Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Universidad y ciencia
versión impresa ISSN 0186-2979
Universidad y ciencia vol.26 no.3 Villahermosa dic. 2010
Artículos
Short–term effects of temperature changes in a pilot plant for the production of biogas from poultry litter
Efectos a corto plazo de los cambios de temperatura en una planta piloto de producción de biogas a partir de pollinaza
T Espinosa–Solares1,2*, M Domaschko2, F Robles–Martínez4, E Durán–Páramo4, G Hernández–Eugenio3, J Bombardiere5
1 Departamento de Ingeniería Agroindustrial, Universidad Autónoma de Chapingo, Chapingo, 56230, Edo de México, MÉXICO. (TES) *Correo electrónico: espinosa@correo.chapingo.mx
2 West Virginia State University, Gus R. Douglass Land–Grant Institute, Institute, WV 25112–1000, USA. (TES)(MD)
3 Departamento de Irrigación, Universidad Autónoma de Chapingo, Chapingo, 56230, Edo de México, MÉXICO. (GHE)
4 Departamento de Bioprocesos, Unidad Profesional Interdisciplinaria de Biotecnología. Instituto Politécnico Nacional. (FRM)(EDP) froblesm@ipn.mx
5 Consultant for Enviro Control Ltd., Singleton Court Business Park, Wonastow Road, Monmouth, UK. (JB)
Artículo recibido: 20 de octubre de 2008
Aceptado: 10 de noviembre de 2010
ABSTRACT
The optimal temperature for anaerobic thermophilic digestion varies from 55 to 65 °C, and in commercial applications it ranges from 50 to 55 °C. The present study took place in a 40 m3 pilot plant, to determine the effect of temperature on methane production, using poultry litter as substrate. The chemical composition of the substrate, the effluent and the biogas were monitored throughout the experiments. The Arrhenius model indicates a strong correlation between the temperature and the specific methanogenic activity (SMA) in the range of 52.2 to 56.7 °C. The activation energy was 146.1 kJ mol–1. No significant differences were observed in the SMA when operations took place in the range of 56.7 to 60 °C.
Key words: Anaerobic digestion, Arrhenius model, specific methanogenic activity.
RESUMEN
La temperatura óptima para la digestión termófila anaerobia varía de 55 a 65 °C, y en aplicaciones comerciarles el intervalo se sitúa entre 50 y 55 °C. El presente trabajo se llevó a cabo en una planta piloto de 40 m3 de capacidad, con el objetivo de estudiar la influencia de la temperatura en la producción de metano, usando como sustrato pollinaza. La composición química del sustrato, el efluente y el biogás fueron monitoreados durante el experimento. El modelo de Arrhenius muestra una correlación relevante entre la temperatura y la actividad metanogénica específica (SMA) en el intervalo de 52.2 a 56.7 °C. La energía de activación fue de 146.1 KJ mol–1. No se observaron diferencias significativas en la SMA cuando se operó en el intervalo de 56.7 a 60 °C.
Palabras clave: Digestión anaerobia, modelo de Arrhenius, actividad metanogénica específica.
INTRODUCTION
The optimal operation temperature for a specific anaerobic biodigester depends on several performance factors, including biogas yield, pathogen reduction in digested feedstock, process stability and operation cost. When a biodigester is considered as a mechanism to reduce pathogens in processed feedstock, higher operational temperatures result in a greater pathogen destruction. In the case of "Class A Biosolids" (according to the EPA classification), materials must be treated at or above 55 °C (EPA 2002). Comparing mesophilic (35 °C) and thermophilic (55 °C) operating temperatures, it is important to note that the solubility of the gases is reduced at higher temperatures. According to the constanst of Henry's Law, reported by Lide and Frederikse (1995) for a water–gas system, the solubility of methane and carbon dioxide is reduced by 30 and 10% respectively when temperature changes from 35 °C to 55 °C. This results in a faster mass transfer under thermophilic conditions. Also, when the thermophilic process is compared with mesophilic conditions using the same fluid, the relative diffusivity of solids in liquids may increase around 88% (Lettinga et al. 2001), while the media viscosity is reduced by almost 50% (El–Mashad et al. 2005). Consequently, the consumption of power for the mechanical manipulation of fluids (mixing, pumping and forced–biogas circulation) is reduced under thermophilic conditions. However, the increase in temperature from a mesophilic to a thermophilic condition requires a greater power input for heating. In order to increase the temperature of the biodigester media, and considering 20 °C as the initial temperature of the fermentation media, the thermophilic process requires at least more than double the energy compared with the mesophilic process. Also, the amount of energy required is greater if the heat released to the environment is taken into account.
