INTRODUCTION
Mollusca is an ancient phylum. Indeed, the first records indicate that mollusks date back to the Cambrian period (Ponder and Lindberg 2008). To date, 85,000 living molluscan species have been described (Chapman 2009), of which ~30,000 species are found only in marine environments. Marine mollusks provide important ecosystem services; they create habitats for benthic organisms (e.g., oyster and mussel beds and vermetid reefs), filter water, biodeposit organic carbon in the seafloor, and serve as food sources for other organisms (Gazeau et al. 2013).
Micromollusks (adults <5 mm, Geiger et al. 2007; adults <10 mm, Barrera and Tunnell 2001) are of great ecological importance. These tiny mollusks are consumed by many organisms in higher trophic levels and constitute important food items in their diets. For example, both blue crabs (Callinectes sapidus) and red octopuses (Octopus maya) consume large quantities of micromollusks. In addition, larger mollusks also consume micromollusks after piercing their shells with their radulae (Naim 1988). Empty micromollusk shells are then used by juvenile hermit crabs, while both occupied and empty shells serve as substrates for bryozoans and other encrusting species. Furthermore, many microherbivorous gastropods feed on seaweeds or epiphytes that grow on micromollusk shells (Shacklock and Doyle 1983, Naim 1988, Narciso et al. 2005, Srivastava and Sinh 2021). Given their importance as food sources within trophic webs and as substrates for other organisms, the roles of micromollusks within ecosystems cannot be ignored.
Information on feeding guilds is needed to understand how benthic communities function and how energy is transferred through food webs (Pimm 1982, Arruda et al. 2003, Pagliosa 2005). As micromollusks are major components of ecosystems in terms of richness and abundance, they play important roles in the energy transfer process as both predators and prey items for other organisms. However, studies of mollusk populations require time-consuming sampling efforts, specific techniques and equipment, and specialized training to identify species properly. This is especially true for micromollusks, as studies of their taxonomic composition show even greater challenges than those of macromollusks due to the limited availability of taxonomic keys and information. Adult micromollusks measure at most 10 mm, and some are almost invisible to the naked eye, thus sorting individuals from marine sediments is laborious. As a result, most information on mollusks has focused on macromollusks and ignored or underestimated the abundance and diversity of micromollusks (Narciso et al. 2005, Middelfart et al. 2016).
Sasaki (2008) points out that finding and describing micromollusk species represents an unlimited frontier for uncovering new insights because these species have only been described in a few habitats, namely submarine caves, hydrothermal vents, sediments, coralline turf, and some cryptic environments like those formed by sunken wood (Olabarria and Chapman 2001). Therefore, the search for micromollusks needs to expand in environments that have yet to be studied well and may harbor a great diversity and richness of species. Some of these environments may even be contained within other organisms. For example, the Eulimidae family contains parasitic species that feed on echinoderms, such as sea cucumbers and starfish, and the Pyramidellidae family contains ectoparasitic species that feed on the bodily fluids of various invertebrates, especially those of polychaete worms or other mollusks (Ankel 1949, Robertson and Mau-Lastovicka 1979, Esparza-Carrera et al. 2018).
Most studies of marine micromollusks in Mexico have been conducted in the Gulf of Mexico such as the study by Blanco et al. (2016) of microgasteropods in Isla Verde, Veracruz, and those by García-Cubas (1969), García-Cubas (1982), and García-Cubas (1991). However, other studies have also been conducted along the Pacific coast of Mexico. In the state of Guerrero, Garcés-Salazar (2011) analyzed microbivalves, and García-Tello (2013) reported 71 species of microgasteropods. Hansen-Bernal (2014) also studied micromollusks from the Pacific coast of Mexico and examined those associated with macroalgae in the intertidal zone. Esparza-Carrera et al. (2018) collected specimens of 52 families and 121 micromollusk species from the tropical Pacific coast of Mexico, 76% of which were gastropods and 13% were bivalves.
