Introduction
The leopard grouper,Mycteroperca rosacea(Streets 1877), is a member of the Epinephelidae family. The distribution of this species spans the Gulf of California to the western Pacific coast of Mexico. Adult leopard groupers form spawning aggregations from April to June in the Gulf of California (Erisman et al. 2007), which increases their vulnerability to fishing pressure (Sala et al. 2004). As of 2018, the leopard grouper has been listed as a species of “Least Concern” on the Red List of the International Union for Conservation of Nature (Erisman and Craig 2018). Due to the quality of its meat, the leopard grouper is a highly valued resource with a high market price, with reported landings of 200-400 t per year (González-Cuellar et al. 2019). Prior to 2000, information on this species was scarce and primarily related to biological aspects such as feeding habits (Peláez-Mendoza 1997). Since then, various studies of leopard grouper reproduction in the wild have been conducted.
In this study, we were able to verify the information that has been generated to date regarding leopard grouper reproduction. The leopard grouper does indeed form large aggregations and spawns for long periods; however, leopard groupers also aggregate outside of the spawning season, and the lunar cycle and spawning do not appear to be synchronized (Erisman et al. 2007). Leopard groupers are gonochoric (Erisman et al. 2008), and this species shows group-synchronous ovarian development(Estrada-Godinez et al. 2011). In the wild, leopard groupers have been observed to spawn from May to June (Estrada-Godinez et al. 2011), March to May (Sala et al. 2003) or April to June (Erisman et al. 2007). In addition, differences in bioindicators and biochemical variables have also been observed throughout the reproductive cycle of this species (Estrada-Godinez et al. 2014).
In recent years, the leopard grouper has been the subject of multiple studies conducted under captive conditions. For example, induced spawning of wild-caught fish was conducted by administering 2 injections of 1,000 and 500 IU·kg-1of human chorionic gonadotropin (hCG; Gracia-López et al. 2004a). In another induction study with hCG and luteinizing hormone-releasing hormone analogue (LHRHa), better results were obtained with females that were induced with hCG (Kiewek-Martínez et al. 2010). Additionally, improvements in larval and juvenile culture have resulted in the production of thousands of juveniles (Gracia-López et al. 2005). The reproductive physiology of captive leopard groupers has also been studied, specifically the roles of sex steroids (estradiol, testosterone, and 11 keto-testosterone) in the reproductive cycle (Maldonado-García et al. 2018).
At the same time, great efforts have been made to increase our current understanding of the different culture phases for this species. For example, Gracia-López et al. (2004a) reported improved hatching rates at temperatures ranging from 24.0 to 30.0 °C and improved yolk sac volume from 26.0 to 28.0 °C. In addition, higher temperatures and the salinity value of 32 resulted in the best larval growth and hatching rates (Gracia-López et al. 2004a). Larvae and juvenile production has also been achieved with fertilization, hatching, and survival rates of 76.8%, 71.2%, and 1.38%, respectively (Gracia-López et al. 2005, Martínez-Lagos and Gracia-López 2009). However, despite these advances, low larval survival at the first feeding remains problematic, which has led to studies of enzymology, probiotics, and disease (Reyes-Becerril et al. 2008, 2011; Martínez-Lagos et al. 2014).
In grouper aquaculture, major limitations have led to inadequate larval survival and inconsistent egg production (Okumura et al. 2002). Capturing fingerlings to be raised in captivity is not an optimal production strategy due to the scarcity of these source organisms (Kungvankij et al. 1986, Lim 1993). As such, reproduction has been studied in a large number of species in captivity with the aim of obtaining eggs. Lee and Yang (2002) and several studies have identified causes that prevent captive females from completing oocyte maturation (Zohar and Mylonas 2001). In most cultured marine fish species, it is sometimes necessary to manipulate abiotic factors, such as the photoperiod or temperature (Carrillo et al. 1989, Zanuy et al. 1995), or rely on exogenous hormone treatments to stimulate final oocyte maturation and ovulation (Mylonas and Zohar 2001).
