INTRODUCTION
Cyclonic gyres and oceanic fronts are recognized as structures that favor biological productivity (Hales et al. 2009, Vidya and Kumar 2013, Mahadevan 2016). In Bay of La Paz, Gulf of California, Mexico (Fig. 1a), the circulation is dominated by a cyclonic gyre that has been observed in late spring (Monreal-Gómez et al. 2001), summer (Sánchez-Velasco et al. 2006, Coria-Monter et al. 2014), and at the end of winter (García-Mirafuentes 2010); therefore, it is considered a semi-permanent gyre (Coria-Monter et al. 2014). Wind stress plays an important role in the formation of the gyre and, via Ekman pumping, promotes nutrient enrichment towards the surface layer (Coria-Monter et al. 2017); thus, the euphotic layer is fertilized and, consequently, phytoplankton biomass increases, which promotes the increase of zooplankton due to the high concentration of food (Durán-Campos et al. 2015).

Figure 1 Bathymetry (isolines, m) in Bay of La Paz, CTD cast hydrographic stations (+), PNF-300 stations for determining chlorophyll a (○), and zooplankton sampling sites ((). The dark solid lines show transects A-B and C-D.
In Bay of La Paz, 24 taxonomic groups have been reported, and copepods are the second most abundant group (Mojica-Ramírez 2008). The genus Centropages belongs to the order Calanoida, family Centropagidae, and inhabits most oceans (Mauchline 1998). Their diet depends on the stage of their life cycle, which consists of egg, 6 nauplius stages, and 6 copepodite stages; the sixth copepodite stage corresponds to the adult stage where females and males can be differentiated (Mauchline 1998). Regarding its diet, during the first copepodite stages, it feeds mainly on phytoplankton and other protists, whereas in the following stages, its feeding regime is more omnivorous and even carnivorous (Conover and Corner 1968, Gaudy and Thibault-Botha 2007). Specifically, Centropages furcatus has the ability to change its diet depending on the quality and quantity of food available; when the concentration of phytoplankton is low, it modifies its habit to carnivorous, but it is considered to be mainly omnivorous (Hernández-Trujillo et al. 2008, López-Ibarra 2008). During the reproductive stage, given the nutritional requirement, C. furcatus shows herbivorous preferences (Ianora 1998, Calbet et al. 2007). The different stages take advantage of physical phenomena and traits to avoid being predated and to have better access to food, to guarantee developmental success until the adult stage (Halsband-Lenk et al. 2002).
Copepod abundance has been linked to physical structures, such as cold or cyclonic gyres in the Sargasso Sea (Eden et al. 2009) and the Arctic Ocean (Llinás et al. 2009). The influence of these physical features on the distribution of zooplankton has been reported for different regions, for example, the influence of oceanic fronts on the Gulf Stream (Anderson and Robinson 2001) and the influence of cyclonic geostrophic circulation (Sánchez-Velasco et al. 2006) and other cyclonic structures in Bay of La Paz, where a differential distribution between herbivores, carnivores, and omnivores has been reported (Durán-Campos et al. 2015). In a cyclonic gyre in the Gulf of California, the vertical distribution of copepods has been related to temperature and chlorophyll a concentrations, and 3 important habitats have been demonstrated as a function of depth: one in the surface mixed layer, dominated by Subeucalanus subtenuis, whose abundances decreased towards the center of the gyre; another in the thermocline, dominated by Nannocalanus minor and Temora discaudata; and the third below the thermocline, where most of the species recorded in the thermocline dominated, but with lower abundance (Cruz-Hernández et al. 2018). In the northeastern Pacific, when the average water temperature increases, subtropical copepods are more abundant and distributed at higher latitudes, whereas when the average water temperature decreases, an overlap of polar and subtropical species occurs and the diversity of copepods increases (Batten and Walne 2011). In other regions, such as the Mediterranean Sea, the presence of copepods that produce resting eggs in shallow areas is regulated by temperature variations and food availability (Halsband-Lenk et al. 2004).
In Bay of La Paz, the most studied calanoid copepods have been Calanus sp. (Aceves-Medina et al. 2007) and Acartia sp. (Lavaniegos et al. 2012), due to their high abundance in the coastal area during the spring-summer season. Most studies reported the presence of C. furcatus, an oceanic and coastal species that is very abundant in summer and winter and dominant in the planktonic community, because it is well adapted to produce eggs even in unfavorable food conditions (Palomares-García et al. 2003, Hernández-Trujillo et al. 2008, Hernández-Trujillo and Esqueda-Escárcega 2016). To the south of the bay, the abundance of eggs and adults of C. furcatus is related to the concentration of chlorophyll a, which is associated with the food source (Aceves-Medina et al. 2007, Hernández-Trujillo et al. 2008). However, studies on the distribution of the different stages of copepodites in the Bay of La Paz are scarce, so the objective of this study was to estimate the influence of a cyclonic gyre and a thermohaline front on the distribution of the population density of the different life stages of C. furcatus in said bay.
