Introduction
The ocellated wrasse,Symphodus ocellatus(Linnaeus 1758), is a common, resident species in the Black, Adriatic, Mediterranean, and Azov seas (Hureau 1986), where it inhabits rocky bottoms and eelgrass beds (Lejeune 1985, Hureau 1986, Harmelin 1987). AlthoughS. ocellatuscan be found from 1 to 30 m depth, these fish generally remain in the benthos throughout their lives, where they feed on mollusks, gastropods, crustaceans, and foraminifera (Bell and Harmelin-Vivien 1983, Lejeune 1985). When they venture away from the benthos,S. ocellatuscan be found in the water column foraging on plankton (e.g., copepods) when they are abundant (Bell and Harmelin-Vivien 1983, Lejeune 1985).Symphodus ocellatusrange from 8 to 12 cm in length and live for 3-5 years (Lejeune 1985, Hureau 1986, Harmelin 1987). The wide distribution and high density ofS. ocellatusin the aforementioned seas has resulted in this ichthyological species being commonly employed to estimate ecological impacts (e.g., anthropogenic or natural disturbances).
In autumn,S. ocellatusjuveniles become plentiful prey for other species given their high abundance (24-85 juveniles per 100 m2; P. Francour, pers. comm.; Levi at al. 2005).Symphodus ocellatusreproduction takes place between May and July in the northwestern Mediterranean Sea (Quignard and Pras 1986), with males exhibiting 3 distinct phenotypes with different behavioral characteristics (i.e., nesting males, satellite males, and sneaker males; Warner and Lejeune 1985, Taborsky et al.1987). Males build nests of algae in rocky depressions, which become spawning sites for females (Alonzo et al. 2000). Embryos within fertilized eggs develop for 3-4 days before hatching and entering the plankton as larvae (Alonzo et al. 2000).
Several studies have investigated the reproductive behaviors ofS. ocellatus(Warner and Lejeune 1985, Taborsky et al. 1987, Bentivegna and Benedetto 1989, Budaev and Zworykin 1998, Alonzo et al. 2000, Alonzo and Warner 2000, Stiver and Alonzo 2013, Sinopoli et al. 2014, Stiver et al. 2014). In addition, Mouillot et al. (1999) evaluated the distribution of this species, while Škeljo and Ferri (2012) evaluated its otolith morphology, and Peskov and Manilo (2018) assessed size-age relationships and sexual plasticity in the Black Sea. However, some information on age and growth comes from studies conducted more than a quarter of a century ago (Pauly 1978, Quignard and Pras 1986).
Fisheries management is important to ensure sustainable fisheries and must be adapted to meet the requirements of different fishing areas (Uzer et al. 2019). Some population parameters, such as fish age and growth, must be studied to improve our understanding of fisheries biology and current management strategies, which are critical to preserve stocks into the future. Several methods are used to determine age in fish (Treble et al. 2008). Otolith growth increments are widely used and can provide information on annual growth (Campana and Thorrold 2001). The biological information ofS. ocellatus, a non-commercial species, is very limited, despite its ecological importance in the region. Thus, the aim of this study was to describe the fundamental biological characteristics ofS. ocellatus, including age, growth, the sex-ratio, fish condition, spawning period, and length-weight relationships in the southeastern Black Sea.
Materials and methods
Sampling
This study was conducted along the Rize coast in the southeastern Black Sea, Turkey (Fig. 1). AllS. ocellatusspecimens were collected from the discards of the commercial fleet, which were obtained using trammel nets (36- and 40-mm mesh) at depths of 10-40 m from June 2015 to May 2016. The number of specimens by sex and combined sexes per month is presented in Table 1. Following transportation to a laboratory of the Fisheries Faculty of Recep Tayyip Erdogan University, Rize (Turkey), the fish samples were immediately measured (total length, TL) to the nearest 0.10 cm and weighed to the nearest 0.10 g. Sex was determined by macroscopic examination of the gonads.
Sex ratio
The monthly length frequency distributions for females and males were calculated using 0.50-cm class intervals and TL. The chi-square test was used to calculate the sex-ratio. The deviations from 1.00:1.00 were tested using the chi-square analysis (Dagnelie 1975).
Length-weight relationships, condition factor, and gonadosomatic index
The length-weight relationship (LWR) was determined with the equationW=aL b (Ricker 1975), whereWis the total weight (g),ais the intercept,bis the slope, andLis the TL (cm). The variation ofbfrom 3 was evaluated with a one-samplet-test. The degree of association between variables was determined withr 2(King 1995).