One successful means to improve fecundity, fertilization, and egg production is related to natural spawning, with the broodstock being maintained in appropriately sized tanks with optimum water conditions and nutritional requirements. This method has been successfully implemented with various grouper species (Toledo et al. 1993, James et al. 1997, Ranjan et al. 2017) and appears to be a logistically feasible means to obtain good quality eggs in adequate quantities (Mylonas et al. 2004, Jerez et al. 2006).
In spite of the results that have been achieved in previous studies with hormonal induction, much remains to be understood about leopard grouper reproduction in captivity. To this end, a long-term study was conducted to evaluate leopard grouper maturation and natural spawning in captivity. This study evaluated egg production and fertilization over multiple spawning seasons and determined which factors may influence reproduction.
Materials and Methods
Broodstock and husbandry
In May 2004, the broodstock used in this study (n= 17) were captured by hook and line in the northern area of San José Island, Baja California Sur (Mexico), and transported to the facilities of the Centro de Investigaciones Biológicas del Noroeste (CIBNOR) in cylindrical tanks (500 L) with supplemental oxygenation. In the laboratory, the fish were anaesthetized with 50 mg·L-1of tricaine methanesulfonate (MS-222), weighed, and dorsally tagged with Spaghetti FloyTags (Floy Tags & Mfg.; Seattle, WA, USA) for subsequent identification.
The fish were stocked in an outdoor circular tank (7 m3, 1 m depth) at a density of 1.40 kg·m-3under natural temperature and photoperiod conditions. The group consisted of 14 females with a mean (SD) weight of 1.17 (0.36) kg and 3 males with a mean (SD) weight of 1.36 (0.15) kg. The fish were fed on alternate days with frozen sardine and mackerel to satiation. From 2004 to 2007, the fish remained outdoors, after which they were transferred to a circular black fiberglass tank (7 m3) in a closed laboratory where they were kept until 2009. Suitable water conditions were maintained by a recirculation system composed of settler, filter, UV lamp, and chiller equipment. With this system, physicochemical parameters, such as temperature, dissolved oxygen, CO2, ammonium nitrogen, nitrites, and nitrates, were controlled. Supplemental oxygenation was provided via blower aeration.
Temperature was continuously measured by submersible HOBO TidbiT temperature loggers (Onset; Bourne, MA, USA); salinity was measured using a refractometer (ATAGO; Bellevue, WA, USA), and oxygen was measured with a YSI 85 oximeter (Yellow Springs Instruments, Yellow Springs, OH, USA). The mean water temperature during the spawning period was 22.9 (1.2) °C and ranged between 21.1 and 23.8 °C. The annual water temperature and photoperiod are shown in Figure 1. Salinity was 37 and dissolved oxygen ranged from 5.3 to 6.2 mg·L-1.
Maturity
Individuals were weighed and sampled to determine the maturation stage and sex in February, July, September, and November 2007 and January 2008. Sperm samples were taken by applying gentle abdominal pressure. Sperm were collected in Eppendorf tubes for subsequent motility observations. Gonad samples were obtained from females by introducing a polyethylene cannula into the oviduct. To determine oocyte diameter and maturation stage, photographs of the samples were taken with a Coolsnap-Pro Color camera (Media Cybernetics; Rockville, MD, USA) installed on a Bx-41 microscope (Olympus; Tokyo, Japan) and analyzed using Image-Pro Plus (v.5.0, Media Cybernetics).
Egg collection and analysis
After spawning, buoyant eggs were passed through the outlet pipe to the collector tank, where they were retained by a mesh net (300 µm). On each spawning day, the eggs were collected in the morning, washed with sterile sea water, and transferred to a tank (35 L) with aeration to estimate the number of eggs released and fertilization rate (%). In this study, the fertilization rate represents the eggs obtained on a spawning day and does not represent the fertilization of a particular female. The total number of eggs was estimated by counting the eggs in 20 samples (15 ml). Mean (SD) egg diameter was measured with Sigma Scan Pro 5.0 (Systat Software; Chicago, IL, USA). The eggs were examined under the microscope to monitor embryonic development, and the fertilization rate was calculated following the early stages of embryonic development. The eggs were individually placed in 96-well microtiter plates (in replicates), with one egg per well, and maintained at 23.0 ± 1.2 °C.