Studies of micromollusks have also been conducted in the Gulf of California. Schwartzlose et al. (1992) gathered bibliographic information of diverse mollusk taxa in the Gulf of California. Among these studies, Baker et al. (1928) described Pyramellidae mollusks, Baker et al. (1930, 1938) collected Rissoidae and Epitoniidae specimens, and Skoglund (1965) studied microgasteropods from Cholla Bay, Sonora, in the upper Gulf of California. In addition, Castillo-Rodríguez (2014) and Tripp-Quezada et al. (2018) studied the composition and structure of micromollusk communities in the Gulf of California. In Nayarit, Tapia-Díaz (2018) collected specimens of 29 families, 41 genera, and 53 species of micromollusks.
Other studies have covered larger areas such as the study by Aguilera-Vilchis and Rivas-Lechuga (2022) that analyzed the state of knowledge of the genus Caecum in Mexico and reported 31 species in the Pacific coast of Mexico and 26 species in the Atlantic coast. Bartsch (1920) described 13 species of Barleeia from San Diego and other coastal areas of California and 4 species of Rissoellidae from Mazatlán at the entrance to the Gulf of California and multiple sites along the Baja California Peninsula, which included the San Ignacio Lagoon, Todos Santos Bay, Punta Abreojos, and Cabo San Lucas. A century later, Raines (2020) reviewed thousands of specimens from Alaska to Chile along the eastern Pacific and described 43 Caecidae species; 5 were new to science.
Although some information is available for micromollusk species in Mexico, it is quite limited. Therefore, it is necessary to improve our knowledge of the distributions and ecology of micromollusk species in Mexico to assess populations properly, especially those in priority regions for the conservation of flora and fauna. Bahia de los Angeles (BLA) is currently protected. This coastal bay in the Gulf of California is ecologically important and biologically productive, and it provides nesting and foraging areas for several species of sea turtles (Seminoff et al. 2008) and an aggregation area for whale sharks (Rhincodon typus) that arrive each summer to feed (Rodríguez-Dowdell et al. 2008). The lack of sufficient information on the micromollusks of BLA has prevented adequate conservation measures from being implemented in this important conservation area. Thus, the objective of this study was to identify the families of microgastropods found in BLA.
MATERIALS AND METHODS
Study site and sample collection
BLA (28°95ʹ N and 113°55ʹ W; Fig. 1) is located in Baja California, Mexico, along the western coast of the Gulf of California in the Bahia de los Angeles, Canal de Ballenas, and Salsipuedes Biosphere Reserve. The tides are semidiurnal, and the climate is arid and highly influenced by the desert regions of the peninsula, with low rainfall and high evaporation rates (Cavazos 2008). BLA has little anthropogenic influence and its varied habitats that shelter diverse benthic organisms are relatively undisturbed.
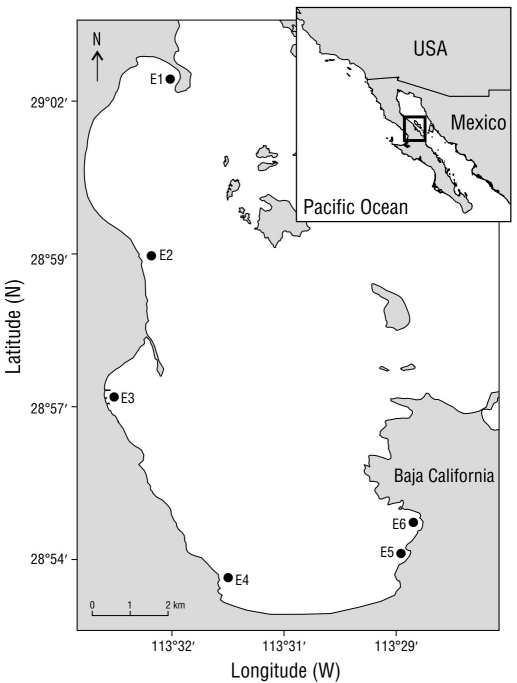
Figure 1 Study area and location of sampling stations (E1-E6, black dots). The stations covered the entire bay and had depths that ranged from 10-15 m.