The condition factor is the ratio between body weight and length, which reflects the interaction between biotic and abiotic variables with regard to the physiological state of the fish (Le Cren 1951). The condition factor (K) was calculated with the equationK=W/aL b (Le Cren 1951), whereWis the observed total weight (g) andaL b is the estimated weight obtained from the LWR (Le Cren 1951). Seasonal age and sex were evaluated to determine how they affect fish condition using a general linear model. The data were statistically analyzed using SPSS (version 15) for Windows.
The spawning season in fish can be estimated from gonadal maturity stages or the gonadosomatic index (GSI), which is a good indicator of reproductive activity (Rizzo and Bazzoli 2020). Sex was recorded, and gonad wet weight was measured to the nearest 0.01 g. Monthly, GSI values were calculated for both females and males with the equation from Avşar (2005): GSI =Wgonad/W× 100, whereWgonadis gonad weight (g) andWis fish total weight (g).
Age and growth
To determine age, the otoliths of 320 fish were removed, cleaned, and stored in Eppendorf tubes containing 70% ethanol for 3-4 weeks. Age was estimated by counting the number of opaque (light = fast growth) and hyaline bands (dark = slow growth) on the sagittal otoliths below reflected light (Panfili et al. 2002).
The age estimations were performed by a single reader who interpreted and counted the hyaline growth bands (annuli; Landa et al. 2014; Fig. 2) following the overall agreed upon otolith margin interpretation scheme for several species (Vitale et al. 2019). The date of birth was taken to be 1 January. The age estimation scheme per quarter is shown in Table 2. Several factors, such as edge type (hyaline and opaque), age resolution, date of capture, theoretical birthday, and the number of annuli, were taken into consideration in the scheme (ICES 2017). Given the aforementioned date of birth, if a hyaline band was observed at the otolith edge during the first half of the year, it was counted as an annulus. On the contrary, if a hyaline band was observed at the otolith edge during the second half of the year, then it was not counted as an annulus (ICES 2012).
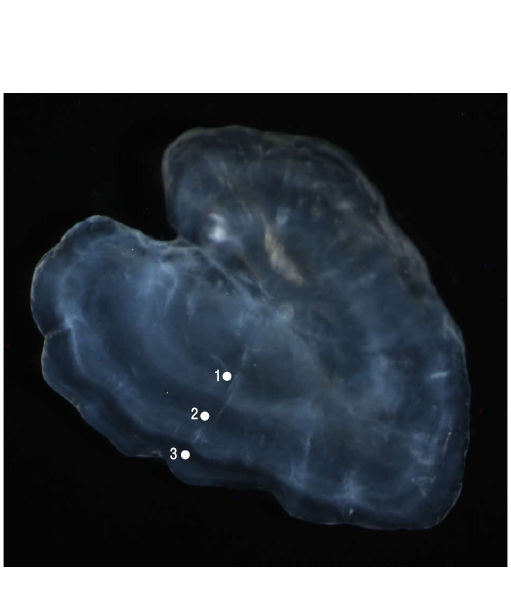
Figure 2. A sagittal otolith from anSymphodus ocellatusindividual caught in July 2015. An opaque edge is apparent, and the estimated age is 3 years.
Table 2. The age estimation scheme forSymphodus ocellatuswith a theoretical birthday on 1 January.
| Date of capture | Otolith edge | Age |
| First and second quarters | Translucent | N |
| Opaque | N+ 1 | |
| Third and fourth quarters | Translucent | N- 1 |
| Opaque | N |
The otoliths were imaged using a Nikon SMZ1000 digital camera connected to a Nikon SMZ1000 stereomicroscope (magnification from ×0.8 to ×8.0; Nikon, Tokyo, Japan) (Fig. 2). The Kolmogorov-Smirnov 2-sample test was used to compare the length frequency for each age between sexes.
Otolith marginal analyses
Two otolith marginal analyses were performed. The nature of the margin (opaque or hyaline) was identified in 182 otoliths. The proportion of individuals with hyaline and opaque edges was evaluated by month to determine if an annual formation pattern was present and by age to evaluate possible ontogenetic differences in the time of opaque edge formation (Holden and Raitt 1974). Then, the absolute marginal distance (AMD), which was estimated using the distance between the end of the last hyaline annulus and the edge, and the distance between the last 2 hyaline annuli (Di, i-1) were used to estimate the relative marginal distance (RMD: AMD/Di, i-1; Panfili et al. 2002, Navarro et al. 2021). Only whole otoliths were selected and measured (μm) using a microscope. Each month, 5-27 otoliths were selected, which amounted to a total of 108 otoliths representative of the entire length range.
