INTRODUCTION
Marine mammals are sentinel species that allow monitoring changes in the structure and function of marine ecosystems due to their conspicuous nature, longevity, fat reserves, and high trophic position (Bossart 2006, Hazen et al. 2019). They incorporate and reflect long-term spatial and temporal scale variations (Moore 2008) and reveal the response of ecosystems to environmental variability (Hazen et al. 2019). Within this group are the pinnipeds, which respond rapidly to changes in prey availability due to climatic oscillations (Le Boeuf and Crocker 2005, Robinson et al. 2012, Páez-Rosas et al. 2020).
The northern elephant seal (Mirounga angustirostris; NES) is distributed in the northeastern Pacific with breeding and haul-out sites located on islands and some continental beaches of Baja California, Mexico, and California, USA (Le Boeuf and Laws 1994). During their annual cycle, adults of both sexes migrate twice from breeding colonies to their main foraging areas located in high-latitude coastal and oceanic waters of the northeastern Pacific (Le Boeuf et al. 2000). The first migration (post-breeding migration) occurs at the end of the breeding season in winter (December-February), and the second (post-molt migration) after molting, which occurs in spring for females and in summer for males (Le Boeuf et al. 2000, Hückstädt 2015). During both migrations, females forage in areas between 40° and 45° N, but their longitudinal movements vary; females remain east of 160° W in the post-breeding migration, whereas, in the post-molting migration, they travel near 180° E (Robinson et al. 2012). In contrast, both male migrations are similar; they reach the Gulf of Alaska and the Aleutian Islands (Stewart and DeLong 1995, Le Boeuf et al. 2000).
During the last decade, large-scale oceanographic events impacted marine environments where NES forage. Since the early 2000s, there have been several El Niño events, which is the warm phase of the climatic and oceanographic phenomenon of El Niño/Southern Oscillation (ENSO), and the 2015-2016 event is one of the strongest on record (Kintisch 2016, L’Heureux 2016). In addition, from late 2013 to mid-2016, the marine heatwave called The Blob developed in the northeastern Pacific, with positive anomalies of up to 4 °C in the sea surface temperature (SST; Kintisch 2015, Peterson et al. 2016). The Blob originated in the southern Gulf of Alaska, extended to the west coast of the Baja California Peninsula, and reached an amplitude of around 2,000 km and 100 m depth (Bond et al. 2015, Kintisch 2015, Peterson et al. 2016). The combination of El Niño and The Blob produced unusual conditions in the northeastern Pacific (Bond et al. 2015, Cavole et al. 2016, Gentenmann et al. 2016): anomalously low chlorophyll a concentrations reflected by low primary productivity, tropical and subtropical species found in mid-latitudes, and increased frequency and intensity of harmful algal blooms (Cavole et al. 2016). As a result of these changes, there were mass mortality events of seabirds and marine mammals (Leising et al. 2015, Cavole et al. 2016, Gentenmann et al. 2016). By the summer of 2019, another marine heatwave referred to as The Blob 2.0 was recorded in the Gulf of Alaska, which had SST anomalies up to 2.5 °C above average (Amaya et al. 2020).
The effect of warm SST anomalies on the body condition of NES females and on pup production has been widely documented in California colonies (Le Boeuf and Crocker 2005, Crocker et al. 2006, Robinson et al. 2012). During strong El Niño events, the weight gain of females from the Año Nuevo colony in northern California has decreased due to an increased foraging effort. That is, females spend more time searching for prey patches but reduced periods on them, decreasing their foraging success compared to “normal” years (Crocker et al. 2006). Changes in the foraging strategy and a depleted female body condition appear not to severely impact the birth rate in the Año Nuevo colony (Le Boeuf and Reiter 1991, Crocker et al. 2006, Robinson et al. 2012). However, the weaning weight of pups born during or after El Niño years is lower, which may decrease the probability of juvenile survival (Le Boeuf and Crocker 2005).
Currently, there are 5 breeding colonies in Baja California (García-Aguilar et al. 2018). The main colonies are those of Guadalupe Island and San Benito Archipelago (Arias-del-Razo et al. 2017). The population size in Baja California for 2009 was estimated at 22,300 individuals (range: 18,600-26,000); however, numbers had declined at an average annual rate of 0.7% between 1970-2009 (García-Aguilar et al. 2018). To date, no information exists on the magnitude of the impact of warm SST events on the NES population of Baja California. Thus, in this paper, we analyze the pup production and birth rate of the San Benito colony over the last 2 decades, a period in which several events of large-scale warm SST anomalies occurred in the northeastern Pacific.
