INTRODUCTION
In bivalve mollusks, broodstock energy reserves notably influence gamete development and maturation as well as the viability and vigor of the embryos and larvae (Chávez-Villalba et al. 2003, Wassnig and Southgate 2012a). These reserves are important during early larval development, when veligers have a limited ability to feed on exogenous sources, and during the late development stage, when these larvae undergo energetically demanding metabolic processes related to metamorphosis and the transition from a pelagic to benthic existence (Gagné et al. 2010, Angel-Dapa et al. 2015). Internal mechanisms of nutrient storage and mobilization that first sustain gametogenesis and then embryonic and larval development are regulated by endogenous and exogenous factors such as disease, stress, and environmental conditions (Saucedo and Southgate 2008, Gireesh et al. 2009). Poor physiological condition of the broodstock can also result in spawning failure, gamete reabsorption, and sub-optimal larval performance, which affect all subsequent steps related to spat production (Gómez-Robles et al. 2013, Mazón-Suástegui et al. 2021). Finally, the presence of anomalous environmental conditions associated with the everchanging relationship between the atmosphere and upper ocean can increase or decrease sea surface temperature (SST; Pastor 2021). This may favor events like La Niña or El Niño, which can also affect the reproductive success of many intertidal bivalve mollusks.
In pearl oysters used for pearl production, studies related to larval cultivation in the laboratory are necessary to complement the erratic collection of spat in the wild (Southgate 2008, 2011; Hoyos-Chairez et al. 2020). However, these types of studies are insufficient for pearl oysters of the genus Pteria and do not guarantee a continuous supply of spat. Studies with Pteria penguin (Röding, 1798), a species of relatively large size that is mostly used for mabé pearl culture in many Pacific Island nations, have focused on evaluating embryonic and larval developmental stages (Wassnig and Southgate 2012a), physical and chemical traits that induce spat settlement (Wassning and Southgate 2012b), stocking densities and microalgal rations (Wassnig and Southgate 2016), and hatchery culture of larvae without living microalgae (Southgate et al. 2016).
In the Gulf of California, Pteria sterna (Gould, 1851) is the only species that is currently used in the commercial culture of pearls, which exhibit a multicolored pattern that is clearly different from those of the pearls produced by Pinctada species (Kiefert et al. 2004, Ruíz-Rubio et al. 2006). Previous studies have reported that the species breeds multiple times during the winter and spring seasons (January to May) when water temperatures decrease and primary productivity increases (Saucedo and Monteforte 1997, Vite-García and Saucedo 2008, Cáceres-Puig et al. 2009). Hatchery rearing experiments with Pteria sterna larvae are scarce (Araya-Nuñez et al. 1991, 1995; Saucedo 2017; Hoyos-Chairez et al. 2020) and still represent the main bottleneck hindering the controlled production of this species. Consequently, the factors exerting the greatest influence on larval development must be determined, especially to understand the relationship between larval performance, broodstock condition, and the environment. This relationship has been analyzed in other bivalve species such as the scallops Pecten maximus (Le Pennec et al. 1990, Gagné et al. 2010), Argopecten purpuratus (Nevejean et al. 2003), Placopecten magellanicus (Pernet et al. 2003), and Argopecten ventricosus (Mazón-Suástegui et al. 2021); the Japanese oyster Crassostrea gigas (Chávez-Villalba et al. 2003); and the penshell Atrina maura (Angel-Dapa et al. 2015).
This study evaluated the seasonal variation in reproductive and larval performance of Pteria sterna due to the influence of environmental factors, evaluating the hypothesis that the transition between La Niña and El Niño events during 2008-2009 would result in a series of anomalies in SST, which would generate warmer conditions that prolonged the summer. We believe that these conditions affected the “normal” timing to collect ripe broodstock in the wild and rear the Pteria sterna larvae under hatchery conditions.
MATERIALS AND METHODS
Origin of broodstock and experimental design
The hypothesis of this study of Pteria sterna larvae was based on the findings of Gómez-Robles et al. (2013) with the same species, which suggest that the internal management of energy reserves associated with gonad maturation and reproductive success in Pteria sterna during 2008-2009 was also affected by the transition from La Niña to El Niño in Bahía de La Paz in the Gulf of California.
Forty adult Pteria sterna (94.4 ± 1.2 mm shell height) were collected from a submarine trestle placed at 10 m depth in Bahía de La Paz, Baja California Sur, Mexico (24°16′ N, 110°19′ W). To test our hypothesis, sampling took place during 2 periods that had been previously reported as reproductive peaks (February and April 2009) as well as a pre-reproductive period (November 2008) and a post-reproductive period (June 2009; Saucedo and Monteforte 1997, Vite-Garcia and Saucedo 2008, Cáceres-Puig et al. 2009). After collection in the field, the oysters were taken to the hatchery, cleaned, and separated into 2 groups with 20 oysters in each group. The first group was used for spawning induction and larval culture, and the second group was used to evaluate indices of broodstock condition. Environmental variables were also monitored during each sampling period.