The von Bertalanffy growth function was determined with the following formula:
wheret 0 is the theoretical age at 0 length,kis the body growth coefficient,L ∞ is the asymptotic mean maximum total length, andLtis the expected total length at agetyears (von Bertalanffy 1938). Munro’s growth performance index,Ø= log(k) + 2log(L ∞ ), was calculated in order to compare the growth results obtained with the findings of other studies (Pauly and Munro 1984). Parameters were determined separately for males, females, and combined sexes (R Foundation for Statistical Computing 2021-11-01) with the ‘FSA’ (Ogle et al. 2021), ‘FSAsim’ (Ogle 2020), and ‘nlstools’ (Baty et al. 2015) packages.
Results
Composition by sex and sex ratio
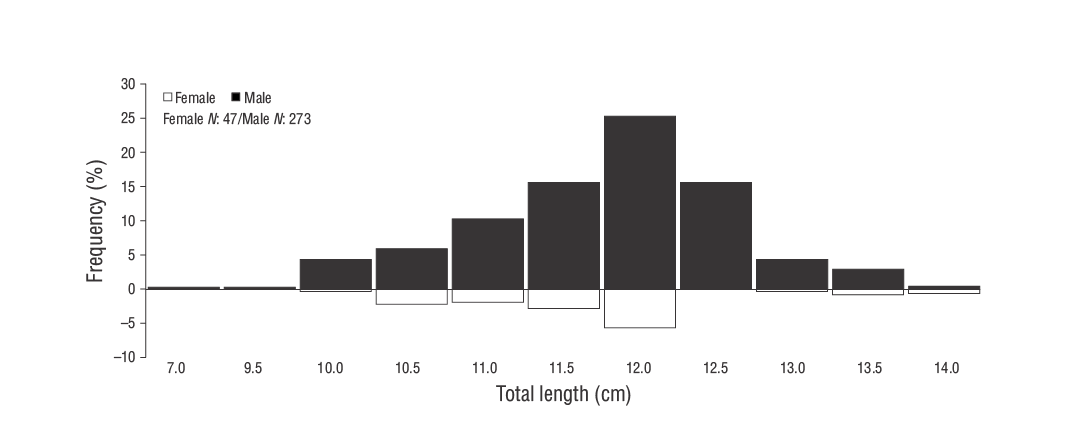
A total of 320S. ocellatusindividuals were collected during the study, of which most were males (males: 273, 85.31%; females: 47, 14.68%). A sex ratio (female:male) of 1.00:5.80 was obtained that was significantly different from the ratio of 1.00:1.00 (P< 0.05). The TL andWvalues of males ranged from 6.90 to 13.70 cm and 6.77 to 33.69 g, respectively, while those of females ranged from 10.00 to 13.70 cm and 15.23 to 33.46 g, respectively. The TL frequency distribution by sex ofS. ocellatusfrom the southeastern Black Sea is given in Figure 3. Monthly variation in the sex ratio was observed throughout the study period, with a vastly greater proportion of males than females from May to September (Table 1).
Length-weight relationships, condition factor, and gonadosomatic index
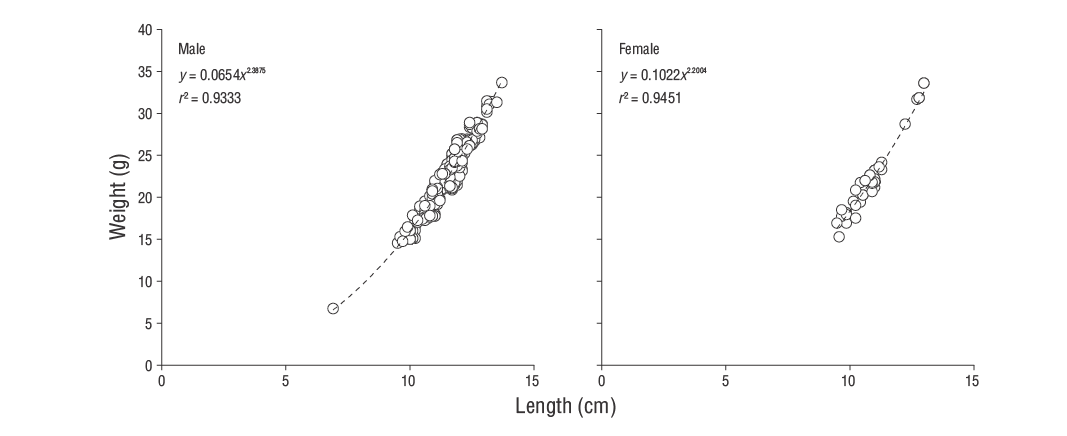
Both male and femaleS. ocellatusexhibited negative allometry (b< 3,t-test:P< 0.050; Fig. 4). The LWR showed a good fit (r 2= 0.93,P< 0.001), and the regression parameters are presented in Table 3.Kwas calculated for all sexes and age-groups (Table 3). No significant effect of age and sex onKwas found according to the general linear model analysis (P> 0.050), although the season had a significant effect (P< 0.050). The highestKvalues were observed in spring and summer, after which they fell sharply in autumn and were at their lowest in winter (Fig. 5a).
