocytes from the extracellular matrix. A mean of 1x106 viable cells were obtained using this process. The chondrocytes were cultivated in monola-yers in a CO2 incubator (NuAire, Inc., MN, USA) at 37 °C with a relative humidity of 5 %, using a commercial culture medium (M-199™ Bio-Whittaker, MD, USA) combined with 10 % bovine fetal serum, penicillin-streptomycin and 1 % amphotericin B (Gibco, NY, USA). The cells were expanded from the primary culture up to the second passage, and reached a mean population of 20x106 cells per mare.
The constructs were manufactured from autologous cells, extracellular matrix, and a mesh of collagen in the shape of an 8 mm diameter disk (Restore soft tissue implant, Porcine SIS, DePuy Johnson & Johnson, MA, USA) using the cell encapsulation technique described by Masri and collaborators (Masri M et al. 2007. Arthroscopy. The Journal of Arthroscopic and Related Surgery 23 (8): 877-883). The average dimensions of the constructs were a 1 cm diameter and a 4 mm thickness.
Treatments
Creation of the chondral defect and subchondral bone perforation
An 8 mm diameter full-thickness chondral defect was created arthroscopically on the medial femoral trochlea of the right stifle of the mares of both groups, with the help of a harvester and a periosteal elevator. A 2.4 mm diameter and 15 mm deep perforation was made in the center of this defect, on the subchondral bone, using an orthopedic drill.
Implantation of the constructs in the experimental group
The procedure described below was performed arthroscopically. A suture anchor (Panalok™ Mitek, MA, USA), joined to an absorbable No.1 polydioxanone suture (PDS™ Mitek, MA, USA), was introduced into the perforated area of the subchondral bone.
The two ends of the suture were left outside the joint and were introduced into two perforations that had been made on the construct with a 20 gauge hypodermic needle. The construct was slipped from the outside into the articular cavity through a 14 mm diameter plastic cannula (Mitek, MA, USA), which was used as a tunnel.
Once the construct had been positioned over the defect, it was held in place with knots, and was thus implanted.
Management of the animals after treatment
Mares from both groups were stabled in individual stalls for a period of two weeks. They were treated with procaine penicillin G (20000 UI/kg) intramuscularly (IM) every 12 h, and gentamicin (6.6 mg/kg) intravenously (IV) every 24 h for five days. In addition, phenylbutazone (4.4 mg/kg) was administered IV every 24 h for three days. Afterwards, the animals were released in a 50 m diameter circular paddock.
Arthroscopic evaluation of the quality of repair tissue
The chondral defects in both groups (experimental and control) were evaluated six months after treatment, using the "Oswestry Arthroscopy Score" for the chondral repair tissue (Smith GD et al. 2005. Arthroscopy: The Journal of Arthroscopic and Related Surgery 21 (12): 1462-1467). Once the repair tissue was evaluated, the mares were allowed to recover from surgery and their participation in the study was ended.
Statistical analysis
The Mann-Whitney test was used to determine the differences between the two groups. The data were analysed using the SPSS™ package (Statistical Package for the Social Sciences SPSS Inc., IL, USA) for Windows™ version 11.
RESULTS AND DISCUSSION
None of the mares presented any type of postsurgical articular complication or rejection of the construct. The results for the Oswestry Arthroscopy Score are shown in Table 1.
The superiority in the results of the experimental group over those of the control group emphasizes the importance of the implanted construct, as this was the only difference between the treatments administered to the groups. These results provide further evidence of the effectiveness of the cell encapsulation technique developed by Masri and collaborators (Masri M et al. 2007. Arthroscopy. The Journal of Arthroscopic and Related Surgery 23 (8): 877-883), that was used to manufacture the constructs. We consider that the cells remained in the construct enough time to induce the formation of repair tissue of a better arthroscopic quality that, in turn, allowed a better integration to the adjacent cartilage.
The statistically significant difference in the observed results (p = 0.001) validates the treatment used in the experimental group, and provides confidence to the continued use of the Oswestry scoring system in further studies. Through the use of arthroscopy, it was possible to detect subtle differences in the repair tissue among individuals, thanks to the optical quality of the technique, combined with the magnification and additional illumination of the tissue. Our results confirm the observation of Ayral (Ayral X 1996. Best Practice & Research Clinical Rheumatology 10 (3): 477-494): "Arthroscopy is considered the golden standard for the evaluation of articular cartilage".
The present study corroborates the findings of Smith and collaborators (Smith GD et al. 2005. Arthroscopy: The Journal of Arthroscopic and Related Surgery 21 (12): 1462-1467) in relation to the need to evaluate the chondral repair tissue using arthroscopy, as a fundamental component of the assessment of treatments. We consider that with such an evaluation, important information is obtained on the prognosis of a repaired chondral lesion.
In relation to the repair of the chondral defects in both experimental groups, the important role of the mesenchymal cells of the bone marrow, when the subchondral bone is perforated, is worth mentioning. No construct was used in the control group to act as a support structure for these cells, which would have allowed them to remain for a longer time over the defect, as occurred in the experimental group. Because of this, we believe that the collagen mesh used in this study positively influenced the results of the experimental group. This observation is supported by the observation of Lubiatowski and collaborators (Lubiatowski P et al. 2006.
Transplantation Proceedings 38 (1): 320-322): "The use of biodegradable polymers such as collagen in the repair of chondral defects improves the quality of the newly formed tissue". An example of this is reported in the study of Steinwachs and Kreuz (Steinwachs M, Kreuz PC 2007. Arthroscopy. The Journal of Arthroscopic and Related Surgery 23 (4): 381387) in which chondral defects in the knee joints of humans were repaired with autologous chondrocytes and a collagen membrane. In this case, the authors observed that the repair tissue improved as the duration of follow-up increased.
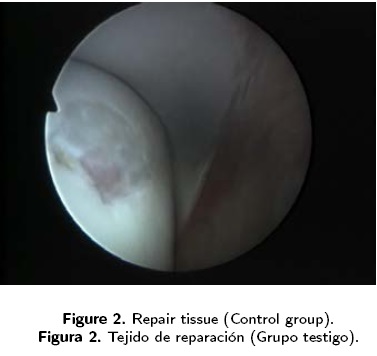
In conclusion, this study has demonstrated that treatment of a full-thickness chondral defect that involves perforation of the subchondral bone and the implantation of a tissue-engineered construct, results in repair tissue of a better arthroscopic quality (Figure 1) than treatments with only subchondral perforation (Figure 2). We consider that the use of tissue engineering techniques and arthroscopy for the repair of chondral defects in equines offers an improved expectation of successful treatment, as the quality of the repair tissue allows a better integration and, therefore, a decreased?— greater permanence of the lesion.

ACKNOWLEDGEMENTS
The Equine Hospital in the School of Veterinary Medicine at UNAM, the National Institute of Rehabilitation (INR), MVZ Alejandro Sigler Rangel for his support as an anesthesiologist, and the Mexican National Council for Science and Technology (CONACyT) for financing the project CONACyT-Salud 2003-C01-98.