Spawning induction and larval rearing
Larval rearing was conducted following the protocol of Saucedo (2017) for Pteria sterna. Broodstock were induced to spawn by thermal shock (20-27 °C; 3 periods of 30 min each). After gamete fertilization, the larvae were reared in 3 conical fiberglass tanks (1,500 L) with filtered (1 µm) and UV-irradiated seawater at 22 °C and 35-36 salinity. The larvae were stocked at 5-7 larvae·mL−1 and fed a 1:1:1 mixture of the microalgae Isochrysis galbana, Pavlova salina, and Chaetoceros calcitrans at 15 × 103 cell·mL−1 (day 1 to 10) and 25 × 103 cell·mL−1 (day 11 onward). The tanks were drained, washed, and refilled with fresh seawater every third day.
Each time the tanks were drained, larvae samples (1 mL) were collected in triplicate and fixed in a 3% formalin solution. The larvae were then counted under a microscope (10×) on a Sedgewick-Rafter chamber to estimate the mean survival rate (%). Groups of 20 larvae were photographed and processed with Image Pro Plus v. 9.0 (Media Cybernetics, Bethesda, MD, USA) to estimate the increase in shell height (0.1 μm) and mean growth rate (μm·d-1). Larval samples (0.5 mL) were also taken in triplicate at the beginning (veliger stage) and end (pediveliger stage) of the larval culture period and stored at -80 °C for further biochemical analyses.
The preserved larval samples were filtered, rinsed with ammonium formate, and decalcified in acetic acid to eliminate the shell as much as possible. The samples were weighed (±0.001 g), lyophilized, re-hydrated in 3.5% cold saline solution, and homogenized to obtain crude extracts. Total carbohydrates were determined following the anthrone-sulfuric acid method (Leyva et al. 2008) using a dextrose solution as the standard (G8270, Sigma-Aldrich, St. Louis, MO, USA). Total proteins were determined by the Bradford (1976) method using Coomassie reagent (B6916, Sigma-Aldrich) and bovine serum albumin (A7906, Sigma-Aldrich) as the standard. Total lipids were estimated with the method of Bligh and Dyer (1959) with modifications, using 20 µL supernatant, 200 µL reagent (Randox Laboratories, Antrim, UK), and lipid Lin-Trol solution (L2648, Sigma-Aldrich) as the standard. Results are expressed in µg·larvae-1. Finally, energy equivalents were calculated for veliger and pediveliger larvae using the conversion factors of Brett and Groves (1979): proteins (20.0 mJ·µg-1), carbohydrates (17.5 mJ·µg-1), and lipids (39.5 mJ·µg-1). Data are reported as J µg-1·larvae-1.
When approximately half of the population reached the pediveliger stage, the larvae were transferred to settlement tanks that were identical to the culturing tanks. The settlement tanks contained artificial collectors made from dark-colored onion bags, including both the outer bag and inner substrate (Saucedo 2017). After 2 weeks, the spat that had settled on the collectors and on the bottom and walls of the settlement tanks were recovered to estimate total recruitment for each larval trial run. During this time, the spat were fed the same mixture of Isochrysis galbana, Pavlova salina, and Chaetoceros calcitrans but at 70-80 × 103 cell·mL-1.
Indices of broodstock condition
Oysters of the second group were separated by sex, and only the females were used in the analyses, as eggs are the best indicator of gamete quality and larval viability. The oysters were measured (±0.1 mm) and weighed (±0.1 g) to first determine a general condition index according to the equation of Gómez-Robles et al. (2013):
In addition, the female oysters were dissected to extract gonad samples. The first section was preserved in Davidson’s solution for 48 h for histological and histochemical analyses, and the second section was preserved at −80 °C for biochemical analysis.
The gonad samples used for histological analyses were dehydrated, embedded in Paraplast-TX (SPI Supplies, West Chester, PA, USA), and thin-sectioned to 4 µm in duplicate. The first set of slides was stained with haematoxylin-eosin (Kim et al. 2006) and examined under a microscope to identify the stages of gonad development (inactive, development, ripe, spawning, and spent) and calculate mean oocyte area (0.1 µm2) according to the methods of Vite-García and Saucedo (2008). This information served as the basis to determine the seasonal changes in broodstock condition and its influence on larval performance (Gómez-Robles et al. 2013). A second set of slides was stained with Sudan Black B (Bayliss 1984) to identify lipid and triglyceride droplets in the gonads, which ranged from dark grey to black. Then, the slides were digitized at high resolution and processed with Image Pro Plus to determine the lipid index of the oocytes (%) according to the equation of Rodriguez-Jaramillo et al. (2008):
For the biochemical analyses, the preserved female gonad samples were weighed (~100 mg), lyophilized, rehydrated in 3.5% saline solution, and homogenized to obtain crude extracts. The total composition of proteins, carbohydrates, and lipids was determined following the procedures previously described for the larvae.