MATERIALS AND METHODS
The hydrographic data and zooplankton samples were obtained during the PALEO-XII research campaign, carried out from 14 to 18 June 2004 in Bay of La Paz, Mexico (Fig. 1b), aboard the R/V El Puma of the National Autonomous University of Mexico. Using a Neil Brown CTD to record conductivity, temperature, and depth, 44 hydrographic stations were sampled (Fig. 1b). Likewise, in order to determine the concentration of chlorophyll a, fluorescence was measured at 26 stations using a PNF-300 natural fluorescence profiler. Salinity and density were calculated according to Fofonoff and Millard (1983). With the hydrographic data, the geostrophic velocity relative to the bottom was calculated according to Pond and Pickard (1995) to determine the circulation pattern. This geostrophic circulation, although it is an approximation of the velocity (since it does not consider the forcing due to the rotational wind stress), generates a qualitative pattern of the circulation (Sánchez-Velasco et al. 2006). According to the vertical distribution of density, the horizontal distribution of temperature, salinity, and the geostrophic circulation pattern at the depth of the pycnocline were analyzed.
Using a General Oceanics rosette equipped with Niskin bottles, Mojica-Ramírez (2008) obtained water samples from different depths in Bay of La Paz to determine nutrients. In a subsequent study, Coria-Monter et al. (2017) showed the horizontal distribution of nutrients. After being filtered through a nitrocellulose membrane, samples were stored in polypropylene bottles and frozen until they were analyzed with a Skalar San-plus continuous segmented flow autoanalyzer following the method developed by Grasshoff et al. (1999) and using a circuit recommended by Kirkwood (1994). The error level was 0.10 µM for nitrate, 0.10 µM for soluble reactive silicate, and 0.04 µM for reactive phosphorus (Coria-Monter et al. 2017). Chlorophyll a was calculated from fluorescence data according to Kiefer et al. (1989), and vertically integrated chlorophyll a was obtained for the upper 20 m layer.
Zooplankton samples were collected at 15 sites by oblique trawls from 200 m depth to the surface, or from near the bottom at stations shallower than 200 m (Fig. 1b). The trawls were carried out for 15 minutes at a speed of 1 m·s-1 using a 60-cm diameter Bongo-type net with a mesh size of 333.0 and 505.0 µm. A General Oceanics flowmeter was placed at the mouth of each net, which made it possible to calculate the volume of water filtered in each trawl and, thus, obtain the density of organisms, which was normalized to the number of individuals per hundred cubic meters (ind·100 m-3). Although the copepodites in stage C-I measure 0.3 to 0.4 mm, the 333.0 µm net was used due to the large amount of organic matter in the bay, which partially saturated the net and enabled the capture of the copepodites in stage C-I. From the samples of both nets, the different stages of C. furcatus (Dana, 1849) were identified and counted (Palomares et al. 1998) according to the morphological development of the body (stages C-I to C-VI) (Dussart and Defaye 2001). The female:male ratio was obtained according to Smith (1999).
To obtain the association between the different copepodite stages of C. furcatus and the physicochemical parameters, a principal component analysis (PCA) was performed. For this, we considered the population density of copepodites in stages C-I to C-V and of females and males in stage C-VI, and the temperature, salinity, magnitude of the geostrophic current, nitrates, phosphates, silicates, and vertically integrated chlorophyll a, at the depth of the thermocline, where these environmental variables describe the hydrodynamics of Bay of La Paz.