Microorganisms under thermophilic conditions exhibit higher metabolic rates, as was reported by Yu and Fang (2003), and thus, greater specific growth rates and, frequently, higher decay rates compared with microorganisms in mesophilic conditions (El–Mashad et al. 2004). It has been reported that alkali–thermophile microorganisms growing at 66 °C and pH 8.5 exhibit doubling times of 10 minutes (Wiegel 1999). Additionally, Demeyer et al. (1981) compared the process stability and the efficiency of mesophilic and thermophilic anaerobic digestions. These advantages indicate that the thermophilic anaerobic process is an interesting option for tropical areas, as the difference between the thermophilic conditions and the temperature of the environment is smaller than that in areas with different climates.
Demeyer et al. (1981) studied several different reactor configurations. Under a steady state, in the daily batch–fed non–mixed single–stage reactor, the yield was greater in the thermophilic process (716 mL g vs –1 d–1) than in the mesophilic process (556 mL g vs –1 d–1).
A study of two–stage biodigesters under thermophilic conditions (68 °C/55 °C) reported (Nielsen et al. 2004) that cattle manure treated at 68°C in the first stage could increase the specific methane yield from 24 to 64 %, depending on the hydraulic retention time in the first reactor (or first stage). The enhanced performance was attributed to an improvement in the hydrolysis. These authors also reported that treating feedstock at 68 °C severely limited the ability of the aceticlastic methanogens and the syntrophic consortia to convert VFA into methane.
Studies on methanogenesis from acetate (Ahring & Westermann 1985) reported that the specific methane production rates, calculated from the exponential growth phase of thermophilic acetate–utilising methanogenic organisms, were optimum near 60 °C. The same research group reported that, for a lab–scale continuously stirred reactor fed with cattle manure (Ahring et al. 2001), an increase in temperature from 55 to 65 °C reduced the specific methanogenic activity (SMA) from 200 to 160 mL g vs –1 d–1, and simultaneously, the level of total volatile fatty acids (VFA) increased from less than 0.3 g L–1 to 1.8–2.4 g acetate L–1. It is important to point out that acetate increased significantly just after changing the temperature from 55 to 65°C, and peaked near 8 g acetate L–1. In addition, a decrease in activity was recorded for glucose–, acetate–, butyrate– and formate–users, and no significant change in activity was registered with propionate. Only the hydrogen–consuming methanogens showed an enhanced activity at 65°C. This indicates that a sudden increase in temperature affects the microbial population, reducing VFA consumption. A reduction in methane yield was observed as a result of a reduced VFA consumption. Finally, Wu et al. (2006) reported that thermophilic microorganisms appear to be highly resilient to temperature fluctuations during the process, however, the longer the low temperature lasted, the greater the decay of methanogenic bacteria. Considering that the claimed optimal temperature for thermophilic anaerobic digestion is still unclear, particularly for poultry litter as the sole feed source, the biodigester performance in this study was quantified in the temperature range near the optimal operation temperature of 55°C.
MATERIALS AND METHODS
Experiments were carried out in the fall of 2003 (run 1) and spring of 2004 (run 2) in a 40 m3 cylindrical anaerobic biodigester, in the campus of West Virginia State University (WVSU), WV, USA. The biodigester was 4.2 m in diameter, 3.5 m in height and had a conical shaped bottom. The feed was prepared in a tank and the biodigester was fed by a pump. The working volume was 27.43 m3 (Espinosa–Solares 2009), the feedstock for the experiments was poultry litter (manure, feathers and wood chips) from wood chip based bedding, and was delivered to the site by a commercial producer in Moorefield, WV. The litter was diluted with fresh water to a total concentration of solids of 57%. The feed slurry was automatically fed into the biodigester every hour. Samples of undiluted fresh litter were collected and analysed for moisture percentage, and total nitrogen (EPA 351.3, described in EPA 1979) and phosphorus (SW 846–6010B, described in EPA 1998). The fatty acid profiles of the litter and biodigester media were obtained by chromatography (Varian 3300 Gas Chromatograph, FID detector; glass column packed with 80/120 Carbopack B–DA/4% Carbowax 20M, Supelco Inc.1975).