Environmental variables
Water temperature (±0.01 °C) and salinity (±0.01) were recorded in situ with a 6920 portable meter (YSI, Yellow Springs, OH, USA). To test the hypothesis, water temperature and SST data for pre-sampling (2006-2007) and post-sampling (2010-2011) periods were obtained from the Aqua MODIS database of the National Oceanic and Atmospheric Association (NOAA; http://coastwatch.pfeg.noaa.gov) and processed following the methods of Reynolds et al. (2002). In addition, water samples were also collected in quadruplicate in situ to determine food content and availability. The first 2 L of the water samples were filtered through Whatman GF/C filters (47-mm diameter), washed with distilled water, dried at 100 °C, burned at 450 °C (4 h), weighed (±0.001 g), and preserved at -20 °C. Seston composition was determined according to the methods of Luna-González et al. (2000). The other seawater samples were filtered, oven-dried at 80 °C for 24 h, weighed (0.001 g), burned to ash at 475 °C for 4 h, and re-weighed to determine inorganic seston content. Organic seston content was determined by the difference in weight between total and inorganic seston content. Data are expressed as mg·L-1. Data related to primary productivity and the concentration of chlorophyll a (mg·m-3) for each of the 4 sampling periods were also obtained from the NOAA Aqua MODIS database.
Statistical analysis
Data of broodstock (condition index, biochemical composition, oocyte size, and lipid index) and larvae (growth rate) condition were checked for normality with the Kolmogorov-Smirnov test. The presence of significant differences in these data over time were evaluated with a one-way analysis of variance (ANOVA; Sokal and Rohlf 1981). Due to the small size of pooled samples (n = 5), data of the biochemical composition of larvae were assessed for significant differences between the April and June 2009 trial runs (superscript letters in Table 2) and veliger and pediveliger stages (superscript numbers) with a Kruskal-Wallis test. As needed, post hoc multiple range comparisons were conducted with the Tukey (HSD) test. Pearson correlation coefficients and Spearman rank correlations were used to evaluate the relationship between broodstock condition, larvae, and environmental variables. When required, the data were arcsin transformed. Analyses were conducted in STATISTICA v. 8.0 (Statsoft, Tulsa, OK, USA).
Table 1 Reproductive and larval performance of the winged pearl oyster Pteria sterna associated with environmental variables during the pre-reproductive, reproductive, and post-reproductive periods of 2008-2009 in Bahía de La Paz, Baja California Sur, Mexico.
| Indicators | 2008 | 2009 | ||
| Nov (Autumn) | Feb (Winter) | Apr (Spring) | Jun (Summer) | |
| Spawning and larvae (num) | ||||
| Spawning response | Unsuccessful | Successful | Successful | Successful |
| Spawned oysters | - | 4 Males; 2 Females | 2 Males; 1 Female | 2 Males; 1 Female |
| Pooled veliger larvae | - | None | 28 × 106 | 1 × 106 |
| Broodstock (female) | ||||
| Main gonad development stage | Ripeness (58%) (Fig. 1b) | Development (63%) (Fig. 1a) | Spawned (83%) (Fig. 1c) | Ripeness (42%) (Fig. 1b) |
| General condition index (%) | 12.5 ± 0.6b | 11.4 ± 0.6b | 11.6 ± 0.7b | 20.3 ± 0.8a |
| Mean size of oocytes (µm2) | 3,935.9 ± 48.1b | 3,453.3 ± 99.5b | 4,597.4 ± 97.2a | 3,786.2 ± 64.3b |
| Mean lipid index of oocytes (%) | 9.7 ± 0.6b | 8.3 ± 1.1bc | 13.1 ± 1.2a | 6.7 ± 0.8c |
| Mean protein content (mg·g-1) | 238.6 ± 23.3a | 199.6 ± 11.8a | 230.5 ± 20.0a | 92.0 ± 8.5b |
| Mean carbohydrate content (mg·g-1) | 17.8 ± 2.4c | 29.2 ± 1.8b | 60.1 ± 5.3a | 30.7 ± 2.7b |
| Mean lipid content (mg·g-1) | 30.8 ± 3.5b | 28.1 ± 1.9b | 39.8 ± 3.2b | 93.6 ± 11.4a |
| Environment | ||||
| Temperature (°C) | 26.2 | 21.1 | 21.8 | 22.5 |
| Sea Surface Temperature SST (°C) | 0.71 | 0.43 | -0.18 | 0.44 |
| Salinity | 35.8 | 36.3 | 36.4 | 36.1 |
| Chlorophyll a (mg·m-3) | 1.4 | 1.9 | 1.3 | 0.8 |
| Total seston (mg·L-1) | 20.8 | 29.1 | 32.3 | 29.5 |
| Organic seston (mg·L-1) | 3.0 | 3.1 | 3.2 | 3.1 |
| Inorganic seston (mg·L-1) | 17.8 | 25.9 | 29.1 | 26.4 |
Mean ± standard errors are shown. Identical superscripts letters within columns denote lack of significant differences at P < 0.001
Table 2 Survival, growth, biochemical composition, and energy equivalents of veliger and pediveliger larvae of the winged pearl oyster Pteria sterna reared at the hatchery in April and June 2009.