Table 3. Von Bertalanffy growth parameters (L ∞ = asymptotic length,k= body growth coefficient, andt 0 = theoretical age), growth performance index (Ø), length-weight relationship parameters (a= intercept andb= slope of the relationship), and number ofSymphodus ocellatus(n) analyzed in the southeastern Black Sea (r 2= coefficient of determination of each function).
| Sex | n | L ∞ | k | t 0 | r 2 | Ø | a | b | r 2 |
| Female | 47 | 14.150 | 0.764 | -0.188 | 0.990 | 2.068 | 0.102 | 2.200 | 0.940 |
| Male | 273 | 12.660 | 0.764 | -1.151 | 0.950 | 1.975 | 0.065 | 2.388 | 0.930 |
| Combined sexes | 320 | 12.630 | 0.791 | -1.080 | 0.950 | 2.000 | 0.070 | 2.362 | 0.930 |
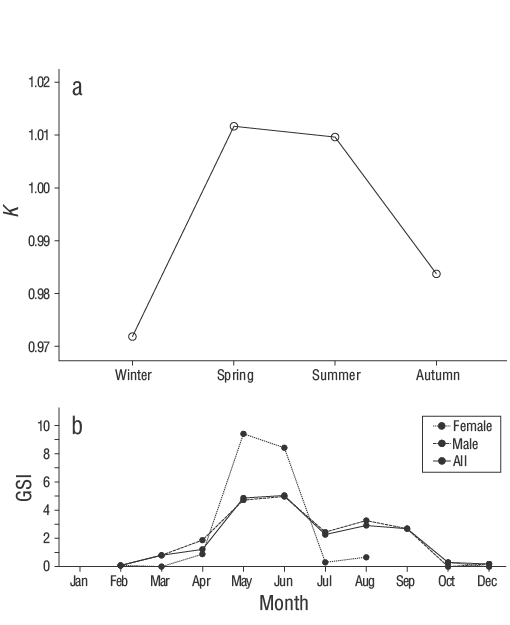
Figure 5. (a) Seasonal condition factor (K) values of combined sexes and (b) monthly gonadosomatic index (GSI) values by sex and combined sexes ofSymphodus ocellatusin the southeastern Black Sea.
The spawning period for males occurs from May to September, with a peak from May to June. The full spawning period for females is not yet known because of the low number of female specimens in this study; however, females also showed a clear spawning peak from May to June. The only conclusion that can be drawn with the limited information available for females is that a peak spawning period for the entire population appears to occur from May to June (Fig. 5b).
Age and growth
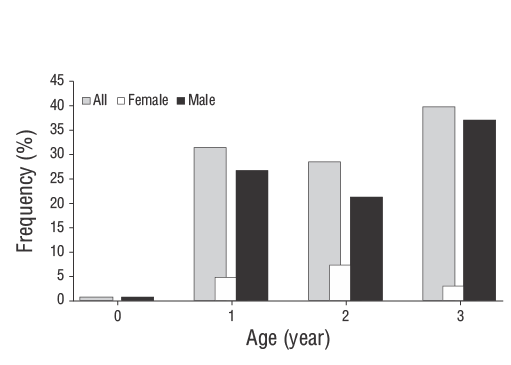
The age composition analysis revealed that most individuals were 1-3 years old (85% male). Only 2 specimens were of age 0. The age composition distribution by sex is given in Figure 6. The minimum length at age 0 was determined to be 6.90 cm, whereas the maximum length at age 3 was determined to be 13.70 cm (Table 4). According to the results of the Kolmogorov-Smirnov test, the difference in TL between sexes at age 1 was not significant (P> 0.050) when length distributions were compared for each age. A significant difference in TL between sexes was found in fish aged 2 (Z= 1.402,P< 0.050) and 3 (Z= 1.560,P< 0.050) years. The age-length keys of males and females are given in Table 5. The mean TL values at ages 2 and 3 were higher in females than in males.