The fertilization rate was evaluated daily and was calculated as the number of fertilized eggs/total number of buoyant eggs. The number of viable eggs was calculated by multiplying the total number of eggs by the fertilization rate. In terms of egg production, the annual relative number of eggs released (eggs·kg-1) and viable egg production (eggs·kg-1) were calculated taking into account the total mass of females (kg) in the tank. The time of day when spawning occurred was determined when the first egg was collected in the collector tank.
Statistical analysis
Monthly and annual differences in the number of eggs released and the fertilization rate for the 2 spawning seasons were analyzed by an analysis of variance (ANOVA,P< 0.05). The effect of the moon on fecundity was determined through a one-way ANOVA, after recording the number of eggs by moon phase and month. Significance was determined with Statistica v.8.0 (StatSoft; Tulsa, OK, USA). We modeled the expected fertilization rate using the generalized linear model logit link function in R v.4.1.2 (RStudio Team 2020) as a function of the spawning date and the number of eggs released per day. We used residual deviance to assess the goodness-of-fit and the chi-square test to evaluate the validity of the model.
Lunar phases were assigned as follows: 0 to the full moon and values up to 29 for each successive date. Days were grouped into 8 lunar phases adapted by Grant et al. (2009) from Stolov (1965) and Bell and Defouw (1966). A circular histogram was plotted with bar length representing the cumulative number of eggs released (×106), the dashed lines and dots representing the spawning frequency, and the color representing the fertilization rate according to lunar phase. Data were analyzed for possible correlations with the lunar phase using Rao’s spacing test. In this case, significance was determined withP< 0.1 (Grant et al. 2009).
This study was conducted following the Guidelines of the European Union Council (2010/63/EU) and Mexican Government (NOM‐062-ZOO‐1999) for the production, care, and use of experimental animals. Experimental protocols and procedures were reviewed and approved by the aquaculture committee of CIBNOR. The authors confirm that the ethical policies of the journalCiencias Marinas, as noted in the author guidelines, have been followed, and approval was awarded by the appropriate ethical review committee.
Results
Spawning season
In 2006, 22 months after the start of the study, a batch of 90,000 unfertilized eggs was obtained, representing the only spawn of that year. No fertile eggs were collected until 2007. In that year, the first and last batches of eggs were collected on 9 March and 29 June, respectively. In 2008, the first spawning date was 12 March, and the spawning season ended on 21 May. The spawning seasons took place during periods in which temperatures increased (21.1 to 23.8 °C in 2007 and 21.2 to 23.6° C in 2008) as the photoperiod increased (Fig. 1). Throughout the duration of this experiment, the first fertilized eggs were collected just before total darkness, between sunset and night, when it was impossible to see any signs of courtship. Egg diameter ranged from 848.0 to 884.0 µm with a mean (SD) of 864.0 ± 22.0 µm.
Maturation
Fish sampled in February 2007 showed evidence of maturity, albeit with males and females in different stages of maturity. Eleven days after this sampling, the first spawning of the year was observed. Oocyte size (mean ± SD) ranged from 107 ± 42 to 554 ± 103 µm. In July 2007, only the males were mature (flowing males); 4 females exhibited vitellogenic oocytes, and 10 females showed previtellogenic oocytes. In September and November 2007, no signs of maturity were observed in any individual. In January 2008, 3 females exhibited hydrated oocytes, although males were not mature at this point.