| Indicators | April | June | ||
| Veliger (day 3) | Pediveliger (day 21) | Veliger (day 3) | Pediveliger (day 21) | |
| Overall performance of larvae and spat | ||||
| Mean survival of larvae (%) | 95.4 | 7.7 | 60.1 | 0.1 |
| Mean shell height of larvae (µm) | 98.6 ± 3.5 | 199.2 ± 7.3 | 72.5 ± 1.5 | 181.5 ± 5.8 |
| Mean growth rate of larvae (µm·d-1) | 6.3 ± 0.6a | 4.9 ± 0.4a | 4.2 ± 0.6b | 2.6 ± 0.4b |
| Pooled pediveliger larvae (num) | - | 184 × 103 | - | < 16,000 |
| Settlement day | - | 23 | - | 32* |
| Final settlement rate of spat (%) | - | 6.6 | - | < 0.1 |
| Harvested spat (num) | - | 17 × 103 | - | < 150 |
| Biochemical composition (mg·larvae-1) | ||||
| Mean protein content | 0.470 ± 0.002a,2 | 6.650 ± 0.710a,1 | 0.410 ± 0.003a,2 | 3.180 ± 0.260b,1 |
| Mean carbohydrate content | 0.0140 ± 0.0001a,2 | 0.7500 ± 0.0010a,1 | 0.0100 ± 0.0001a,2 | 0.2300 ± 0.0010b,1 |
| Mean lipid content | 0.0040 ± 0.0002b,2 | 0.1400 ± 0.0020a,1 | 0.0300 ± 0.0002a,2 | 0.0500 ± 0.0010b,1 |
| Energy equivalents (J µg-1·larvae-1) | ||||
| Mean protein energy | 0.044a,1 | 0.003b,2 | 0.050a,1 | 0.010a,1 |
| Mean carbohydrate energy | 1.310a,1 | 0.020b,2 | 1.290a,1 | 0.080a,2 |
| Mean lipid energy | 10.700a,1 | 0.300b,2 | 12.000a,1 | 0.800a,2 |
Mean ± standard errors are shown. Identical superscripts letters within columns denote lack of significant differences between the April and June runs at each developmental stage; identical superscripts numbers denote lack of significant differences between veliger and pediveliger stages at each month; (*) larvae reached day 32 in culturing tanks, but most of them died and only a few of them settled
RESULTS
Spawning response in relation to broodstock condition and environmental factors
The spawning response related to broodstock condition and environmental factors is shown in Table 1. The oysters collected in November 2008 did not respond to induced spawning. During this time, the water temperature was the highest, total seston content was the lowest, and only 63% of the broodstock had ripe gonads. Conversely, oysters successfully spawned in February 2009 (4 males, 2 females), April 2009 (2 males, 1 female), and June 2009 (2 males, 1 female). However, the gonads were only of optimal condition for reproduction in April 2009 (20% ripe, 63% spawned, largest oocytes; Fig. 1) when favorable environmental conditions were present (lowest water temperatures, highest total seston content; Table 1). The seasonal relationship between water temperature and total seston content was inverse (r = -0.87). The relationship between total seston and chlorophyll a content was not significant (r = -0.44).