RESULTS
In Bay of La Paz, the density of the water depends mainly on the temperature, so the thermocline and the pycnocline appear at the same depth with very similar shapes. Along the A-B transect, which runs from the southwest to the northeast of the bay, the pycnocline was found above 30 m depth (Fig. 2a), which evidenced the uplift of the isopycnes. The pycnocline was bell-shaped, with the dome close to station 30. At the ends of the vertical section of this transect, the pycnocline was notably found between 20 and 30 m depth, whereas at station 30, the central part of the dome, it was found between 10 and 15 m depth; this hydrographic structure is characteristic of a cyclonic gyre. Figure 2b shows the geostrophic velocity through transect C-D in Boca Grande, and it evidenced the entry and exit of water in the northwest portion and the southeast portion of the transect, respectively. The water entered with a geostrophic velocity of ~50 cm·s-1, which decreased markedly with depth, whereas in the southeastern portion of the transect, the water exited with lower velocity, which was very uniform with depth (Fig. 2b). The temperature at 20 m depth increased from the center of the bay (16.5 °C) towards the coastal area (19.5 °C) and towards the Boca Grande region, where it reached 22.0 °C. The cold core located at 24.55° N and 110.55° W is typical of a cyclonic gyre (McGillicuddy 2016). Another hydrographic feature observed during this study was the temperature gradient (0.95 °C·km-1) near Boca Grande (Fig. 3a). Salinity at 20 m depth showed a distribution similar to that of temperature, with a range of values from 34.90 to 35.18 and a low salinity core (34.90) close to the cold core, and the maximum salinity was observed in Boca Grande, where the largest gradient occurred (0.29 km-1) (Fig. 3b). With both gradients, a thermohaline front was identified in the Boca Grande region (Fig. 3a, b). The geostrophic current pattern confirmed a clear cyclonic circulation with geostrophic velocities of ~50 cm·s-1 near Boca Grande (Fig. 3c).
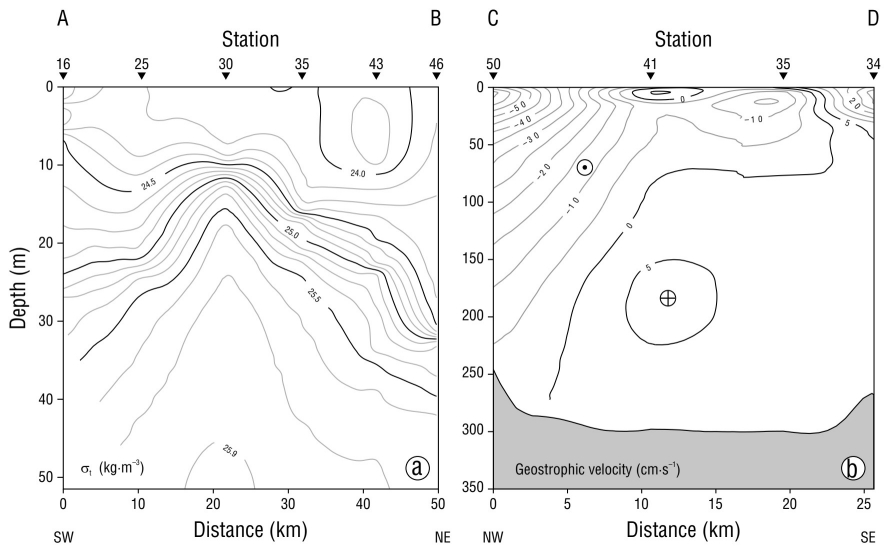
Figure 2 (a) Vertical section of density (σt) in the upper 50-m layer along transect A-B. (b) Geostrophic velocity across transect C-D.

Figure 3 Horizontal distribution at 20-m depth: (a) temperature (°C), (b) salinity, (c) geostrophic velocity (cm·s-1) relative to the bottom, (d) nitrate (µM), (e) soluble reactive phosphorus (SRP, µM), (f) soluble reactive silicate (SRSi, µM). The 3 bottom panels were taken from Coria-Monter et al. (2017).
Regarding nutrients at 20 m depth, the distribution of nitrates with a range of 4.0 to 16.0 µM showed the highest concentration in the Alfonso basin area (Fig. 3d). Phosphates were observed in a range of 0.4 to 1.8 µM (Fig. 3e), with 3 nuclei; the one with the highest concentration was located in the Alfonso basin and the other 2, with lower concentrations, in Boca Grande and in the southern region of Bay of La Paz. Silicates ranged from 4.0 to 18.0 µM, with the highest concentrations in the southern part of the bay and a core of 14.0 µM at Boca Grande (Fig. 3f).
Vertically integrated chlorophyll a distribution up to 20 m depth showed 2 nuclei of high concentration. One of the nuclei was located in the central part of the bay at a concentration of 24 mg·m-2, which coincided with the center of the cyclonic gyre. The other nucleus was located in the Boca Grande area at a concentration of 21 mg·m-2 and was associated with the sill and the thermohaline front (Fig. 4a).