MATERIALS AND METHODS
Study area and data collection
The San Benito Archipelago is composed of 3 volcanic islands, namely East, Middle, and West, and a group of islets (Fig. 1). The archipelago, which is part of the Baja California Pacific Islands Biosphere Reserve, is located in the Pacific off Mexico (28°18ʹ N, 115°32ʹ W), 31.5 km west of Cedros Island and 130.0 km from the Baja California Peninsula. The climate is arid with an average annual rainfall of 65.1 to 121.3 mm, and the most amount of rain falls between December through February. The average annual air temperature varies between 19 to 20 °C (Junak and Philbrick 1999).
NES aggregate on all 3 islands during the breeding season in winter, but since there is a constant exchange of seals between the 3 islands (García-Aguilar 2005), we considered San Benito as a single reproductive unit, so the analyses were done at the colony level, not at the island level. Births occur from early December to early February (García-Aguilar 2004), and lactation lasts about 27 days (Le Boeuf 1972).
We compiled published and unpublished counts of live pups (suckling and weaned) and adult females for the period 2002-2019 (Table 1). Since pups remain on land up to ~2.5 months of age (Reiter et al. 1978), counts of this age class were carried out after the end of the birth season, assuming that all living pups were detected during counts. On the other hand, since NES females are asynchronous, they are not simultaneously found on land at any time during the breeding season; therefore, they cannot be counted in a single survey. The temporal distribution of females in the San Benito colony was described by García-Aguilar (2004) using the Rothery and McCann (1987) model, which allowed us to obtain correction factors based on the count date to estimate the total number of females that arrived each season to the colony (Table 1).
Table 1 Counts of northern elephant seal pups and adult female in the San Benito Archipelago, 2002-2019, and total number of adult females estimated based on correction factors.
| Pups | Adult females | |||||
| Season | Date | Count | Date | Count | Correction factor | Total |
| 2001-2002 | February 16-18, 2002a | 2,024 | January 19-25, 2002a | 2,944 | 0.795 | 2,341 |
| 2002-2003 | February 17-20, 2003a | 2,050 | January 18-21, 2003a | 3,009 | 0.808 | 2,430 |
| 2003-2004 | February 8-10, 2004b | 1,771 | January 15-18, 2004b | 3,016 | 0.791 | 2,387 |
| 2008-2009 | January 22-24, 2009c | 1,689 | January 22-24, 2009c | 2,352 | 0.792 | 1,862 |
| 2012-2013 | February 8-12, 2013d | 1,504 | February 8-12, 2013d | 2,061 | 0.315 | 650 |
| 2013-2014 | February 6-11, 2014d | 1,097 | February 6-11, 2014d | 1,907 | 0.361 | 688 |
| 2014-2015 | February 18-20, 2015d | 1,205 | February 18-20, 2015d | 1,964 | 0.140 | 275 |
| 2016-2016 | February 12-15, 2016e | 1,317 | February 12-15, 2016e | 1,579 | 0.283 | 447 |
| 2018-2019 | February 13-14, 2019 | 1,114 | February 13-14, 2019 | 1,713 | 0.278 | 476 |
a García-Aguilar (2005), bGarcía-Aguilar (unpublished data), cFranco-Ortiz (2012), dElorriaga-Verplancken et al. (2015), eElorriaga-Verplancken and García-Aguilar (2018).
Colony trend and birth rate
The instantaneous rate of change (r) was obtained through a linear regression, in which the natural logarithm (ln) of the number of animals was the dependent variable and the time (years) was the independent variable. The annual rate of increase was calculated as λ = e r , where r is the slope of the linear regression (Caughley 1977). An analysis of variance (ANOVA) was performed to test the null hypothesis r = 0. The average annual rate of increase was obtained as (λ - 1) × 100 and expressed as a percentage. This analysis was performed separately for adult females and for pups.
The colony size was estimated for 2019 multiplying the number of pups produced by the factor M calculated for the NES population in California (Lowry et al. 2014), which reflects the population:pup ratio and varies according to λpups.
The birth rate, which represents the proportion of adult females that give birth in a single year (Croxall and Hiby 1983), was calculated for each season dividing the number of pups produced by the total number of adult females. A Z-test was performed to analyze variations in the birth rate over the study period.
Sea surface temperature anomalies
The potential foraging area for NES adult females of the San Benito colony, delimited by Aurioles et al. (2006) based on stable isotope analyses, is located ~8° south of those in California, between 31° and 43° N, and 126° to 175° W (Fig. 2). Monthly SST values with a 1-degree spatial resolution within this polygon were downloaded from the climate data library of the International Research Institute for Climate and Society (http://iridl.ldeo.columbia.edu/SOURCES/.NOAA, accessed 2022 September 01) for the period 1986-2020. Since SST trends are positive across the global ocean (L’Heureux et al. 2013), we first analyzed the trend in our study area. Quarterly average values (December-January-February, January-February-March, and so on) were calculated, which in turn were averaged to obtain annual estimates. The trend for SST for the period 1986-2020 was determined using the Mann-Kendall test (MK test) at a significant level of 0.05.