Egg production
There were 43 and 27 spawning days in 2007 and 2008, respectively, with a similar number of spawning days (9 to 13 days) and eggs obtained each month, except in March 2008, when only 5 spawning days were recorded because the spawning season began in the middle of that month. The monthly and annual mean (SE) number of eggs per spawning day and mean (SE) fertilization rate obtained in 2007 and 2008 are shown in Table 1. Large variation in daily egg release was observed, with 18,560 to 471,000 eggs released per day in 2007 (mean [SE]: 101,705 [13,876]) and 800 to 145,000 eggs released per day in 2008 (mean [SE]: 40,510 [7,874]) (Fig. 2). No consistent trend was recorded throughout the spawning season. In all, 4.37 × 106and 1.07 × 106eggs were released in 2007 and 2008, respectively (Fig. 3, Table 2). The annual relative number of eggs released was 288,750 eggs·kg-1and 72,919 eggs·kg-1in 2007 and 2008, respectively, while viable egg production was 184,800 and 25,375 eggs·kg-1in 2007 and 2008, respectively.
Table 1. Number of spawning days and eggs obtained per month. Mean (SE) eggs per release and fertilization rate (F) per month obtained in 2007 and 2008.
| Year | Month | Spawning days | Eggs | Eggs/release (×103) | F(%) |
| 2007 | |||||
| March | 11 | 1,045,667 | 95.0 (54.8)a | 16.8 (20.0)a | |
| April | 13 | 886,111 | 68.2 (28.6)a | 76.7 (24.0)b | |
| May | 10 | 1,139,828 | 114.0 (132.0)a | 80.6 (16.3)b | |
| June | 9 | 1,301,692 | 144.6 (121.0)a | 71.0 (28.0)b | |
| 2008 | |||||
| March | 5 | 194,870 | 38.9 (40.8)a | 23.1 (38.5)a | |
| April | 12 | 428,373 | 35.7 (35.5)a | 30.3 (33.1)a | |
| May | 10 | 470,537 | 47.0 (46.8)a | 39.2 (34.6)a | |
| Total | 2007 | 43 | 4,373,298 | 101.7 (91.0)x | 61.1 (34.2)x |
| Total | 2008 | 27 | 1,093,780 | 40.5 (40.9)y | 32.3 (33.8)y |
Means within a column followed by different letters are significantly different (P< 0.05).
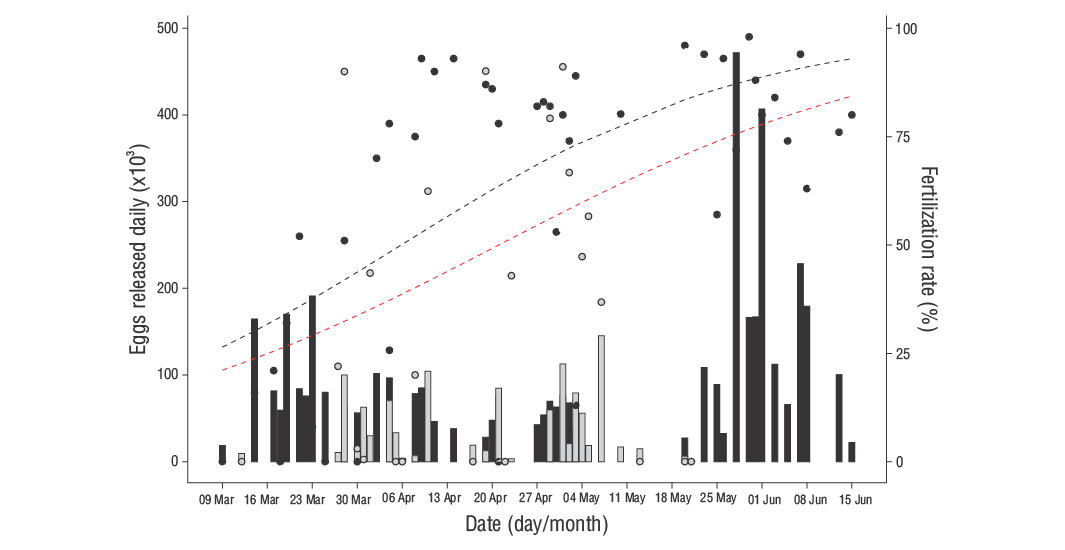
Figure 2. Eggs released each day in 2007 (black bars) and 2008 (grey bars) and the fertility rate in 2007 (black spots) and 2008 (grey spots) based on leopard grouper natural spawning. The relationship between the number of eggs released and spawning date in 2007 (black dashed line) and for the combined values of 2007 and 2008 (red dashed line) is shown based on the generalized linear models logit link function.