Table 4. Basic biometric data by sex ofSymphodus ocellatusin the southeastern Black Sea.
| Total length (cm) | Total weight (g) | Condition factor | ||||||||
| Age group | Sex | N | Mean ± SD | Range | Mean ± SD | Range | Mean ± SD | Range | ||
| 0 | Male | 2 | 8.20 ± 0.65 | 6.90-9.50 | 10.66 ± 2.93 | 6.77-14.56 | 1.85 ± 0.08 | 1.65-2.06 | ||
| 1 | Female | 15 | 10.56 ± 0.22 | 10.00-11.10 | 18.35 ± 1.03 | 15.23-20.78 | 1.53 ± 0.02 | 1.38-1.68 | ||
| Male | 85 | 10.64 ± 0.09 | 9.60-11.40 | 18.59 ± 0.44 | 14.77-22.97 | 1.48 ± 0.01 | 1.26-1.69 | |||
| 2 | Female | 23 | 11.50 ± 0.17 | 11.00-11.60 | 21.94 ± 0.78 | 20.60-23.11 | 1.43 ± 0.02 | 1.34-1.62 | ||
| Male | 68 | 11.53 ± 0.10 | 11.00-11.80 | 22.41 ± 0.49 | 17.79-25.20 | 1.42 ± 0.01 | 1.25-1.58 | |||
| 3 | Female | 9 | 12.92 ± 0.29 | 11.80-13.70 | 29.03 ± 1.26 | 23.20-33.46 | 1.33 ± 0.03 | 1.27-1.43 | ||
| Male | 118 | 12.28 ± 0.08 | 11.80-13.70 | 26.23 ± 0.37 | 21.47-33.69 | 1.37 ± 0.00 | 1.22-1.58 | |||
Table 5. Age-length keys by sex ofSymphodus ocellatusin the southeastern Black Sea.
| Length (cm) | Females (N) | Males (N) | |||||||||||
| Age (year) | Age (year) | Total | |||||||||||
| N | 0 | 1 | 2 | 3 | N | 0 | 1 | 2 | 3 | N | |||
| 7.00 | 0 | 1 | 1 | 1 | |||||||||
| 9.50 | 0 | 1 | 1 | 1 | |||||||||
| 10.00 | 1 | 1 | 14 | 14 | 15 | ||||||||
| 10.50 | 7 | 7 | 19 | 19 | 26 | ||||||||
| 11.00 | 6 | 5 | 1 | 33 | 28 | 5 | 39 | ||||||
| 11.50 | 9 | 2 | 7 | 50 | 24 | 26 | 59 | ||||||
| 12.00 | 18 | 15 | 3 | 81 | 37 | 44 | 99 | ||||||
| 12.50 | 0 | 50 | 50 | 50 | |||||||||
| 13.00 | 1 | 1 | 14 | 14 | 15 | ||||||||
| 13.50 | 3 | 3 | 9 | 9 | 12 | ||||||||
| 14.00 | 2 | 2 | 1 | 1 | 3 | ||||||||
| Total | 47 | 0 | 15 | 23 | 9 | 273 | 2 | 85 | 68 | 118 | 320 | ||
| Mean length | 10.76 | 11.80 | 13.05 | 8.20 | 10.86 | 11.73 | 12.46 | ||||||
The von Bertalanffy growth curve was fitted to the lengths-at-age for all fish and both sexes (Table 2). The estimated von Bertalanffy growth parameters for combined sexes wereL ∞ = 12.63 cm,k= 0.791 year-1, andt 0 = -1.080 years. The difference ofL ∞ ,k, andt 0 values between sexes was significant according to the fit1LT model selected based on the Akaike information criterion (P< 0.001). Females reached a larger asymptotic TL (L ∞ = 14.15 cm) than males (L ∞ = 12.66 cm) and also grew slightly faster (Ø= 2.068 in females) than males (Ø= 1.975).
Otolith marginal analyses
In Figure 7a, it can be seen that most otoliths showed hyaline edges in the first and second quarters and opaque edges in the third and fourth quarters. Some opaque edges were also observed in the first half of the year in April. The percentage of opaque otolith edges began to increase in February, and more than 50% of otoliths had opaque edges in April, after which this percentage decreased until July and subsequently increased from July to December (Fig. 7a). The hyaline growth increment inS. ocellatusotoliths is therefore formed in winter in the southeastern Black Sea. The AMD and RMD values were lowest from January to February and highest from March to September, although they decreased from June to July (Fig. 7b). After analyzing the edge type by age group, a downward trend was observed from March to July at ages 2 and 3, followed by a steep rise until October. For 1-year-old individuals, December exhibited a percentage of opaque edges greater than 50% (Fig. 7c).