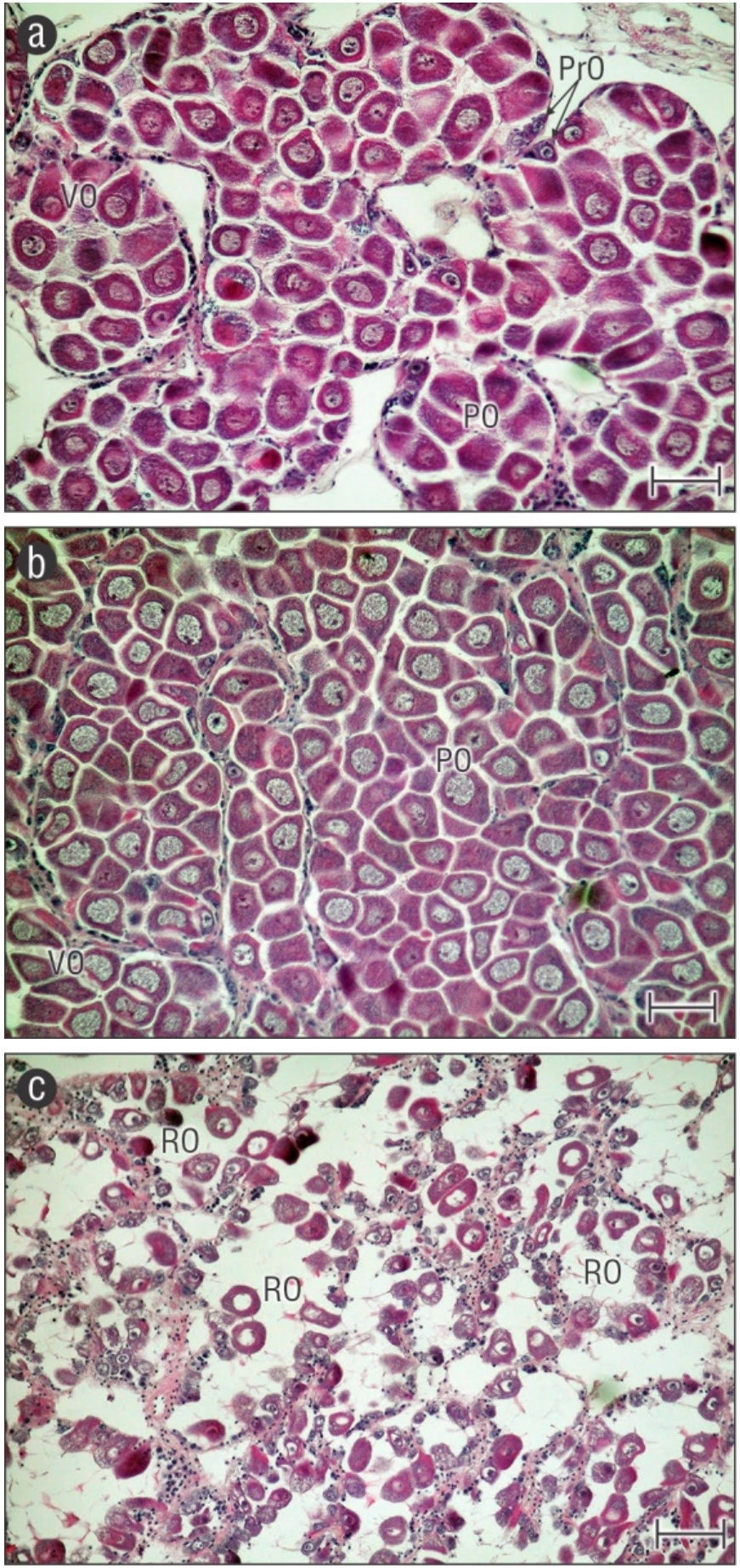
Figure 1 Photomicrographs of female gonads (10×) of the winged pearl oyster Pteria sterna stained with hematoxylin-eosin showing the stages of gametogenic cycle that prevailed during each sampling period: (a) development stage in February 2009, depicting pre-vitellogenic oocytes (PrO), vitellogenic oocytes (VO), and post-vitellogenic oocytes (PO) filling the acini; (b) ripeness stage in November 2008 and June 2009 that mostly show PO and a few VO; and (c) spawning stage in April 2009, in which only residual oocytes (RO) are observed within acini. Scale bar is 50 µm.
Larval performance in relation to broodstock and environmental conditions
The spawning of February 2009 failed to produce veliger larvae. Although the water temperature remained low and the oocyte size and lipid index ranked second among all periods, most of the broodstock exhibited developing gonads and the lowest condition index values of the study period (Fig. 1, Table 1). In April and June 2009, spawning yielded viable veliger larvae with no shell deformities and active swimming behavior. However, only pediveliger larvae of the April run completed metamorphosis, settled at day 23, and developed into healthy spat (Table 2). These larvae originated from broodstock with low condition index values but high percentages of ripe-spawned gonads, the largest oocytes, and the highest lipid index values of all periods. These values also coincided with low water temperatures and the highest total seston values (but not chlorophyll a and the SST that showed no anomalies in April 2009). Even though the June trial run also resulted in a few viable larvae, most of them stopped growing between day 15 and 17, and only ~150 small spat were harvested on day 32 (Table 2). The broodstock in this period exhibited the highest condition index values, yet only 50% of their gonads were ripe (Fig. 1). In addition, their oocytes were small, and their lipid index values were the lowest in the study, although both water temperature and total seston values remained favorable.