Figure 3. Leopard grouper eggs accumulated during the spawning season of 2007 and 2008) in captivity.
Table 2. Cumulative spawning parameters during 2 consecutive leopard grouper spawning seasons (2007 and 2008).
| 2007 Mean (SE) | 2008 Mean (SE) | |
| Eggs released daily (×103eggs) | 101.70 (13.9)a | 40.50 (7.8)b |
| Total eggs released (×106eggs) | 4.37 | 1.07 |
| Annual relative number of eggs released (103eggs·kg-1) | 288.00 | 73.00 |
| Fertilization (%) | 61.10 (5.2)a | 32.30 (6.5)b |
| Viable egg production (103eggs·kg-1) | 184.80 | 25.30 |
Statistically significant differences between the 2 years were present in the parameters examined (P< 0.05).
Statistical Analysis
The number of eggs collected per spawning day ranged from 68,200 (April) to 144,600 (June) in 2007 and 38,900 (March) to 47,000 (May) in 2008. There were no statistically significant differences within each year (P> 0.05; Table 1), although significant differences were found between 2007 and 2008 (P< 0.05). A low fertilization rate was recorded at the start of the spawning season that gradually increased, with significant differences between months in 2007 (P< 0.05). The fertilization rate exhibited higher daily variations in 2007 than in 2008, and significant differences (P< 0.05) were found between those 2 years, with the fertilization rate decreasing from 61.1% in 2007 to 32.3% in 2008.
For 2007, a significant interaction between the fertilization rate and spawning date was detected, but no relationship was present between the fertilization rate and daily egg release (Fig. 2). The same significant interaction between fertilization rate and spawning date was detected for the combined data of 2007 and 2008. The expectation did not deviate from zero. Also, most residuals exhibited absolute values smaller than 2. There appeared to be no influential data points, and only 3 outliers without influence. The chi-square test for residual deviance returned aPvalue of 0.99 for both models, so we can conclude that the model fits well. For 2008, no relationship was observed between factors.
The spawning frequency was significantly different according to the moon phase. More spawning days (9 days) were observed during the full moon phase compared to the waning gibbous (4 days), waning crescent (3 days), or first quarter (2 days) phases. Moreover, the number of eggs released was significantly higher during the full moon (1,067,696 eggs) and waning gibbous (742,876 eggs) phases and significantly lower during the first quarter (107,222 eggs) phase when compared to those of the other moon phases (371,540 to 637,046 eggs). No significant difference was observed in the fertilization rate. Nonetheless, a tendency toward greater fertilization from waxing gibbous to third quarter phases can be observed in Figure 4.
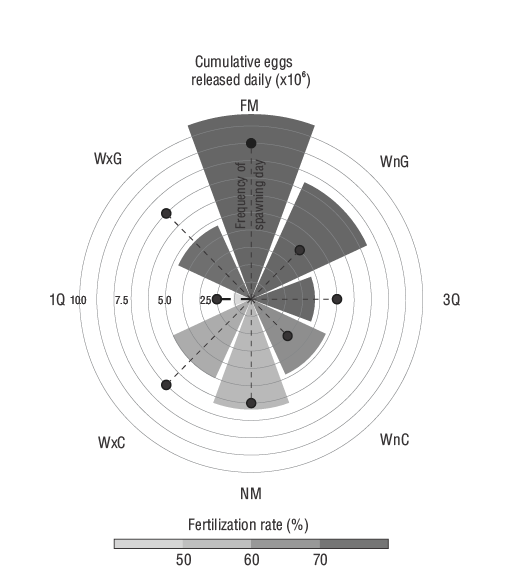
Figure 4. Fecundity (cumulative number of eggs ×106), spawning frequency (number of spawning days), and fertilization rate of leopard grouper natural spawning according to moon phase. FM: full moon; WnG: waning gibbous; 3Q: third quarter; WnC: waning crescent; NM: new moon; WxC: waxing crescent; 1Q: first quarter; WxG: waxing gibbous.