The biodigester was heated by an external shell and tube heat exchanger and recirculation pump. The biodigester liquid was pumped through the tubes and back into the tank, and a heated glycol/water mixture (70°C) was pumped through the shell. The pumps were computer controlled. Heating occurred when the internal temperature of the biodigester, measured by an internal thermocouple, dropped 0.1°C below the target. Biogas in the headspace was pulled by a blower through a bubbling ring located at the base of the biodigester for 5 minutes each hour. Biogas was discharged from the biodigester by differential pressure, and cooled to remove water prior to flow and composition measurement. A detailed description of the system has been reported elsewhere (Espinosa–Solares et al. 2006). A Coriolis (Emerson, #CMF025M319NABAEZZZ) mass flowmeter measured biogas discharge from the tank. A Drager Multiwarn II methane detector was used to measure the methane percentage in the biogas. Feed and effluent samples were collected twice per week. TS (total solids) and VS (volatile solids) were determined using the Standard methods for the examination of water and wastewater (APHA et al. 1998). The VA (volatile acids), COD (chemical oxygen demand) and AMM (ammonia) were analysed using methods 8196, 8000 and 10031 reported in the Hach Water Analysis Handbook (Anonymous 2004).
The pilot plant had been running for four months at 56.7°C prior to the experiment. Two runs were performed. In the fall of 2003, the biodigester was operated at five different temperatures: 56.7, 55.5, 54.4, 53.3 and 52.2 °C. After data collection at 56.7°C, the temperature was changed by 1.1°C and data collection began after a minimum three–day adaptation period. After data was collected for at least four days, the temperature was again changed by 1.1°C. After run 1, the pilot plant was operated at 56.7°C until feed input was stopped for the winter. The digester was not fed from the end of the 2003 experiment until four weeks before the 2004 experiment began. In the spring of 2004, run 2 started and the digester was operated at four different temperatures: 56.7, 57.8, 58.9 and 60 °C. The retention time was 40 days and temperature changes were introduced during this period.
The Arrhenius model (Equation 1) was used to explain the effect of the temperature on methane yield:
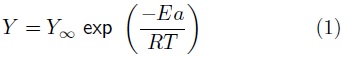
where Y represents the specific methanogenic activity (SMA) (m3 kg vs –1), Y∞ is the pre–exponential factor (m3 kg vsEa is the activation energy (kJ mol–1), T is the absolute temperature (K) and R is the universal gas constant with a value of 8.314 x 10–3 kJ mol–1 K–1.
RESULTS
Table 1 shows the changes in feed, operation and performance of the biodigester during the experiments. The first run reduced the temperature from 56.7 to 52.2 °C, and the second run began at 56.7 °C and finished at 60 °C. As may be seen in Table 1, feeding was constant during the two runs in terms of feed rate and volatile solids. The average influent COD was 52 g L–1, while the ammonia concentration was 17 g L–1 throughout the experiment. The average VFA concentration was 2.6 g L–1.
Table 1 also indicates a clear influence of temperature on the biogas yield in the experiments performed in the temperature range of 52.2 to 56.7 °C. Biogas production decreased when temperature decreased. However, no important changes were observed when temperature changed from 56.7 to 60 °C. In order to quantify the observed effect, the model expressed in Equation 1 was successfully applied to the SMA in run 1. The experimental data in the predicted model are presented in Figure 1. The energy of activation during this run was 146.1 kJ mol–1. This constant could be considered relatively high. In fact, the performance depended strongly on the temperature in the range of 52.2 to 56.7°C. On the other hand, when the temperature increased from 56.7 to 60 °C in run 2, no effect was observed on the SMA. In fact, the performance during the experiment remained almost constant and was easily explained using the average of the four treatments.

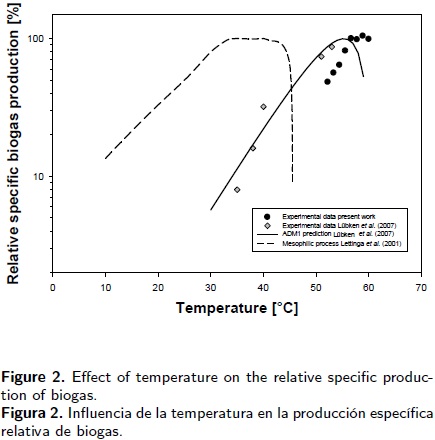
The relative performance was evaluated in this study in order to quantify the effect of the temperature on the methane yield production. Figure 2 presents these parameters for the experiments. The corresponding yield recorded at 56.7 °C was taken as a reference in both cases. Thus, the average methane production in each treatment, as a function of temperature, was used to evaluate the relative performance.