Figure 2 shows the variation in water temperature and SST anomalies for the 2008-2009 cycle compared to those of previous (2006-2007) and subsequent (2009-2010) cycles. In general, the effects of the El Niño 2006-2007, La Niña 2007-2008, and El Niño 2008-2009 events were apparent in Bahía de La Paz, with temperatures during the 2008-2009 cycle being at least 1.5-2.0 °C warmer than those of the previous cycle. In 2009, the variation in SST anomalies ranged from 0.7 °C in February and -0.2 °C in April to 0.5 °C in June and continued to increase to 1.7 °C in January-February 2010. Thereafter, SST drastically decreased in 2009-2010 and favored another cold La Niña event.
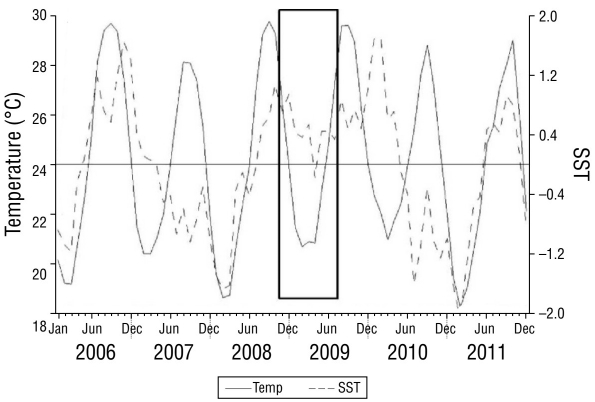
Figure 2 Variations in water temperature and sea surface temperature (SST) anomalies for the 2008-2009 reproductive cycle in Bahía de La Paz (solid rectangle) and those of the previous (2006-2007) and subsequent (2010-2011) cycles.
The general condition index of the broodstock was significantly higher (F = 41.4, P < 0.001) in June 2009 than in February and April and maintained an inverse relationship with water temperature (r = -0.75) and a direct relationship with total seston content (r = 0.90). Significant increases in mean oocyte area (F = 26.9, P < 0.001) and the lipid index (F = 9.9, P < 0.001) were also found in April 2009 when compared to those of other months. Both indicators were significantly correlated with each other (r 2 = 0.88) and showed an inverse relationship with water temperature (r = -0.71) and a direct relationship with total seston content (r = 0.62). At the same time, significantly higher total protein, carbohydrate, and lipid content were found in November 2008 (F = 13.5, P < 0.001), April 2009 (F = 18.6, P < 0.001), and June 2009 (F = 22.5, P < 0.001), respectively. However, the lipid content of female gonads was inversely related with the lipid index (r = -0.91) and oocyte area (r = -0.77).
Larval survival, growth, and biochemical and energy composition
Larval survival and growth from the April and June 2009 trial runs are shown in Figure 3 and Table 2. At the veliger stage (day 3), survival was 95.4% in April 2009 and 60.1% in June 2009. At the umbonate stage (day 10-11), survival drastically decreased to 16.1% in April and 10.3% in June, whereas the final survival percentage of eyed pediveligers at day 21 was 7.7% in April and 0.1% in June.
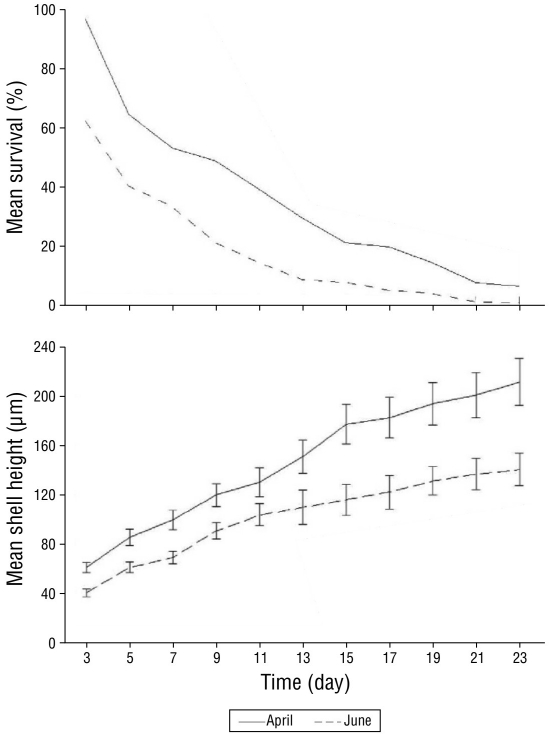
Figure 3 Survival and growth based on the shell height of larvae of the winged pearl oyster Pteria sterna reared at the hatchery in April and June 2009.