Blanco-Betancourt et al. (2004) described sea surface temperatures (SST) in BLA that ranged between 28.7 °C in summer and 15.3 °C in winter. Amador-Buenrostro et al. (1991) indicated that the configuration of the bay and its bathymetry are responsible for its circulation; they detected a persistent gyre off Punta La Gringa in the northern and central areas of the bay and found that the strongest currents (3 cm·s-1) are located at Punta La Gringa and in the southern region of the bay. However, wind-induced currents can reach speeds of 25 cm·s-1.
A total of 24 sediment samples were collected from 6 stations distributed along the coastline (10-15 m depth) in September 2013 and February 2014, which were considered to be representative of summer and winter conditions, respectively. The 6 stations were relatively equidistant from each other (Fig. 1). During both summer and winter, 4 samples were collected in each station using a Petite Ponar Grab (Wildco, Yulee, FL, USA). Samples were sieved using a 1-mm mesh (Couto et al. 2010) and fixed with a 5% formaldehyde solution. Additional samples were taken to determine organic matter (OM) content, which was evaluated with the ignition loss method (Byers et al. 1978). SST and salinity (SL) measurements were also taken at each station using a YSI Pro2030 meter (Yellow Springs, OH, USA). For the granulometric analysis, 20-g sediment samples were dry-sieved through a series of mesh sieves (90 µm to 1 cm) and mechanically shaken for 15 min. The sediment retained in each sieve was weighted with a Denver balance (resolution of ± 0.01 g). The granulometric classification was obtained with Sysgran v.3.0 (Folk and Ward 1957, Rendón-Márquez 1995).
Faunistic analysis
The samples were washed in the laboratory with tap water using a 500-µm mesh screen, and all fauna were stored in 70% ethanol. Organisms were grouped into the following taxa: crustaceans, echinoderms, mollusks, polychaetes, and miscellaneous. Sediment samples were processed to separate macrofauna and micromollusks. Organisms were counted and identified to the family level according to the criteria of Abbott (1974), Brusca (1980), and McLean and Gosliner (1996) using a Wild M10 Stereo Zoom Microscope (Leica, Wetzlar, Germany). The feeding guilds of each family were determined using the information available in the literature (Tripp-Quezada et al. 2018, Arvizu-Ruiz and Reyes Bonilla 2021, Srivastava and Singh 2021).
Data analyses
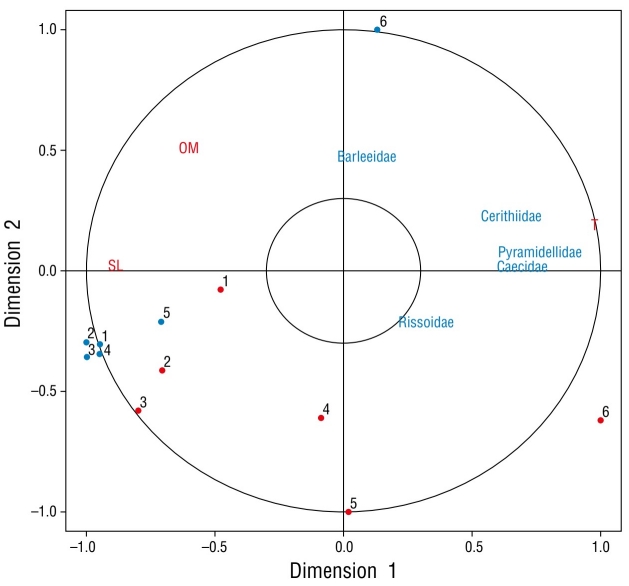
We used a Bayesian analysis of variance (ANOVA) due to its probabilistic nature (Box and Tiao 2011) to detect differences in micromollusk density between summer and winter. A canonical correlation analysis (CCA) was performed to analyze the composition of benthic groups at the family level and the potential relationships with environmental variables (SST, SL, and OM). All analyses were conducted in R v.4.0.0 (R Core Team 2020). For the CCA, we did not consider the Tornidae family because, when we included this family, the data did not meet the requirements for the analysis, which was probably due to the low abundance of this family in both seasons.
