Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Universidad y ciencia
versão impressa ISSN 0186-2979
Universidad y ciencia vol.26 no.1 Villahermosa Abr. 2010
Nota Científica
Optimal concentration of kanamycin as a selective agent for the transformation of Musa cv. "Grand nain"
Concentración óptima de kanamicina como agente selectivo para la transformación de Musa cv. Enano Gigante
BH Chi–Manzanero2, POM Acereto–Escoffié1, Ε Castaño3, LC Rodríguez–Zapata2*
1 Facultad de Ingeniería Química, Universidad Autónoma de Yucatán. (POMAE)
2 Unidad de Biotecnología, Centro de Investigación Científica de Yucatán, Mérida, Yucatán. (BHCM)(LCRZ)*Correo electrónico: lcrz@cicy.mx
3 Unidad de Bioquímica y Biología Molecular de Plantas, Centro de Investigación Científica de Yucatán, Mérida, Yucatán. (EC)
Recibida 4 de agosto de 2008
Aceptada 26 de noviembre de 2009
ABSTRACT
The effect of different kanamycin concentrations on the growth and morphogenesis of meristematic tissue in the banana "Grand Nain" is presented in this study. The results obtained allowed us to establish the kanamycin concentration to be used for the selection of explants transformed by the nptll gene. This result will allow us to select the banana explants transformed by the integration of the nptll gene.
Key words: Kanamycin, Musa, meristematic tissue.
RESUMEN
En este trabajo se presenta el efecto de diferentes concentraciones de la kanamicina sobre el crecimiento y la morfogénesis de tejido meristemático del banano cv. Enano Gigante. Los resultados obtenidos permitieron establecer la concentración de kanamicina a usar para la selección de explantes transformados con el gene nptll. Este resultado permitirá la selección de explantes de banana transformados por la integración del gen nptll.
Palabras clave: Kanamicina, Musa, tejido meristemático.
INTRODUCTION
The molecular breeding of the banana plant is necessary considering its long life cycle and triploidy, as well as the sterility of most of the edible cultivars. Despite being one of the important food crops in the developing world, economically important cultivars of banana, including Cavendish and Lady Finger, have not benefitted from traditional plant breeding practices because of these major impediments (Khanna H, Becker D, Kleidon J, Dale J, 2004. Molecular Breeding 14: 239–252). Hybrids produced by classical breeding programmes often lack one or more important characteristics such as flavour and aroma, a suitable post–harvest physiology, fruit bunch shape, dwarfness, a short cycle, etc. Genetic engineering as an alternative method of banana improvement has the advantage that new characteristics can be brought into the plant without changing the desirable characteristics of the existing cultivar and, as the plant is vegetatively propagated,these characters, if expressed, offer an immediate genetic gain. Most transformation protocols require a tissue culture step, which allows the generation of transgenic plants through organogenesis or somatic embryogenesis using plant growth regulators and other compounds in the culture medium. Species including soybean, banana and sugar beet may be regenerated by both methods (Hansen G, Wright MS, 1999. Trends in Plant Science 4: 226–231). Kanamycin is the selective agent most frequently used in the selection of transformed plants. Thus, knowledge on the sensibility to this antibiotic of particular explant species is a key element in the development of a transformation system integrating the gene for kanamycin resistance (nptll). Kanamycin has previously been used in the selection of transformed Musa AAA Grand Nain explants. The use of 100 mg L–1 of kanamycin sulphate as a selective agent has been reported. Although ca. 50% of the apical meristems and 40 % of the corm slices formed shoots on the selective micropropagation medium, only 40 % of the tissues that formed shoots presented a vigorous root growth when transfered to a kanamycin–containing rooting medium. This result implied that ca. 60% of the shoots may have been chimeric and did not contain transformed meristematic tissue that would give rise to kanamycin–resistant roots (May GD, Afza R, Mason HS, Wiecko A, Novak FJ, Arntzen CJ, 1995. Biotechnology 13: 486–492). In other studies, embryogenic cells of Musa Grand Nain were bombarded and selected on a medium containing 100 mg L–1 of kanamycin. Subsequently, kanamycin was omitted from an embryo germinating medium, but was re–introduced at the rooting stage when germinated embryos formed roots (Becker DK, Dugdale B, Smith MK, Hardin RM, Dale JL, 2000. Plant Cell Reports 19: 229–234). Additionally, a kanamycin stimulant effect has been reported for the in vitro culture of several species (Costa MGC, Nogueira FTS, Figueira ML, Otoni WC, Brommonschenkel SH Cecon PR, 2000. Plant Cell Reports 19: 327–332), as well as phytotoxicity with both undifferentiated calli and organogenesis and regeneration of shoots (Tang H, Ren Z, Krczal G, 2000. Plant Cell Reports 19: 881–887). Both kanamycin and streptomycin favour shoot differentiation in tobacco leaf sections, and kanamycin also stimulates shoot production from carrot calli (Holford P, Newbury HJ, 1992. Plant Cell Reports 11: 93–96).