Discussion
Conditions for natural spawning in captivity
The acclimatization of organisms to captive conditions, maturation, and successful natural spawning are influenced by a large number of biological, physicochemical, and environmental factors. For example, farmed fish can exhibit reproductive problems if their environmental conditions are suboptimal (Mustafa et al. 2015). As such, maintaining individuals of different species under appropriate temperature and tank conditions with proper feeding and care can encourage spontaneous reproduction in captivity. Our results have shown that the recirculating system employed with the tanks (7 m3) used in this study was efficient and produced desirable conditions for leopard grouper maturation and natural breeding. Moreover, the feeding and photoperiod regimes and oxygen, salinity, and temperature conditions properly maintained the wild-caught broodstock and allowed for reproduction with spontaneous spawning to develop in this species. Spontaneous spawning has also been reported in a large number of fish species including the amberjack,Seriola dumerili(Jerez et al. 2006), and the fine sole,Paralichthys adspersus(Ángeles and Mendo 2005). There are also many examples of grouper species in which individuals were maintained in fiberglass tanks, cages, or concrete tanks with recirculation systems (Toledo et al. 1993, James et al. 1997, Jagadis et al. 2006, Mathew 2010, Ranjan et al. 2017).
The diet provided during this study was mainly composed of sardine, mackerel, and squid. This diet is similar to those observed in natural feeding studies of wildM. rosaceapopulations with organisms of the same size as those in the present study (Peláez-Mendoza 1997, Pérez Rojo 2016). The diet was sufficient for maintaining healthy broodstock and achieving maturation and spawning. However, more studies are needed to develop artificial diets that improve the quality and number of spawns. Grouper nutrition has not been adequately studied, although its importance to reproductive organisms, the quality of eggs and larvae, and fecundity is understood (Luo et al. 2005). During a natural spawning study withEpinephelus tauvina, broodstock were fed a variety of trash fish, cephalopods, vitamins E and B12, and other nutritional supplements to provide eicosapentaenoic acid, docosahexaenoic acid, and polyunsaturated fatty acids (Mathew 2010).
The acclimatization time and maturation in captive fishes depend on a variety of factors, which include facility conditions, management, nutrition, and aspects related to the biology of the species. Marine fishes likeSolea solea(Ofelio et al. 2020) andS. dumerli(Jerez et al. 2006) can take 2 and 6 years to mature in captivity, respectively. Groupers have been described as species that can mature and naturally spawn in captivity after a post-capture acclimatization period, which has been found to be relatively short inEpinephelus suillus(Toledo et al. 1993),Epinephelus polyphekadion(James et al. 1997), andEpinephelus merra(Jagadis et al. 2006).
Reproductive cycle, spawning season, and the influence of temperature, photoperiod, and moon phase
In the wild, leopard groupers have been observed to spawn from May to June (Estrada-Godinez et al. 2011), March to May (Sala et al. 2003), or April to June (Erisman et al. 2007). Estrada-Godinez et al. (2011) observed temperatures ranging from 21 to 25 °C and approximately 13 h of daylight in the spawning season. The differences reported between spawning seasons could be due to differences in temperature or other variables between study sites. Indeed, Sala et al. (2003) suggested that latitudinal differences in the Gulf of California, which are related to water temperature, could result in differences in leopard grouper spawning periods. In both the wild and captivity, the reproductive cycle of the leopard grouper exhibits differences in reproductive phases throughout the year (Erisman et al. 2008, Estrada-Godinez et al. 2011). A period of rest occurs from August until the oocytes begin to develop in November (Kiewek-Martínez et al. 2010). This is followed by a maturation period of 4 months, which begins as the daylight hours and temperature begin to progressively increase.