RESULTS
A total of 20,353 specimens were collected with 15,310 in summer and 5,043 in winter. We identified 7 micromollusk families in the samples in both seasons: Barleeidae, Caecidae, Cerithiidae, Eulimidae, Pyramidellidae, Rissoidae, and Tornidae. As Eulimidae was represented by very few organisms in both seasons (<30 individuals), this family was discarded from the statistical analyses to avoid bias arising from an excess of zeros. Table 1 shows the total abundance per family in both periods and the corresponding feeding guilds and trophic levels. Density values also showed a similar pattern, with 679,200 ind·m-2 and 223,111 ind·m-2 in summer and winter, respectively. In summer, Caecidae showed the maximum density (278,044 ind·m-2) among all families, whereas, in winter, Barleeidae showed the highest density (142,222 ind·m-2) among all families. During both seasons, Tornidae showed the lowest densities (1,867 and 1,411 ind·m-2 in summer and winter, respectively). Station 6 showed the highest mean density for both periods (~69,288 and 39,807 ind·m-2 in summer and winter, respectively), whereas station 2 showed the minimum mean density for both periods (15,556 and 4,467 ind·m-2 in summer and winter, respectively).
Table 1 Total abundance per family in summer and winter as well as the corresponding feeding guilds. c = carnivore, d = detritivore, e = ectoparasite, and h = herbivore.
| Family (Feeding guild, Trophic level) |
Summer (abundance) |
Winter (abundance) |
| Barleeiidae (h, d) | 4,821 | 3,200 |
| Caecidae (h, d) | 6,256 | 470 |
| Cerithiidae (h, d) | 2,488 | 807 |
| Eulimidae (c, e) | 28 | 21 |
| Pyramidellidae (c, e) | 1,454 | 412 |
| Rissoidae (h, d) | 221 | 88 |
| Tornidae (h, d) | 42 | 45 |
| Total | 15,310 | 5,043 |
During summer, SST ranged from 30.0 to 29.4 °C, whereas, in winter, it ranged from 15.8 to 16.4 °C. In contrast, salinity had lower values in summer (~32) compared to those in winter (~34). In addition, OM concentrations ranged between 1.00% and 2.22% in summer and 1.28% and 2.70% in winter (Table 2). The results from the granulometry analysis for summer (fine to course) and winter (medium to coarse) sands are shown in Table 3.
Table 2 Mean (± SD), temperature (°C), salinity, and organic matter (%) for summer and winter in Bahia de los Angeles (BLA).
| Temperature | Salinity | Organic matter | ||||
| Summer | Winter | Summer | Winter | Summer | Winter | |
| Mean ± SD | 30.1 ± 0.4 | 15.8 ± 0.7 | 33.2 ± 0.1 | 34.5 ± 0.7 | 1.6 ± 0.4 | 2.04 ± 0.6 |
| Minimum | 29.4 | 15.2 | 32.0 | 33.1 | 1.0 | 1.3 |
| Maximum | 30.0 | 16.4 | 32.3 | 35.0 | 2.2 | 2.7 |
Table 3 Summer and winter granulometry results for each sampled station.
| Station | Summer | Winter |
| E1 | Fine sand | Medium sand |
| E2 | Medium sand | Medium sand |
| E3 | Fine sand | Medium sand |
| E4 | Medium sand | Medium sand |
| E5 | Medium sand | Coarse sand |
| E6 | Coarse sand | Coarse sand |
The results of the Spearman correlation analysis showed a significant correlation between SST (r = 0.890, P < 0.05) and abundance in summer, although no significant correlations with any abiotic variable were observed in winter (r < 0.305, P > 0.05 for all abiotic variables). The results of the Bayesian ANOVA indicated a low probability of differences in mean density among stations in winter (BF = 0.25). Conversely, some stations had high probabilities of being different in summer (Table 4). Moreover, most stations, with the exception of station 6, had high probabilities of showing differences between seasons (Table 4). The CCA found a high canonical correlation coefficient (r = 0.880, P < 0.01) between temperature and the linear representation of the families Caecidae, Cerithiidae, and Pyramidellidae; Barleeidae, Rissoidae, and Tornidae were not specifically associated with any station or abiotic variables (Fig. 2).