Figure 2 Potential foraging area (PFA) of northern elephant seal adult females of the San Benito colony (SBA). Based on Aurioles-Gamboa et al. (2006).
Given that the SST had a positive and significant trend in the study area (see below), we calculated the monthly anomalies for the period 2001-2019 following the methodology of the Climate Prediction Center for the Oceanic Niño Index (http://cpc.ncep.noaa.gov, accessed 2022 September 10), which consists of using 30-year base periods to detect anomalies in successive periods of 5 years. Thus, for the years 2001-2004 we used the base period 1986-2015; for 2005-2009, the base period 1991-2020. However, for the last two 5-year periods (2010-2014 and 2015-2019), since the time series corresponding to the base periods 1996-2025 and 2001-2030 are not complete, we used the base period 1991-2020.
RESULTS
Between 2002 and 2019, the number of pups and adult females decreased, with average annual rates of 3.58% and 3.78%, respectively (Table 2, Fig. 3). Since λpups = 0.964, the colony size for 2019 was estimated by multiplying the number of pups produced in that year by 4.24 (the multiplicative factor M). Thus, an abundance of 4,723 individuals (95% CI: 3,821-5,615) was estimated. The West Island congregated the largest number of seals (56% of the total), followed by the Middle (33%) and East (11%) islands.
Table 2 Average annual rate of increase (λ) of pups and adult females of northern elephant seals in the San Benito Archipelago, 2002-2019. N = number of counts, r = instantaneous rate of change, R2 = coefficient of determination.
| N | r | R2 | λ | |
| Pups | 9 | -0.036 | 0.87 | 0.964 |
| Adult females | 9 | -0.039 | 0.94 | 0.962 |
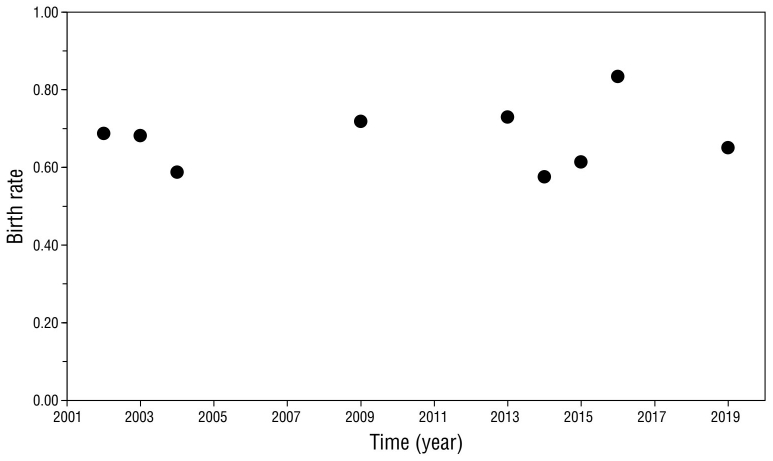
The birth rate remained stable throughout the study period, with a mean of 0.68 ± 0.08, varying from 0.58 in 2014 to 0.83 in 2016 (Fig. 4). Z-scores were not significant (P > 0.05), except for the 2016 season, which was slightly above average (Z = 1.96, P = 0.049).
The MK test revealed positive and significant SST trends in the potential foraging area of San Benito females for the period 1986-2020 (Z = 3.18, P < 0.01; Fig. 5), with a mean increment of 0.18 °C per decade. SST anomalies revealed that 2003, 2006-2007, 2009-2010, and 2016 were years in which cold conditions (SST anomaly less than or equal to -0.25 °C) prevailed, whereas in 2001, 2004, 2008-2009, 2011-2015, and 2019 warm conditions (SST anomaly ≥ 0.25 °C) predominated (Fig. 6).
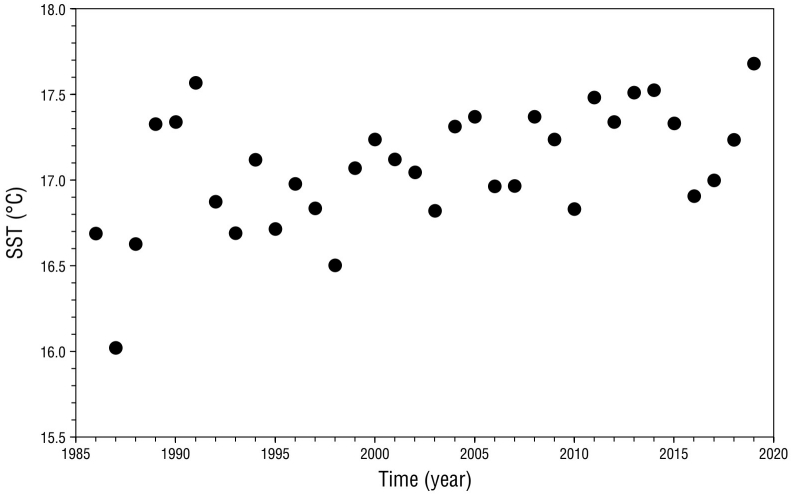
Figure 5 Mean annual sea surface temperature (SST; 1986-2020) in the potential foraging area of northern elephant seal adult females from the San Benito colony.