In the present study, final maturation in females and full maturation in males was observed in February 2007 and January 2008 through oocyte analyses. Based on the collected eggs, the spawning began in March and carried through until May-June. We noted that the onset of the spawning season occurred as the photoperiod increased, which may be what triggers gonadic maturity after the winter solstice. However, additional follow-up studies are needed to confirm this hypothesis. Indeed, few studies have been conducted on photoperiod and temperature in groupers despite these variables being highly important to the reproductive process (Rimmer and Glamuzina 2019). Kanemaru et al. (2012) observed gonadal maturity inE. merraafter induction with gonadotropin-releasing hormone agonists (GnRHa) and a long photoperiod. The influence of temperature on maturation and spawning remains largely unknown, although temperature alone was insufficient to control gonadal development inEpinephelus fuscoguttatus(Rimmer and Glamuzina 2019).
The spawning and daily egg release frequencies were significantly different according to moon phases, although no significant difference was observed in the fertilization rate after analyzing 70 spawning days throughout the spawning season. It should be noted that these results are based on the effect of the moon on a group of fish and are not the result of observations of individual fish. The lunar rhythm has been found to influence the natural spawning ofE. merra(Jagadis et al. 2006) andE. tauvina(Mathew 2010) in captivity. In another study, the relationship between spawning and the lunar cycle was found to decrease throughout the experimental period (Ranjan et al. 2017).
Egg production
Groupers generally acclimatize well to confinement and can reproduce spontaneously under these conditions (Tucker 1994). The efficiency of egg production per animal was very clear, which supports natural reproduction as an important component of leopard grouper reproduction, especially when considering the additional associated benefits such as lower personnel, facility, and energy costs and less animal handling. In 2007 and 2008, the total number of eggs released during the spawning season was 4.3 × 106and 1.0 × 106, respectively. In addition, natural spawning also resulted in successful egg production.
In previous studies, a total of 47.00 × 106eggs were collected in a study withEpinephelus coioides(Ranjan et al. 2017), while 9.20 × 106eggs were collected withE. tauvina(Mathew 2010), and more than 17.00 × 106eggs were collected withE. polyphekadion(James et al. 1997). Great differences in egg production are apparent among these studies, as they focused on different species, individual sizes, confinement conditions, feeding methods, and facility conditions. In this study, differences were present between 2007 and 2008, including significant differences in the number of eggs collected and their fertilization rates. These differences may be the result of the fish being moved to a closed laboratory in 2007 after the spawning season. Nonetheless, the reasons for these differences are not clear given that physicochemical parameters, feeding, and handling were similar in both years.
It is difficult to compare the results of the present study to those of studies that have employed hormonal induction. In this study, the results were obtained from a group of fish and did not come from the data of individuals. However, the collective results of all studies on this topic elucidate the improvements that have been made with natural spawning when compared to those of hormonal induction. The fecundity and fertilization rates obtained after hormonal induction with hCG and LHRHa inM. rosaceaare highly variable and range from 3 × 103to 571 × 103eggs per spawning (Kiewek-Martínez et al. 2010), which resulted in the production of 3.70 × 106viable eggs with 97 females and a low fertilization rate. In general, studies of leopard grouper reproduction have made the benefits of natural reproduction apparent given that natural reproduction yields higher egg production with a smaller number of specimens than does hormonal induction.
Estimated egg production was 288 × 103and 73 × 103eggs·kg-1in 2007 and 2008, respectively. Interspecific variation in fecundity has been observed with other species that show higher fecundity than the leopard grouper. For example, annual relative fecundity values greater than 400,000 eggs·kg−1and between 2.80 to 4.90 × 106eggs·kg−1were observed with the red porgy (Pagrus pagrus; Mylonas et al. 2004) and sharpsnout sea bream (Diplodus puntazzo; Papadaki et al. 2008), respectively.