Table 4 Given that we did not find differences in winter stations, the first 2 columns show differences between summer stations and the Bayes factor (BF10) in favor of the alternative hypothesis. The last 2 columns show differences of the same station between the seasons with its respective Bayes factor (BF). The BF indicates the times that the probability of differences is bigger than the probability of no differences.
| Differences | BF10 | Differences | BF10 |
| E2S vs. E4S | 16.072 | E1W vs. E1S | 6.643 |
| E2S vs. E5S | 5.719 | E2W vs. E2S | 5.719 |
| E3S vs. E4S | 5.930 | E3W vs. E3S | 4.579 |
| E3S vs. E5S | 3.245 | E4W vs. E4S | 24.406 |
| E5W vs. E5S | 6.156 |
DISCUSSION
Overall, a total of 7 families were identified in BLA: Barleeidae, Caecidae, Cerithiidae, Eulimidae, Pyramidellidae, Rissoidae, and Tornidae. Of these, the most abundant families were Caecidae and Barleeidae. This is likely because Barleeidae mollusks are known to be abundant and widely distributed (Hansen-Bernal 2014), which may be in part because these mollusks exhibit direct development without a planktotrophic stage (Ponder 1983) that may also contribute to their relatively high diversity. The Tornidae and Eulimidae families were the least abundant micromollusks in this study.
Raines (2019) points out that even though caecids are not very diverse, they tend to be very prolific in shallow waters. This could have resulted in the very high abundance observed in our study (6,256 organisms). Along the northwestern Pacific coast of the United States, Bartsch (1920) reported the presence of Caecidae, and Raines (2019) added a new species of this family to those reported by McLean (1978) for the same region. Aguilera-Vilchis and Rivas-Lechuga (2022) analyzed the Caecidae family and listed 31 Caecum species in the Pacific coast of Mexico.
Although we only found 7 micromollusk families in our study site, our abundances were higher than those reported previously. Tapia-Díaz (2018) conducted an extensive study of micromollusks in Nayarit in the southern Gulf of California during winter 2013 and found only 288 micromollusks. In contrast, 20,353 individuals were collected in this study. We collected 5,043 organisms during winter alone, which is far more than what Tapia-Díaz (2018) collected. García-Tello (2013) collected 9,440 organisms (51 families) in Acapulco in the Pacific coast of Mexico, which is also far less than the total number of organisms collected in this study. This indicates that the Gulf of California is a highly productive system.
Esparza-Carrera et al. (2018) collected micromollusks in Tenacatita, Jalisco (11 families), and Manzanillo, Colima (9 families), whereas Esparza-Carrera and Esqueda-González (2019) collected micromollusks in Manzanillo, Colima (4 families), Tenacatita, Jalisco (5 families), Bahía Banderas, Jalisco (11 families), and Mazatlán, Sinaloa (13 families). Hansen-Bernal (2014) collected micromollusks in Michoacán, Jalisco, and Guerrero in areas with macroalgae and found 12 micromollusk families, with Barleeia and Fossarus being the most abundant genera. In our study, we collected 7 families of microgasteropods. The family richness of our study is slightly lower, although we only collected in 6 stations in BLA. Nonetheless, the family richness in our study is higher than those of Manzanillo and Tenacatita.