DISCUSSION
Angelidaki et al. (2004) reported that 1.5 g L–1 total VFA may be taken as an indication of a healthy process in full–scale biogas plants. Espinosa–Solares et al. (2006) used the same set–up as the one discussed here, and reported a healthy process using chicken litter as the only feed source, with an average total VFA of 3.7 g L–1 and a range of 2.2 to 5.6 g L–1. An average VFA of 2.6 g L–1 was obtained in this study, and it is possible to consider that the experimental processes were performed under healthy conditions. Thus, the variations detected in the different treatments could be attributed to changes in operational conditions rather than to a VFA concentration effect. It is clear from Table 1 that the amount of biogas and the percentage of methane were greater in the experiments where the temperature was periodically reduced. This may be attributed to the age of the poultry litter feedstock used in the experiments. Feedstock for run 1 was obtained from a poultry house and fed into the digester after 1 month of storage. Feedstock for run 2 was stored for 6 months prior to input into the digester. During storage, the quality of the feedstock decreases due to the loss of available carbon.
The effect of different temperatures on the SMA, regarding the temperature range used, could be attributed to several factors:
a) The typical fatty acids in the chicken litter used in this study include acetate (61.5%), butyrate (30.8%) and propionate (7.7%). It is important to note that other fatty acids are not detectable. In the case of the fermentation media during the biodegradation process, the predominant fatty acids include acetate (50.2%) and propionate (41 %), along with small amounts of iso–butyrate (3.4%), iso–valerate (2.1%), butyrate (1.6%) and valerate (1.6%). It has been reported that methane formed from propionate occurs most rapidly at 55°C, whereas 60°C is the optimal temperature for conversion of acetate, butyrate or formate (Ahring 1994). An increase in temperature to more than 60°C stopped methane production in tests carried out with these substrates. In contrast, formation of methane from hydrogen and CO2 was fastest at 65°C. In our case, the fatty acid profile inside the biodigester indicated that methane production depended mainly on acetate and propionate. These fatty acids have different optimum temperatures for methane production, thus neither 60°C nor 55°C was the best for the chicken litter fermentation media. We found an optimum temperature of 56.7°C in our study, which agrees with the reports of Shiratori et al. (2008) and Ollivier et al. (1985). These authors worked with manure treatments and recorded an optimal temperature of 55 to 58 °C for the growth and metabolic activity of the thermophilic microflora in methanogenic anaerobic digesters.
b) Another factor that could contribute to explain the best response at that temperature is the fact that the digester had been operating at 56.7 °C for two years; therefore it is possible that the consortia were adapted and selected naturally to that operation temperature. Thus, the equilibrium between the increase in performance and the death rate due to rising temperatures was established at 56.7 °C. Besides, an hourly feed input could promote the activity of the consortia, on a minor scale, that produced methane from acetate and butyrate. An enhanced performance of methane production was observed in the experiments as temperature increased up to 56.7°C. After this point, performance was practically the same as at the highest temperature evaluated here (60 °C). There were no significant differences during the second treatment. It is clear that an important reduction in performance took place when temperature changed from 56.7°C to 52.2 °C. A 51.6% SMA reduction was recorded throughout the entire range. The most significant reduction, of 35.8% in SMA, occurred between 56.7 and 54.4 °C, and a 15.8% decrease was observed when changing from 54.4 to 52.2°C. Figure 2 also presents our results compared with those of Lettinga et al. (2001) and Lübken et al. (2007). While Lettinga et al. (2001) reported data for mesophilic conditions, Lübken et al. (2007) presented data obtained for faecal sludge and a substrate of forage and cellulose. As may be seen, the behaviour is very similar. Our experimental data mimics part of those reported for mesophilic anaerobic methane yield (Lettinga et al. 2001; Henze & Harremöes 1983). Data reported in the 60, 70 and 80s has shown that a plateau is reached after an increase up to 35°C, and remains constant up to 40°C(Figure 2). Beyond this temperature, performance decreases approximately 10% when temperature increases to around 43°C, and is followed by a drastic reduction in biogas production at 45°C.
The slopes for this temperature range may be compared with the 3.76 %°C–1 calculated from data reported in 1983 (Lettinga et al. 2001; Henze & Harremöes 1983) for mesophilic methane production (Figure 2). Thus, for the 52.2 to 54.4°C temperature range, the thermophilic process is 1.9 times more sensitive to changes in temperature than the mesophilic process. However, temperature plays a more important role in methane production in the 54.4 to 56.7°C range. The sensitivity to temperature changes was 4.3 times greater than that observed under mesophilic conditions. A small temperature change substantially modified the performance of the biodigester.