Figure 7. Results of otolith marginal analysis ofSymphodus ocellatusin the southeastern Black Sea: (a) monthly proportion of opaque (black) and hyaline (gray) edges; (b) monthly mean values of relative marginal distance (RMD) and absolute marginal distance (AMD); (c) monthly proportion of opaque edges by age group.
Discussion
This study constitutes a first approach to describing the biological aspects ofS. ocellatusin the southeastern Black Sea based on monthly samples collected from June 2015 to May 2016. Nonetheless, additional studies that include longer sampling times are needed to determine whether the seasonal differences found in this study remain consistent over time.
Composition by sex and sex ratio
The largestS. ocellatusTL value obtained in the present study (13.70 cm) was clearly lower than the largest TL value of 18.50 cm previously recorded within its distribution (Altın et al. 2015), although our value was higher than those reported for the Aegean, Mediterranean, and Marmara seas (6.60-10.70 cm; Valle et al. 2003, Özaydın et al. 2007, İlhan et al. 2008, Gürkan et al. 2010, Keskin and Gaygusuz 2010, Kasapoglu and Duzgunes 2013, Bilge et al. 2014, Kurtele et al. 2019). This difference in TL is probably not due to sample size because variable numbers of specimens were analyzed in other studies. Thus, the reasons for this difference may be biogeographical variability in the growth rate and the differences in local fishing pressure (Pallaoro and Jardas 2003), which likely influence the structure of populations in different areas.
Sex ratio information is necessary to understand relationships between population dynamics, individuals, and the environment (Vicentini and Araújo 2003). The sex ratio varies among species, populations, and periods within the same population, and many factors, such as environmental conditions, food availability, and reproductive behavior, may cause the sex ratio to differ from 1.00:1.00 (Nikolsky 1963, Vandeputte et al. 2012). The large deviation away from the theoretical and expected sex ratio of 1.00:1.00 found in this study indicates that male individuals were clearly dominant over females. A higher, although not very high, proportion ofS. ocellatusmales has also been found in other areas. For example, a sex ratio of 1.11:1.00 was obtained forS. ocellatuscaptured along the Ukrainian coast of the Black Sea (Peskov and Manilo 2018) based on 80 specimens collected from 1958 to 2013. Similarly, males ofSymphodus tinca, a congener, were also predominant during reproductive periods throughout the sampling period in studies conducted along the Croatian coast (1.43:1.00; Pallaoro and Jardas 2003) and in northeastern Tunisia (1.38:1.00; Ben Slama et al. 2010). In the goldsinny wrasse (Ctenolabrus rupestris), Sayer et al. (1995) reported a sex ratio of 1.12:1.00 in different coastal areas of Scotland.
The sex ratio may vary seasonally and regionally depending on the habitat preferences of each sex. For example, sex-based flocking tendencies and differential sex- and age-based migration patterns have been observed (Nilsson 1970). However, additional research based on larger sampling sizes and times is required to fully address this issue in the southeastern Black Sea. The vastly greater proportion of males than females observed from May to September in this study coincides with a portion of the estimated spawning period, which could indicate differential sex-based behavior ofS. ocellatuswith regard to its distribution and thus reproduction.
Symphodus ocellatusexhibits 3 male phenotypes (nesting, satellite, and sneaker) with different behavioral and physical characteristics (Taborsky et al. 1987). Nesting males court females, build nests and defend them, and provide parental care. Sneaker males do not defend or care for nests and do not court females (Taborsky et al. 1987, Alonzo et al. 2000). Satellite males associate with a nesting male and court females but do not build nests or provide parental care (Alonzo and Warner 2000, Alonzo 2008). Numerous males are found in nesting areas during the spawning season, and the proportions of these 3 male phenotypes can vary between sites (Alonzo and Warner 2000, Alonzo and Heckman 2010).
Length-weight relationships, condition factor, and gonadosomatic index
The slope value (b) from the LWRs for combined sexes estimated in this study (2.36) was very low compared to those of other studies conducted in other Mediterranean areas (3.08-3.22; Table 6). Two studies with the largest sample sizes and TL values representative ofS. ocellatus(Keskin and Gaygusuz 2010, Altın et al. 2015) reported similarly highbvalues (3.10-3.20) in Mediterranean areas relatively close to our study area. By not including specimens smaller than 9.50 cm (except one), the present study is limited when compared to other studies, which could be responsible for the lowbvalue we obtained. Carlander (1977) and Froese (2006) demonstrated that values ofb<2.5 or >3.5 are often derived from samples with narrow size ranges. The differences in condition between large and small specimens can vary between years, localities, and seasons and thus result in different weight-length relationships (Froese 2006).