DISCUSSION
The reproductive success of a species is strongly related with the success of its foraging strategies, which can be affected by anomalous environmental conditions (Crocker et al. 2006). NES females consume wide-ranging epi- and mesopelagic prey (Riofrío-Lazo et al. 2012), mainly squid and myctophid fish (Antonelis et al. 1994, Goetsch 2018), and environmental stress conditions could impact their foraging success, which may reduce pup production. Nevertheless, our results suggest that, as observed in the Año Nuevo colony, this was not the case for females from the San Benito colony. However, there is a sustained negative trend in pup production since the late 1990s (García-Aguilar et al. 2018, present study), which appears to be unrelated to the presence of large-scale events of warm SST anomalies, in contrast to what has been observed for other pinnipeds from the California Current ecosystem, such as the California sea lion (Zalophus californianus) or the Guadalupe fur seal (Arctocephalus townsendi) (Elorriaga-Verplancken et al. 2016, Delong et al. 2017). In fact, the stable NES birth rates observed in our study period (2002-2019) indicate that there was no impact despite the occurrence of several El Niño events and The Blob.
The persistent decrease of the San Benito colony, like that observed in the Guadalupe Island colony, could be due to the movements of seals towards the colonies in southern California in response to the sustained increase in air temperature in the region, as proposed by García-Aguilar et al. (2018). The fact that both the number of adult females and the number of pups has decreased at a similar average annual rate (~3.6%) during the last 2 decades with a birth rate that has not declined, suggests that the reproductive success of females (expressed exclusively in terms of pup production) has been constant over this period. Therefore, the negative trend could be explained by the proposed migration to the north. Nevertheless, further research is needed to expand our knowledge on this regard.
How the distribution of animals in the San Benito Archipelago has changed over time is noteworthy. East Island was apparently the first to be occupied in the late 1910s, and for several decades the largest number of NES congregated there (Williams 1941). By the mid-1960s, animals were observed moving to the West and Middle islands (Rice et al. 1965). In the early 2000s, the largest number of animals congregated on the Middle Island, followed by the West Island and East Island (García-Aguilar 2005). The recent distribution is different: in 2019 most of the NES congregated on the West Island. The gradual abandonment of the Middle Island in recent years could be associated with increasingly frequent heatwaves (i.e., days with an average maximum air temperature >25 °C) during winter and spring (García-Aguilar et al. 2018) that severely impact pup survival in harems without access to the sea (Salogni et al. 2016), which were common 20 years ago but now no longer exist.
Two non-exclusive explications were proposed regarding why birth rates are not severely impacted by large-scale warm anomaly events (Crocker et al. 2006). The first concerns the foraging strategy of NES females, which forage for prolonged periods in deep (up to ~700 m) oceanic waters, where the effect of these events is weaker than in coastal waters. The second explanation concerns the lagged effect of warm anomalies along the food web, hence the lagged impact on top predators. Considering these 2 explanations, it is unsurprising that the reduction observed in the birth rate was not drastic in the San Benito colony during the strong El Niño event of 2015-2016 or The Blob in 2013-2016. In fact, stable isotope (δ15N and δ13C) analyses showed that NES females from the San Benito colony did not change their habitat use throughout these events, except towards the end of the 2014 and 2018 post-molting migration, when they apparently expanded their foraging range (Rodríguez-Rafael 2021).
Our results confirm the persistent decline of the San Benito colony since the end of the 20th century. However, there was no evidence that this decline was caused by a decrease in female reproductive success associated with anomalous warming events in the northeast Pacific. Instead, the results support the scenario in which animals are migrating from colonies of Baja California to colonies of California. These types of assessments must continue over time, complemented by additional approaches such as stable isotope analyses or telemetry, to strengthen our knowledge of how NES use marine and terrestrial habitats, especially given the recent increase in the frequency of warm anomalies in the Pacific Ocean (Freund et al. 2019) and the positive trend in air temperature in the region (Cayan et al. 2008).
This study provides information exclusively on the current status of the San Benito NES colony. However, the present study is part of the comprehensive effort of different Mexican academic institutions to understand the ecological processes that determine the population dynamics of this and other pinniped species that inhabit the Baja California Pacific Islands Biosphere Reserve, such as the California sea lion, the Guadalupe fur seal, and the Pacific harbor seal (Phoca vitulina). The conjunction of the information generated is an essential tool to create a program aimed at the management and conservation of these species in the region.











 texto em
texto em