On day 11, the mean growth rate of umbonate larvae was significantly higher (F = 18.1, P < 0.001) in April 2009 than in June 2009. At day 23, differences in the growth rate of pediveliger larvae were also significant (F = 24.5, P < 0.001) between April and June (Table 2, Fig. 3). The relationships between larval shell height and oocyte area and between shell height and the lipid index were not significant (r = 0.30 in both cases).
At the veliger stage, differences in larval protein and carbohydrate content were not significant between April and June 2009 (H = 2.3, P > 0.050), although these differences were significant at the pediveliger stage (H = 6.8, P < 0.050). Similarly, variations in protein and carbohydrate content were significant between the veliger and pediveliger stages in April (H = 19.2, P < 0.001) and June (H = 20.5; P < 0.001). The lipid content of the larvae varied significantly between runs in April and June in the veliger (H = 24.1, P < 0.001) and pediveliger (H =16.5, P < 0.001) stages and between both developmental stages in April (H = 20.3, P < 0.001) and June (H = 6.2, P < 0.050). When compared to the energy equivalents of total proteins and carbohydrates, those obtained from lipid reserves significantly increased from April to June (H = 11.3, P < 0.001) and significantly diminished from the veliger to pediveliger stages (H = 27.3, P < 0.001; Table 2).
DISCUSSION
This study provides evidence that both confirms and contradicts the present working hypothesis based on the clear relationship that exists between reproductive condition, larval viability, and the environment (Chávez-Villalba et al. 2003, Mazón-Suástegui et al. 2021). In Pteria sterna, first we observe that environmental variations affected reproductive performance more than larval performance, as all trial runs were carried out under controlled temperature and diet conditions in the hatchery and not in the wild, which would expose the larvae to the prevailing conditions at the time of broodstock collection. For example, November is considered to be a pre-reproductive period for Pteria sterna (Saucedo and Monteforte 1997, Vite-García and Saucedo 2008, Cáceres-Puig et al. 2009), and the failure of the broodstock to spawn and produce larvae during this month appears to be directly related to the presence of the highest water temperatures, lowest seston content, and SST anomalies. These anomalies, which reflect cyclic oceanographic events that occurred during 2006-2007 (El Niño), 2007-2008 (La Niña), and 2008-2009 (El Niño), produced positive thermal oscillations (+0.5 to + 1.0 ºC) from August to December 2008 that prolonged warm summer conditions. In turn, this scenario likely resulted in the production of immature or over-ripe (atretic) oocytes that were unable to sustain larval development in November 2008 and February 2009 (Gómez-Robles et al. 2013). This is notable given that one of the main breeding peaks for Pteria sterna occurs during February. These SST anomalies continued to increase until reaching values of 1.7 °C in January-February 2010 (El Niño event) and then drastically dropped to -1.6 °C in July 2010 and -2.1 °C in February 2010 (La Niña event; Fig. 2). García-Cuellar et al. (2004) also reported an anomalously long summer caused by the 1997-1998 El Niño in Bahía de La Paz that extended the spawning season of the Panamic pearl oyster Pinctada mazatlanica, which unlike Pteria sterna, only breeds during summer.
The results of this study also indicate that the environmental conditions from February to June 2009 (low water temperature, high seston content, and SST anomalies between -0.5 to +0.5 °C) were favorable for gamete nutrition and larval development throughout the spring. However, April 2009 was the only period in which the larvae were viable and contained sufficient energy reserves (mostly lipids) to complete metamorphosis and settle, which coincided with the highest percentages of ripe and spawning gonads, the largest oocytes, the highest lipid index values, low water temperatures, and the highest seston content. According to Saucedo and Monteforte (1997), Vite-García and Saucedo (2008), and Cáceres-Puig et al. (2009), April is another peak breeding period for Pteria sterna, which coincides with the optimal use of energy reserves during gametogenesis to maximize reproductive performance (Gómez-Robles et al. 2013). Finally, June is recognized as a post-reproductive period for Pteria sterna, which is mainly due to increasing temperatures and decreasing food availability (Saucedo and Monteforte 1997, Vite-García and Saucedo 2008, Cáceres-Puig et al. 2009). In this period, the thermal anomalies remained within a normal range (+0.5 °C), although temperatures above 24.0 °C from May onward once again resulted in adverse conditions for optimal gamete development (only 50% of gonads were ripe; oocytes were small; and lipid index values were the lowest of all periods). Thus, the oysters successfully spawned in June, yet the only viable larvae that survived and settled (<150) died when they were moved to the sea for the final growth phase.