Table 6. Length-weight relationship parameters ofSymphodus ocellatusestimated in the present study and from other studies in the Mediterranean and Black Seas.
| N | Total length range (cm) | Total weight range (g) | a | b | r 2 | |
| Mediterranean | ||||||
| Valle et al. (2003) | 456 | 3.00-9.00 | - | 0.009 | 3.170 | 0.960 |
| Kurtela et al. (2019) | 118 | 2.20-7.80 | 0.10-7.00 | 0.012 | 3.010 | 0.970 |
| Aegean Sea | ||||||
| Özaydın et al. (2007) | 216 | 4.70-9.70 | - | 0.009 | 3.220 | 0.960 |
| İlhan et al. (2008) | 328 | 4.70-9.20 | 1.10-10.56 | 0.009 | 3.190 | 0.970 |
| Gürkan et al. (2010) | 10 | 4.30-6.60 | 0.59-2.79 | 0.004 | 3.480 | 0.980 |
| Bilge et al. (2014) | 274 | 4.60-9.00 | - | 0.010 | 3.130 | 0.950 |
| Altın et al. (2015) | 1922 | 1.22-18.50 | 0.01-81.69 | 0.010 | 3.200 | 0.980 |
| Marmara Sea | ||||||
| Keskin and Gaygusuz (2010) | 575 | 1.80-10.70 | - | 0.010 | 3.080 | 0.970 |
| Black Sea | ||||||
| Present study (females) | 47 | 10.00-13.70 | 15.23-47.53 | 0.102 | 2.200 | 0.940 |
| Present study (males) | 273 | 6.90-13.70 | 6.77-79.97 | 0.065 | 2.380 | 0.930 |
| Present study (combined sexes) | 320 | 6.90-13.70 | 6.77-79.97 | 0.069 | 2.360 | 0.930 |
The meanKvalues ranged from 0.880 to 1.100. A highKvalue implies that the environmental conditions are suitable for a given fish population (Blackwell et al. 2000). As theKvalue in our study was higher than 1.000, the environmental conditions in the southeastern Black Sea are suitable forS. ocellatus. However, the number of females in our study was quite low and varied monthly. This can influenceKgiven that theKvalue is affected by variables such as fish length, sampling method, environment, food source, spawning period, and growth rate (Le Cren 1951).
In this study, the lowestKvalues were observed in winter, while the highest values were observed in spring and summer. Le Cren (1951) also reported that theKdecreases in winter, increases in spring and summer, and decreases again in autumn. Low sea temperature is a factor that negatively influences metabolic processes (e.g., assimilation, digestion, ingestion) and eventually the growth rate in fish. Thus, overwintering is a particularly important period for fish (reviewed by Hurst 2007). During periods of low food availability and/or temperatures,Kdeclines. TheKvalues estimated in this study increased as the spawning season approached, which was probably related to the onset of favorable environmental conditions, and then drastically decreased after summer.
Hermaphroditism, namely a change of sex with age, has been reported in someSymphodusspecies (Quignard 1966, Remacle 1970), such asSymphodus melanocercus, although this has not been observed inS. ocellatus( Warner and Lejeune 1985). MaleS. ocellatushave been reported to display different reproductive behaviors (Lejeune 1985). For example, some males build nests and care for the eggs (Gerbe 1864, Šoljan 1930, Taborsky et al. 1987), while females visit these nests and lay their eggs in multiple nests (Taborsky et al. 1987).
Fiedler (1964), Voss (1976), and Lejeune (1985) reported that the breeding period ofS. ocellatuscan last up to 2 months and occurs between May and June in the Mediterranean Sea. In addition, Quignard and Pras (1986) reported that the spawning season ofS. ocellatustakes place over 4 months in spring and summer (April-July) in the Mediterranean Sea, while Bentivegna and Benedetto (1989) reported that the spawning season takes place from May to August in the Bay of Naples in the Mediterrenean Sea. Salekhova (1971) reported that the spawning ofCrenilabrusspecies is multiportional, and reproduction in the Black Sea occurs from April to August when water temperatures range from 12 to 26 °C, with a spawning peak from the end of May to the end of June. The May-June spawning peak reported in this study for the Rize coast in the southeastern Black Sea agrees with those of previous studies in nearby areas. Along the coasts of Tunisia, the reproductive periods of the congenerS. tincaand the Labridae family were reported to occur from April to June (Ouannes-Ghorbel et al. 2002, Ben Slama et al. 2010) and March to June (Dieuzeide et al. 1954).