Several papers have dealt with the optimal conditions for the thermophilic operation of a biodigester. The best range for thermophilic consortia was reported as 55 to 60°C in 1981 (Demeyer et al. 1981). Lettinga et al. (2001) considered the optimum to be above 60°C, while an optimum thermophilic biodigester operating temperature of 50 to 65 °C was reported in 1992 (Gendebien et al. 1992). In Denmark, commercial thermophilic biodigester operational temperatures vary from 50 to 55°C (Ahring 1994). This apparent disagreement could be attributed to the fact that optimal growth depends on other factors including the chemical composition of the biodigester media, the fluid–dynamic conditions, the chemical composition of the feed, the adaptation of the consortia to the operation temperature, the feedstock and the initial source of microorganisms. All these factors contributed in this study to obtain an optimal thermophilic range that was, for instance, different from that reported by Lübken et al. (2007) (Figure 2). Chicken litter was used as feed in this study, with most of the microorganisms unknown, as Balagurusamy (2007) reported in a preliminary study on the molecular characterisation of eubacteria that was carried out in the same thermophilic anaerobic digester used in this study. Balagurusamy's (2007) results showed that more than 75 % of the clones represented uncultured bacteria. Thus, until now it has been difficult to know exactly which micoorganisms are working, as well as what the optimal conditions are when different materials are fed into an anaerobic reactor.
In light of the present findings, the performance of the anaerobic digestion of chicken litter showed a strong dependence on temperature under thermophilic conditions. A temperature reduction from 56.7 to 52.2°C decreased methane production around 50 %. Applying the Arrhenius model to the specific methanogenic activity, an activation energy value of 146.1 kJ mol–1 was obtained. No important changes in performance were observed when temperature changed from 56.7°C to 60°C. Thus, the optimal operation temperature for the thermophilic anaerobic digestion of poultry litter slurry was 56.7 °C. The influence of temperature on the performance of methane production under thermophilic conditions was between two and four times greater than that with respect to the production obtained through the mesophilic process. This indicates that the thermophilic anaerobic digestion is less stable than the mesophilic digestion when temperature changes take place. Further research is needed in order to evaluate the influence of temperature on the performance and the energy cost of biodigesters, and this will be dealt with in future communications.
ACKNOWLEDGEMENTS
The authors gratefully acknowledge the financial support provided by the West Virginia State University, Division of Agricultural, Consumer, Environmental and Outreach Programs, and the Autonomous University of Chapingo, Agroindustrial Engineering Department. The study was funded by USDA Administrative Grant 2004–06200. We would also like to thank Mike Easter and Chris Postalwait for their contributions.
REFERENCES
Ahring BK (1994) Status on science and application of thermophilic anaerobic digestion. Water Sci. Technol. 30(12): 241–249. [ Links ]
Ahring BK, Westermann P (1985) Methanogenesis from acetate: physiology of a thermophilic, acetate–utilizing methanogenic bacterium. FEMS Microbiol. Lett. 28(1): 15–19. [ Links ]
Ahring BK, Ibrahim AA, Mladenovska Z (2001) Effect of temperature increase from 55 to 65 °C on performance and microbial population dynamics of an anaerobic reactor treating cattle manure. Water Res. 35(10): 2446–2452. [ Links ]
American Public Health Association/American Water Works Association/Water Environment Federation (1998) Standard Methods for the Examination of Water and Wastewater, 20th ed., Washington DC, USA. 1220 pp. [ Links ]
Angelidaki I, Boe K, Ellegaard L (2004) Effect of operating conditions and reactor configuration on efficiency of full–scale biogas plants. En AD2004 Conference (IWA) 29 Aug. to 3 Sept. Montreal, Canada. [ Links ]