Considering the above, the purpose of this study was to evaluate the effect of kanamycin on the growth and organogenesis of banana explants in order to determine the optimal concentration to be used as a selective agent in the development of a transformation system for the banana Musa acuminata cv "Grand Nain".
MATERIALS AND METHODS
Plant material
Highly meristematic tissue of the banana Musa acuminata cv "Grand Nain" derived from the meristems of in vitro–grown shoots and "scalps" was obtained from the Laboratory for Tropical Crop Improvement, Catholic University of Leuven, Belgium.
Tissue culture
Scalps were maintained on a semi–solid Murashige and Skoog medium (Murashige T, Skoog F, 1962. Physiologia Plantarum 15: 473–497) containing 2% sucrose, 100 μΜ bencilaminopurine (BAP), 1 μΜ indolacetic acid (IAA) and 0.8% agar. The pH was adjusted to 5.6 prior to autoclaving. Before use, scalp cultures were maintained at a constant 15 °C, with a 12 h photoperiod of white light (Phillips fluorescent white light) in order to minimise sub–culturing cycles. For proliferation, scalp cultures were incubated at 25 °C in a 16 h day–1 photoperiod of white light, and sub–cultured every 15 days. Kanamycin was dissolved in water, filtersterilised with Millipore 0.22 μm filters, added to an autoclaved medium, cooled to 50 °C, and poured into Gerber jars as a selective medium. The medium volume per Gerber jar was 20 ml.
Experimental method
The following levels of kanamycin were evaluated: 0, 75, 150, 300 and 600 mg L–1 . Sample size was four explants per petri dish, with four repetitions per treatment. Samples were incubated at 25 °C in a 16 h day–1 photoperiod of white light and sub–cultured every 15 days. Fresh weight and morphological development were recorded. The fresh weight data were transformed to relative growth (RG) using the following formula: Ps–Pi/Pi, where Ps is the explant weight recorded in each sub–culture and Pi is the initial weight.
RESULTS AND DISCUSSION
The relative growth (RG) of the explants cultivated with different levels of kanamycin for 60 days, as well as that of the control (without kanamycin), are shown in Figure 1. Large differences may be observed in the increase in explant weight, 30 days after inoculation. The control showed a RG close to 20, while the different treatments applied (75, 150, 300 and 600 mg L–1 kan) showed RG values between 20 and 50 % of the value recorded for the control. The inhibitory effect of kanamycin on tissue growth was clearly visible at day 45; the control registered a RG of 75 in relation to its initial weight, while the treatments with 75 and 600 mg L–1 of kanamycin presented a RG of 19 and 5.5, presented a RG above 400 and 800, at days 75 and 90 respectively, while the explants had a maximum RG of 30 in the presence of kanamycin. However, no necrosis was observed in the explants (data not shown) other than a partial necrosis in the presence of 600 mg L–1 of kanamycin presented a RG of 19 and 5.5, respectively.
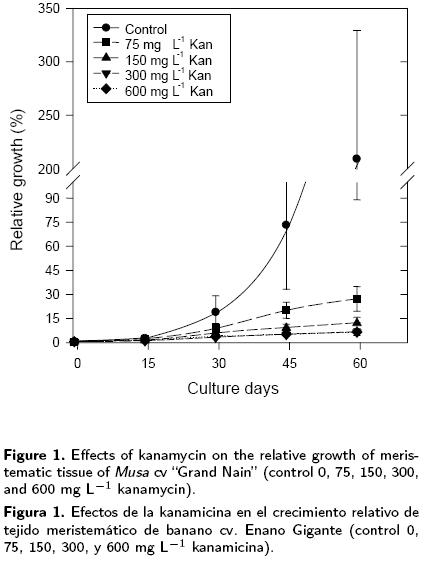
The treatment with 75 mg L–1 of kanamycin was the least inhibitory of growth, while there was no difference between treatments in the range of 150–600 mg L–1 of kanamycin. A RG above 200 was recorded for the control at day 60, whereas the RG increased from 1 to 7 times the initial weight in the treatments with kanamycin. The difference between the treatment with 75 mg L–1 of kanamycin and those with kanamycin levels above this, was maintained. For the last sub–cultures, the control presented a RG above 400 and 800, at days 75 and 90 respectively, while the explants had a maximum RG of 30 in the presence of kanamycin. However, no necrosis was observed in the explants (data not shown) other than a partial necrosis in the presence of 600 mg L–1 of kanamycin.