Sea temperature is a critical environmental factor that affects fish growth, embryonic and larval development, and reproductive activity (Avşar 2005). Seasonal changes in sea temperature affect fish reproduction. For example, increases in temperature stimulate reproduction in fish species that lay eggs in spring, while decreases in temperature stimulate reproduction in species that lay eggs in autumn (Pankhurst and Munday 2011).
Age and growth
The growth parameters ofS. ocellatusin the Black Sea were estimated in this study for the first time. Moreover, we present a first approach to establishing the age and growth of this species in the Black Sea based on otolith data. Given the absence of the other age studies ofS. ocellatusin the Black Sea, a comparison was performed with the only other study available, which was conducted four decades ago (Pauly 1978) in the neighboring Mediterranean Sea (Table 7). The maximum estimated age (5 years) ofS. ocellatusof Quignard and Pras (1986) was higher than the maximum age of 3 years estimated in this study.
Table 7. A comparison of the von Bertalanffy growth parameters ofSymphodus ocellatusandSymphodus mediterraneusin the present study in the Black Sea and in other Mediterranean areas.
| Reference | Species | Study area | Sex | L ∞(cm) | k(year-1) | t 0 (year) | Ø |
| Pauly D. (1978) | Symphodus ocellatus | Mediterranean Sea | Female | 10.300 | 0.645 | - | 1.840 |
| Male | 10.800 | 0.949 | - | 2.040 | |||
| Present study | Symphodus ocellatus | Black Sea | |||||
| Female | 14.150 | 0.764 | -0.188 | 2.068 | |||
| Male | 12.660 | 0.764 | -1.151 | 1.975 | |||
| Combined | 12.630 | 0.791 | -1.080 | 2.000 | |||
| Škeljo et al (2015) | Symphodus mediterraneus | Mediterranean Sea | Female | 14.110 | 0.330 | -1.420 | 1.810 |
| Male | 16.090 | 0.500 | -0.830 | 2.110 | |||
| All | 13.760 | 0.460 | -1.080 | 1.930 |
A performance index can be used to determine the average growth parameters for a given species (Sparre and Venema 1992) and is beneficial when evaluating growth under environmental stress (Pauly 1991). The slightly faster growth rate in females with larger asymptotic lengths than males in this study contrasts with what has been reported in previous studies ofS. ocellatusin French Mediterranean waters (Pauly 1978) and of the congenerSymphodus mediterraneus(Škeljo et al. 2015), which exhibited higherØ,L ∞ , andkvalues in males than in females. Peskov and Manilo (2018) investigated size-at-age and sex variability inS. ocellatusalong the Ukrainian coast of the Black Sea. According to their results, males were characterized by significantly higher values than those of females. Sayer et al. (1995) pointed to the large difference in the relative size of males and females and annual gonadal development to explain the different growth rates between sexes.
Differences in growth may be due to latitudinal differences in oceanographic variables, such as temperature, or to biological differences in maintenance metabolism and diet among populations (Pauly 1994, Landa et al. 2002). Water temperature affects fish physiology and food availability, which in turn affect biological production (Weatherley and Gill 1987) and growth (Elizarov 1965). Thus, regional ecological conditions may explain the regional variation in growth, although it is not possible to discern the causes behind the differences in sexual growth between this study and that of Pauly (1978). However, the difference in growth observed inS. ocellatusbetween the coasts of France and the Black Sea could be based on differences in trophic resources and water temperature.
The analysis of otolith edges revealed that the periodicity of annulus formation inS. ocellatusis likely annual. Thus, opaque edges predominate in autumn and hyaline edges predominate during the first months of the year (although no information was available for January). The formation of opaque or hyaline bands can be attributed to variables such as seasonal sea temperature cycles, light conditions, fish feeding habits, and reproductive cycles (Beckman and Wilson 1995).
Symphodus ocellatusis not a commercially important fish species and is not a target fish species in the Black Sea. As such, no minimum landing size limits have been defined for catches. The present study provides the first data on the growth and reproduction ofS. ocellatusin the southeastern Black Sea of Turkey, which may be of great interest for future stock assessments to sustainably manage its remaining populations. Further research is needed to increase the accuracy of the estimations presented in this study regarding the period of female reproduction.











 texto en
texto en