Figure 4 (a) Horizontal distribution of integrated chlorophyll a (mg·m-2) at 20-m depth. Horizontal distribution of temperature (°C, isolines) at 20-m depth and density distribution for the different copepodite stages of Centropages furcatus (ind·100 m-3): (b) C-I, (c) C-II, (d) C-III, (e) C-IV, (f) C-V, (g) female, (h) male. Diameter of the circles is proportional to the population density value.
Regarding the distribution of the population density of the different copepodite stages of C. furcatus, 2 areas with different population characteristics were distinguished. The first was located inside the bay and coincided with the presence of the cyclonic gyre, and the second coincided with the thermohaline front.
In the Alfonso basin, stage C-I of copepodites showed the highest population density at the center of the cyclonic gyre, where low temperatures, low salinities, maximum concentrations of nitrates and phosphates, and high concentrations of chlorophyll a were recorded. From this region onwards, population density decreased towards the periphery of the gyre and increased again in the coastal zone (Fig. 4b). The C-II development stage showed a marked decrease in population density with respect to the C-I stage, although its distribution was similar, with a higher population density at the center of the gyre and a decrease towards the periphery (Fig. 4c). The highest density of copepodites in stage C-III was located in the station near the western coast of Bay of La Paz and in the southernmost station, with a tendency to decrease towards the center of the cyclonic gyre; in the southern region off Espiritu Santo Island, no organisms were recorded (Fig. 4d). Stage C-IV showed a higher population density at the center of the cyclonic gyre with respect to the southern area of the bay (Fig. 4e). As for the C-V stage, its density was high at the center of the gyre, decreased towards the periphery, and tended to increase off the coast and at southern stations in the bay (Fig. 4f). In the case of stage C-VI, stage in which the ontogenetic development of organisms has ended, differences in density were observed between females and males. Females and males followed the same distribution pattern as that of stages C-III to C-V (Fig. 4g), but their population density was lower (Fig. 4h), with a ratio of females to males of 4:1 for the Alfonso basin.
Regarding the region of the thermohaline front, the population density of copepodite stages C-I to C-VI was higher than in the area of the cyclonic gyre. The C-I stage distribution indicated higher densities at stations located to the north, close to the Gulf of California, and a density decrease towards the thermohaline front (Fig. 4b). The density of copepodites in stage C-II showed a marked decrease, and their distribution coincided with that of copepodites in stage C-I, with the highest density in the station located to the north (Fig. 4c). For stage C-III, 2 points with greater density were recorded, the first close to the front and the second in the northernmost station (Fig. 4d). The highest density of copepodites in stage C-IV occurred close to the front and decreased northward and southward (Fig. 4e). The C-V stage had its highest density at a single station near the front, and the density decreased towards the south and north (Fig. 4f). Females showed the highest density at the front, which decreased towards the north (Fig. 4g). Males (Fig. 4h) showed the same density distribution as the C-IV stage. The ratio of females to males was 2:1 and lower than that observed in the gyre area.
According to the results of the PCA and the factor loading matrix, nitrates, phosphates, and silicates showed the highest negative loadings on axis 1. Salinity, temperature, and magnitude of the geostrophic current showed the highest positive loading. On axis 2, integrated chlorophyll a showed the highest positive loading, followed by phosphates (Table 1a). The eigenvectors indicate the loading and geometric projection (Table 1b).
Table 1 Factor loadings (a) and eigenvectors (b) obtained from the principal component analysis of the copepodite stages in relation to physicochemical variables and integrated chlorophyll a.