Studies dealing with hatchery reared Pteria sterna larvae are scarce and report final survival rates that notably vary from 2-4% (Araya-Núñez et al. 1991, 1995) to 17% (Saucedo 2017). In this study, the final survival rates of pediveliger larvae were 7.70% in April and <0.10% in June. In particular, the final survival rate of larvae in April 2009 was higher than the value of 0.32% reported by Hoyos-Chairez et al. (2020) for Pteria sterna and the value of 4.70% reported by Southgate et al. (2016) for Pteria penguin, which is a species larger than Pteria sterna that begins settling on day 16-17 at a shell height of ~240 μm (Wassnig and Southgate 2012b, Wassnig and Southgate 2016). Similarly, the day and size at which Pteria sterna larvae settle vary greatly among studies: day 19 at ~218 μm (Saucedo 2017), day 23 at ~210 μm (this study), day 28 at ~290 µm (Hoyos-Chairez et al. 2020), and day 39 at ~252 µm (Araya-Nuñez et al. 1995).
The delay or inability of bivalve larvae to complete metamorphosis may be due to 3 possible causes: (1) unsuitable rearing conditions, including poor seawater quality and diet, although the protocols for water supply, microalgae production, and larval culture were the same as those previously employed with Pteria sterna (Saucedo 2017, Hoyos-Chairez et al. 2020); (2) differences in settlement cues related to the material, surface, color, and nature of the substrate (Doroudi and Southgate 2002, Wassnig and Southgate 2012b), although all factors were consistent in our study; and (3) poor physiological condition of the wild broodstock at the moment of collection (Chávez-Villalba et al. 2003, Gómez-Robles et al. 2013), which was only suboptimal in November 2008. Several authors have highlighted the important relationship between egg size/composition and larval survival and vigor in bivalve species such as Mercenaria mercenaria and Argopecten irradians (Kraueter et al. 1982), Pecten maximus (Le Pennec et al. 1990, Gagné et al. 2010), Crassostrea gigas (Chávez-Villalba et al. 2003), Agropecten purpuratus (Nevejean et al. 2003), Atrina maura (Angel-Dapa et al. 2015), and Pteria sterna (Gómez-Robles et al. 2013).
The strategies of carbohydrate, protein, and lipid reserve utilization vary among bivalve mollusks based on location (i.e., tropical, subtropical, and temperate zones) and larval development stage (Nevejean et al. 2003, Pernet et al. 2003, Gireesh et al. 2009). In Pteria sterna, proteins represented the major constituent of veliger (~96%) and pediveliger (~90%) larvae in April and June 2009, which was likely related to the formation of the shell and vital organs from an early stage. Despite this, the energy equivalents calculated for proteins and carbohydrates were very low in both months and developmental stages (<1.3 J µg-1·larvae-1), which indicates that energy expenditure associated with both energy sources is minimal in Pteria sterna. In contrast, the energy supplied from lipids not only was significantly greater (8 to 9 times) in April and June but decreased 10 times faster in the pediveliger stage than in the veliger stage. This indicates that lipids are far more important than proteins and carbohydrates when meeting the metabolic demands associated with the metamorphosis success of the pediveliger larvae. Several authors have confirmed that lipids provide between 70-90% of the total energy expenditure associated with larval development in bivalve mollusks. These authors have also used the lipid content of eggs as a reliable indicator of larval performance in species such as M. mercenaria and Crassostrea gigas (Gallager et al. 1986), Pecten maximus (Le Pennec et al. 1990; Gagné et al. 2010), Agropecten purpuratus (Nevejean et al. 2003), Placopecten magellanicus (Pernet et al. 2003), Atrina maura (Angel-Dapa et al. 2015), Paphia malabarica (Gireesh et al. 2009), and Agropecten ventricosus (Mazón-Suástegui et al. 2021).
Our results confirm that broodstock (egg) condition is vital during initial larval development, but it also has enduring effects until metamorphosis. Although the conditions for larval culture and the production of Pteria sterna spat were only optimal in April, it is likely that environmental anomalies associated with SST continued to occur, which affects the reproductive performance of this species. In addition to collecting ripe broodstock in winter-spring to ensure larval viability, we recommend including a brief conditioning period at the hatchery immediately following the main spawning event to promote nutrient recycling and natural gamete recovery, and another in late autumn to hasten final ripening. Both strategies could increase the chances of producing healthy spat outside of the main breeding season, thus supporting natural spatfall and pearl farming operations in the Gulf of California.











 texto en
texto en 