Anonymous (2004) Hach Water Analysis Handbook, 4th ed. Hach, Colorado, USA. 1700 pp. [ Links ]
Balagurusamy N (2007) A preliminary study on molecular characterization of the eubacteria in a thermophilic, poultry waste fed anaerobic digester. Revista Mexicana de Ingeniería Química 6(003): 237–242. [ Links ]
Demeyer A, Jacob F, Jay M, Menguy G, Perrier J (1981) La conversion bioénergétique du rayonnement solaire et les biotechnologies. Ed. Technique et Documentation, Paris, France. 328 pp. [ Links ]
El–Mashad HM, Zeeman G, van Loon WKP, Bot GPA, Lettinga G (2004) Effect of temperature and temperature fluctuation on thermophilic anaerobic digestion of cattle manure. Biores. Technol. 95(2): 191–201. [ Links ]
El–Mashad HM, van Loon WKP, Zeeman G, Bot GPA (2005) Rheological properties of dairy cattle manure. Bioresur. Technol. 96(5): 531–535. [ Links ]
EPA (Environmental Protection Agency) (1979) Methods for Chemical Analysis of Water and Wastes, EPA 600/4–79–020, USA. [ Links ]
EPA (Environmental Protection Agency) (1998) Solid Waste Procedures, USA. [ Links ]
EPA (Environmental Protection Agency) (2002) Standards for Use and Disposal of Sewage Sludge, 40 Code of Federal Regulations, Part 503, USA. [ Links ]
Espinosa–Solares T, Bombardiere J, Chatfield M, Domaschko M, Easter M, Stafford DA, Castillo–Angeles S, Castellanos–Hernandez N (2006) Macroscopic mass and energy balance of a pilot plant anaerobic bioreactor operated under thermophilic conditions. Appl. Biochem. Biotechnol. 132(1/3): 959–968. [ Links ]
Espinosa–Solares T, Valle–Guadarrama S, Bombardiere J, Domaschko M, Easter M (2009) Effect of heating strategy on power consumption and performance of a pilot plant anaerobic digester. Appl. Biochem. Biotechnol. 156(1/3): 35–44. [ Links ]
Gendebien A, Pauwels M, Constant M, Ledrut–Damanet MJ, Nyns EJ, Willumsen HC, Butson J, Fabry R, Ferrero GL (1992) Landfill Gas: From Environment to Energy. Directorate General for Energy, Commission of the European Communities. [ Links ]
Henze M, Harremöes P (1983) Anaerobic treatment of wastewater in fixed film reactors literature review. Water Sci. Technol. 15(8/9): 1–10. [ Links ]
Lettinga G, Rebac S, Zeeman G (2001) Challenge of psychrophilic anaerobic wastewater treatment. Trends Biotechnol. 19(9): 363–370. [ Links ]
Lide DR, Frederikse HPR (1995) CRC Handbook of Chemistry and Physics, 76th ed., CRC Press, Inc., Boca Raton, FL. 2712 pp. [ Links ]
Lübken M, Wichern M, Letsiou I, Kehl O, Bischof F, Horn H (2007) Thermophilic anaerobic digestion in compact systems: investigations by modern microbiological techniques and mathematical simulation. Water Sci. Technol. 56(10): 19–28. [ Links ]
Nielsen HB, Mladenovska Z, Westermann P, Ahring BK (2004) Comparison of two–stage thermophilic (68 °C/55 °C) anaerobic digestion with one–stage thermophilic (55 °C) digestion of cattle manure. Biotechnol. Bioeng. 86(3): 291–300. [ Links ]
Ollivier BM, Mah RA, Ferguson TJ, Boone DR, Garcia JL, Robinson R (1985) Emendation of the genus Ther–mobacteroides: Thermobacteroides proteolyticus sp. nov., a proteolytic acetogen from a methanogenic enrichment. Int. J. Syst. Bacteriol. 35(4): 425–428. [ Links ]
Shiratori H, Ohiwa H, Ikeno H, Ayame S, Kataoka N, Miya A, Beppu T, Ueda K (2008) Lutispora thermophila gen. nov., sp. nov., a thermophilic, spore–forming bacterium isolated from a thermophilic methanogenic bioreactor digesting municipal solid wastes. Int. J. Syst. Evol. Microbiol. 58(2): 964–969. [ Links ]
Supelco, Inc. (1975) Supelco Bulletin 749E. Bellefonte, PA, USA. [ Links ]
Yu HQ, Fang HHP (2003) Acidogenesis of gelatin–rich wastewater in an upflow anaerobic reactor: Influence of pH and temperature. Water Research 37: 55–66. [ Links ]
Wiegel J (1999) Ecology and diversity of anaerobic alkali–thermophiles, En: 8th International Symposium on Microbial Ecology, Halifax, Canada. Atlantic Canada Society for Microbial Ecology: 1–8. [ Links ]
Wu MC, Sun KW, Zhang Y (2006) Influence of temperature fluctuation on thermophilic anaerobic digestion of municipal organic solid waste. J. of Zhejiang Univ. SCIENCE B 7(3): 180–185. [ Links ]