| a | |||||||
| Factor loadings | |||||||
| PC1 | PC2 | PC3 | PC4 | PC5 | PC6 | PC7 | |
| Temperature (°C) | 0.88313 | -0.03905 | -0.04187 | 0.38699 | 0.23552 | -0.09467 | -0.05112 |
| Salinity | 0.92684 | 0.03311 | 0.08196 | 0.19691 | -0.17901 | 0.20642 | 0.14044 |
| Integrated chlorophyll a (mg·m-2) | -0.02758 | 0.96362 | -0.00478 | 0.10686 | -0.16733 | -0.17673 | -0.00156 |
| SRP (µM) | -0.76253 | 0.55406 | -0.03484 | 0.11110 | 0.13650 | 0.27246 | -0.07167 |
| SRSi (µM) | -0.70349 | -0.27299 | 0.60903 | 0.21513 | -0.09815 | -0.02718 | -0.05486 |
| Nitrate (µM) | -0.95486 | 0.01496 | -0.01550 | 0.10526 | 0.15256 | -0.07475 | 0.21871 |
| Geostrophic current speed (cm·s-1) | 0.71727 | 0.38354 | 0.48510 | -0.25756 | 0.18683 | 0.00652 | 0.04256 |
| b | |||||||
| Eigenvectors | |||||||
| PC1 | PC2 | PC3 | PC4 | PC5 | PC6 | PC7 | |
| Temperature (°C) | 0.43391 | -0.03232 | -0.05334 | 0.66762 | 0.52408 | -0.23419 | -0.18059 |
| Salinity | 0.45539 | 0.02740 | 0.10441 | 0.33970 | -0.39832 | 0.51064 | 0.49614 |
| Integrated chlorophyll a (mg·m-2) | -0.01355 | 0.79749 | -0.00609 | 0.18436 | -0.37234 | -0.43718 | -0.00553 |
| SRP (µM) | -0.37466 | 0.45854 | -0.04439 | 0.19167 | 0.30375 | 0.67402 | -0.25318 |
| SRSi (µM) | -0.34565 | -0.22593 | 0.77586 | 0.37114 | -0.21841 | -0.06725 | -0.19380 |
| Nitrate (µM) | -0.46916 | 0.01238 | -0.01975 | 0.18159 | 0.33947 | -0.18491 | 0.77262 |
| Geostrophic current speed (cm·s-1) | 0.35242 | 0.31742 | 0.61798 | -0.44434 | 0.41574 | 0.01613 | 0.15034 |
The first component (PC1) explained 59.2% of the variance, whereas the second component (PC2) explained 20.9% (Fig. 5), so the first 2 principal components explained 80.0% of the total variance. Stage C-I was associated with integrated chlorophyll a, which indirectly depends on nutrients (phosphates, nitrates, and silicates). The rest of the stages were associated with salinity, temperature, and current speed. According to the PCA, the most effective variables for PC1 were salinity, temperature, current speed, nitrates, phosphates, and silicates; on the other hand, integrated chlorophyll a defined PC2. Therefore, PC1 can be identified as the hydrodynamics and nutrients and PC2 as the integrated chlorophyll a (Fig. 5).

Figure 5 The first 2 components of a principal component analysis showing the relationship between the copepodite stages (C-1 to C-V, female, and male; blue arrows) and environmental variables (nitrate, vertically integrated chlorophyll a, geostrophic current speed, temperature, salinity [red arrows]). Soluble reactive phosphorus, SRP; soluble reactive silicate, SRSi.
DISCUSSION
In addition to corroborating the existence of a cyclonic gyre, this study shows the presence of a thermohaline front in the Boca Grande area that coincided with that reported by Durán-Campos et al. (2019), who associated it with the presence of a cyclone-anticyclone dipole.
The results of this study showed that the entry of water from the Gulf of California with maximum velocity values in the northern part of Boca Grande and the outflow of water near the Islands Espíritu Santo and Roca Partida produced the cyclonic circulation, which coincides with what was reported by Coria-Monter et al. (2017), Durán-Campos et al. (2019), Coria-Monter et al. (2020), and Rocha-Díaz et al. (2022). Monreal-Gómez et al. (2001) reported that the cyclonic gyre in the Boca Grande area is produced, in part, by the exchange of water masses with the Gulf of California. It is in this area where the exchange between both basins develops and occurs. In the sill area in Boca Grande, the thermohaline front is produced by the upwelling of water caused by the abrupt change in depth. In addition to the presence of the sill in Boca Grande, nutrient-rich water from the upper gulf penetrates into the bay due to the direction of the current (Lavín and Marinone 2003, Bustos-Serrano and Castro-Valdez 2006). This produces 2 maxima of chlorophyll concentration, the first at the center of the bay below 50 m depth and the second at the sill of Boca Grande due to the thermohaline front (Coria-Monter et al. 2017, Durán-Campos et al. 2019).
The distribution of the different development stages of C. furcatus showed 2 areas. The first of these was located in the cyclonic gyre, where the temperature of 15 °C is optimal for the development of the C-I stage (Carlotti et al. 2007); this temperature occurred at 20 m depth and, together with a high concentration of chlorophyll a, produced the ideal conditions for the reproduction of the species and the survival of the copepodites in stage C-I. The second area corresponds to Boca Grande, with the highest population density of copepodites in all stages, probably because the adults come from the Gulf of California and are introduced into the bay through Boca Grande. Likewise, the topographic feature (sill) can produce an upwelling of nutrients to the surface layers that, combined with the temperature in this area, favors the development of C. furcatus; this coincides with Trasviña-Castro et al. (2003), who reported a retention similar to that recorded in this study for the El Bajo Espíritu Santo Seamount, where they observed a high diversity of species of fish larvae and zooplankton due to the shock of the ebb current of the Bay of La Paz on one of the flanks of the mountain that disturbed the isotherms and isohalines and produced a thermohaline front in that area.