From these results it is concluded that this type of banana explant has a high natural resistance to kanamycin, as has been reported previously for other monocotyledons. For example, concentrations of kanamycin above 500 mg L–1 are required to inhibit the growth of rice calli (Colby SM, Meredith CP, 1990. Plant Cell Reports 9: 237–240), and more than 800 mg L–1 are required to inhibit the growth of several Gramineae cell cultures incubated in darkness (Hauptmann RM, Vasil V, Ozias–Akins P, Tabaeizadeh Z, Rogers SG, Fraley RT, Horsch RB, Vasil IV, 1988. Plant Physiology 86: 602–606). In the case of transformed M. truncatula cv. Jema–long lines, the effect of kanamycin was observed only with 300 and 400 mg L–1 on the first pair of leaves which became white. Considering the high level of resistance to kanamycin of the seedlings, it is believed that the greatest concentration should be used to assure selection efficiency. This optimised antibiotic selection scheme eliminates the regeneration of non–transformed escapes and discriminates between resistant and non–resistant seedlings, confirming the inheritance of the phenotype (Roldão–Lopes AD, de Sousa Araujo AS, 1 S., Metelo–Fernandes dos Santos DM, Salema–Fevereiro MP, 2004. Plant Cell, Tissue and Organ Culture 78: 277–280). In addition, a high kanamycin dose in apple foliar explant cultures has been associated with a change in the colour of the explants to yellow or white (Norelli JL, Aldwinckle HS, 1993. J. Amer. Soc. Hort. Sci. 118:311–316). This response is possibly related to the mode of action of kanamycin, since this antibiotic acts by inhibiting the initiation of plastid translation during the binding to the 30S ribosomal sub–units present in plastids (Kapaun JA, Cheng ZM, 1999. HortScience 34: 727–729). This may explain why explants, lacking functional plastids after being hete–rotrophically cultivated, are resistant to high levels of kanamycin.
With respect to morphology, shoots began to differentiate from the control explants after the third week. These began to appear green in ca. the fifth week when foliar primordia differentiation was evident (see Figure 2). However, the development of the shoots was notably delayed in the explants cultivated in the presence of kanamycin (Figure 2). Although some foliar primordia developed in the presence of kanamycin, they finally became black and necrotic (data not shown). This may be attributed to the inhibitory effect of kanamycin on the translation process of the plastids (Kapaun JA, Cheng ZM, 1999. HortScience 34: 727–729). Similar effects on organogenesis have already been reported. For example, in the case of apple explants, a total inhibition of shoot regeneration from foliar explants has been observed and the tissues turned chlorotic, and eventually necrotic, with 5 mg L–1 of kanamycin (Yepes LM, Aldwinckle HS, 1994. Plant Cell Tissue and Organ Culture 37: 257–269).
In Vitis vinifera, concentrations of at least 4 mg L–1 of kanamycin inhibit root formation in shoots obtained from micro cuttings (Perós JP, Torregrosa L, Berger G, 1998. Journal of Experimental Botany 49: 171–179). The inhibitory effect of kanamycin has also been demonstrated during the somatic embryogenesis of walnuts, through the inhibition of the formation of secondary embryos cultivated in the presence of 100 mg L–1 of kanamycin (Tang H, Ren Z, Krczal G, 2000. Plant Cell Reports 19: 881–887).
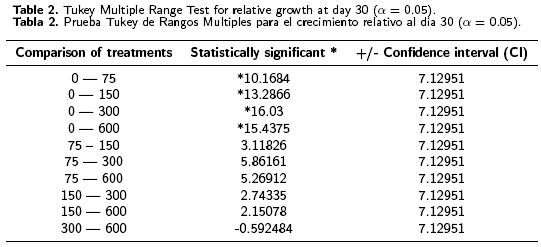
One–way ANOVA tests applied to the results obtained in this study indicated a statistically significant difference at day 15 between the relative growth means from one level to another, at the 95 % confidence level (see Table 1). The Tukey multiple range tests indicated statistically significant differences between the control and the treatments with 150, 300 and 600 mg L–1 of kanamycin, as well as between the treatment with 75 and those with 150, 300 and 600 mg L–1 of kanamycin (data not showed). There was a statistically significant difference after day 30 between the control and all the levels assayed, although there was no statistically significant difference between these (see Table 2). In spite of there being no statistically significant difference between treatments after day 30, it was obvious that 150 mg L–1 of kanamycin is the optimal concentration for the selection of transformed banana cells, as this was the minimum concentration at which inhibition of growth and of organogenesis were observed. This contrasts with the treatment with 75 mg L–1 of kanamycin in which, notwithstanding that morphogenesis was inhibited, RG was 60–70 % higher than in the other treatments that allowed some escapes. The concentration of 150 mg L–1 reported here is higher than that reported (100 mg L–1) for explant regeneration by organogenesis (May GD, Afza R, Mason HS, Wiecko A, Novak FJ, Arntzen CJ, 1995. Biotechnology 13: 486–492). In that study, the kanamycin concentration did not allow a good selection of transformed explants. Another study reported 100 mg L–1 of kanamycin for the selection and regeneration of bombarded embryogenic cells. This concentration made it possible to select individual transformed cells (Becker DK, Dugdale B, Smith MK, Hardin RM, Dale JL, 2000. Plant Cell Reports 19: 229–234).
The inhibition of bud formation in banana explants in the presence of kanamycin, and their delayed growth in relation to the control, indicated that this antibiotic is a good selective agent for the development of transformation protocols for banana. It is probable that the transformed cells will be able to metabolise the kanamycin and form new buds, even at high kanamycin concentrations, and in this way generate transformed plants.