Number of spawning events
Leopard grouper females show group-synchronous ovarian development (Maldonado-García et al. 2018). Group synchronous fish have ovaries with different types of oocytes at the same time. These fish may ovulate once or over many days or weeks during a reproductive cycle. In the present study, 43 and 27 spawning days were observed in 2007 and 2008, respectively, and the number of spawning days per month remained relatively constant (9-13 spawning days per month) except in March 2008. In this month, the leopard groupers began to spawn on 12 March, which resulted in only 5 spawning events in that month.
In general, grouper species that reproduce naturally in captivity spawn several times a month during several months of the year. Natural spawning has been observed in almost all months during 2 consecutive years (Mathew 2010). During the spawning season, the spawning frequency has been observed to increase from 5 to 13 spawns per month (Ranjan et al. 2017). In addition, multiple spawning events were observed between August and October in 2004, with the number of eggs per spawning event ranging from 22,000 to 180,000 (Jagadis et al. 2006). Finally, groupers that had spawned only a few times for 3 consecutive years were able to continuously spawn 2-3 times a month over 4 months after changes to the nutrition of the broodstock were implemented (James et al. 1997). A future research goal should be to incorporate wild-caught reproductive organisms into captive breeding programs to take advantage of their genetic makeup to avoid inbreeding, which can negatively impact subsequent generations (Bright et al. 2016, Superio et al. 2021).
Time of spawning
The lack of light at the time of courtship and spawning did not allow for direct observations of these 2 actions. However, direct observations throughout the duration of this experiment allowed us to confirm that the first fertilized eggs obtained in the collector appeared just before total darkness between sunset and night. Erisman et al. (2007) studied the spawning behavior of the leopard grouper in the wild and indicated that spawning occurred in the late afternoon a few hours before sunset. Spawning as dark approaches is a regular behavior that has been frequently observed in other species, like the brown grouper (Epinephelus marginatus), which was found to spawn one hour before and half an hour after sunset around the new moon period (Zabala et al. 1997). This spawning behavior has also been observed with the Nassau grouper (Epinephelus striatus; Whaylen et al. 2004). Spawning amongst 10 to 17 fish was observed in the evening a few days after the full moon (Rowell et al. 2019). Natural spawning in captivity has often been observed during the afternoon, at sunset, and overnight (Jagadis et al. 2006, Mathew 2010, Ranjan et al. 2017), but it can also occur in the morning (James et al. 1997).
Size of fertilized oocytes
The mean ± SD egg diameter from natural spawning was 864.0 ± 22.0 µm (848.0 to 884.0 µm). Similar egg diameters have been found via hormonal induction, namely 859.0 ± 18.0 μm (Gracia-López et al. 2005), 860.0 ± 30.0 μm and 880.0 ± 20.0 μm (Gracia-López et al. 2004a), and 872.0 ± 15.0 μm (Gracia-López et al. 2004b).Epinephelus merraeggs were found to be spherical and buoyant with diameters ranging from 710.0 to 730.0 μm (Jagadis et al. 2006). The fertilized eggs ofE. coioideswere round and measured about 857.7 μm (Fourooghifard et al. 2017). InE. polyphekadion, egg diameter was 757.3 ± 37.4 μm (James et al. 1997). It is important to mention that the resulting egg diameter was higher in eggs that were naturally spawned than in those obtained with hormonal induction (Papanikos et al. 2003).
In conclusion, with our methodology, we can ensure constant egg production in captivity with wild-caught fish that are maintained for approximately 2 years without manipulating the parameters that commonly affect fish reproduction. Leopard groupers spawn during 3-4 months and can produce 184 × 103viable eggs·kg-1. The spawning season in this study (March to May-June) was related to increases in temperature and the photoperiod, although further studies are needed to confirm the effects of these variables on leopard grouper reproduction. Lastly, the moon phase affected fecundity throughout the spawning season.











 texto en
texto en 