The analysis of feeding guilds revealed that 5 of the 7 families were comprised of herbivores and deposit-feeders. The remaining 2 families, Pyramidellidae and Eulimidae, were comprised of carnivores and ectoparasites. These 2 families were more abundant in summer than in winter. This may be because mollusks were highly abundant in the summer of 2013 (Ángeles-González et al. 2021), and Pyramidellidae species feed mainly on other mollusks and annelid worms (Robertson and Mau-Lastovicka 1979).
Ángeles-González et al. (2021) found significant differences in the abundance of benthic fauna between summer and winter in BLA during the same years as those of our study, with higher abundances for summer than for winter. This same trend was also reported in other studies (Gulf of California, Brusca 1980; BLA, Barnard and Grady 1968). Nevertheless, our results contrast with those from the Pacific. For example, Olabarría et al. (2001) found higher abundances in winter than in summer along the coast of Sinaloa. As stated by Alvarez-Borrego (2008), the tidal currents of the Midriff Islands Region are very intense. Indeed, the energy dissipation rates in the region exceed 0.3 W·m-2 (Argote et al. 1995). Intense mixing creates a similar situation to that of constant upwelling. The resulting cold surface and subsurface waters could be responsible for the higher abundances observed in summer (15,310) compared to those observed in winter (5,043) in this study.
Ángeles-González et al. (2021) showed a direct relationship between SST and SL and the abundance of macrobenthic fauna in BLA. We found similar results for most micromollusk families, although the CCA did not reveal any associations between the other abiotic variables and the abundance of microbenthic fauna in BLA. In addition, the Spearman correlation analysis indicated a significant relationship between abundance and temperature (SST; r = 0.89, P < 0.05) in summer but not in winter. We found no significant correlations between abundance and any other abiotic variable (r < 0.305, P > 0.05). Moreover, the CCA and correlation analyses did not indicate any associations between OM and abundance for any season, which could be because most families in this study are deposit-feeders. As a detritus pool is composed of dead organic matter that has variable nitrogen and carbon content and nutritional value (Kenneth 1988), we assumed that OM was always available, and thus abundance should not be correlated with OM.
Deposit-feeders regenerate nutrients that can be used by microalgae, macroalgae, and seagrasses through their foraging activities. These activities favor the presence of herbivore species that can exploit the phytobenthos. The sedimentary food of deposit-feeders includes drifting macroalgal debris, benthic diatoms, and algal remains. An increase in deposit-feeders has been found to enhance bioturbation activity, which in turn provides nutrients for macroalgae and seagrasses while favoring an increase in herbivore abundance (Byren 2004, Andrade-Díaz 2016).
To the best of our knowledge, there are no records of micromollusks in BLA, although records exist for the mollusk families of Caecidae, Cerithiidae, Eulimidae, Tornidae, Pyramidellidae, and Rissoidae in BLA and throughout the Gulf of California (Coan 1968, Hendrickx et al. 2007). However, the Barleeidae family has only been reported by Hendrickx et al. (2007) in the Gulf of California and along the Pacific coast of Mexico in Tijuana, Baja California, by Tapia-Díaz (2018) in Puerto Vallarta, Jalisco, and more recently by Gama-Kwick et al. (2021) and GBIF (2020) in the coasts of Guerrero.
In Mexico, micromollusk studies are in the early stages, and malacologists have much ground yet to cover. It is likely that numerous micromollusk species are already endangered due to pollution and climate change, as they are calcifying organisms that must build shells to survive (Doney et al. 2012, Gazeau et al. 2013, Díaz-Castañeda et al. 2019). Therefore, it is important to study these benthic organisms to understand how entire communities may respond to changing environmental conditions. Studies of benthic communities require great effort, as they involve special sampling techniques and time-consuming laboratory work and organism identification, yet they are sorely needed. In this study, the proximity of the sampling sites to the coast and their shallow depths may have influenced the number of families and their abundance in the samples. Nevertheless, due to the ecological importance of micromollusks, we believe that this study contributes valuable information to address the knowledge gaps associated with this important group.











 texto en
texto en