The distribution of the different stages of C. furcatus marks 2 areas with different population characteristics. In the first, which corresponds to the cyclonic gyre in the Alfonso basin, a marked concentric distribution of the different stages of copepodites was observed due to the influence of the gyre. The C-I stage was concentrated mainly at the center of the gyre, since food sources such as diatoms, dinoflagellates, and prasinophytes concentrate there; their abundance is a consequence of the pumping of nutrients and the conditions that are suitable for their development (Saavedra-Rojas 2003, McGillicuddy et al. 2007, Mojica-Ramírez 2008, Coria-Monter et al. 2014). The distribution associated with the presence of a cyclonic gyre for the genus Centropages has also been observed in the Ligurian Sea, where the highest abundance of Centropages typicus occurred both to the southwest and at the center of a gyre (Molinero and Nival 2004). Pinca and Dallot (1997) associated the distribution of organisms with a high concentration of phytoplankton and the high abundance of copepods with the divergence zone of the gyres that occur in the Mediterranean Sea. The areas within the bay that coincided with the center of the gyre showed the highest density values of copepodites in stage C-I, and in the proximity of the center, the highest population density of adults was observed because the concentrations of both dinoflagellates and diatoms required for quality feeding and reproduction are found in this area (Brand-Schmidt et al. 2009). Because the diet of the genus Centropages is dominated by phytoplankton and other protists, the central zone of the gyre shows favorable conditions for the herbivorous diet in the first stages, which later changes to omnivorous and cannibalistic (Gaudy and Thibault-Botha 2007).
The second area, which corresponds to the thermohaline front, according to the distribution and population density of the stages, is a region where the species can obtain the food and temperature required for its ontogenetic development and an ideal site for reproduction. This coincides with what was reported by Durán-Campos et al. (2019), who described the front in Bay of La Paz as the area where phytoplankton fertilization is promoted due to the transport of nutrients from subsurface layers to surface layers. The difference in proportion between females and males is due to the fact that females of the genus Centropages require a specific diet and find it mainly in the proximity of the gyre center, where the concentration of dinoflagellates is high. According to van Someren-Gréve et al. (2017), female behavior is to optimize their diet, so they are “passive” partners, whereas males seek to cover their food and reproductive needs simultaneously. These male requirements, together with the barrier created by the front, favor their greater density in this area, in contrast to the cyclonic gyre where the turbulence created by the Ekman pump causes males to catch food inefficiently and affects their survival.
In addition to optimal conditions for feeding, each stage requires specific environmental conditions. Temperature affects each stage differently; in general, Centropages requires an optimal temperature range of 10 to 20 °C to fully develop, so temperature is the main environmental variable, apart from salinity, that delimits the distribution of the developmental stages of C. furcatus (Smith and Lane 1985, Ianora 1998, Halsband-Lenk et al. 2002, Carlotti et al. 2007), which was corroborated with the PCA. It is important to mention that during this study there were very few copepodites in stage C-II, probably because conditions were inadequate for their survival. According to the life cycle of C. furcatus, during the summer season, the nauplius stage molts into C-I (Hernández-Trujillo et al. 2008); in this stage and in stages C-II and C-III feeding habits change from herbivore to omnivore (Carlotti et al. 2007) and, as a consequence, these show the lowest survival, whereas the stages C-IV and C-V prepare for reproduction and egg production that will occur when chlorophyll a concentrations are low (Hernández-Trujillo et al. 2008).
The distribution of the different stages of copepodites showed 2 groups, the first mainly in the inner bay, associated with the cyclonic structure, and the second in the Boca Grande area, at the northern limit of the bay, associated with the thermohaline front, which is the ideal site with appropriate characteristics for the development and survival of C. furcatus in Bay of La Paz.
The results of this study showed, at the end of spring 2004, in the Bay of La Paz, 2 areas of higher density of C. furcatus, one in the cyclonic gyre in the Alfonso basin and the other in the thermohaline front located in the Boca Grande area. The density of the different stages of C. furcatus both inside the bay, where a cyclonic gyre was observed, and in Boca Grande, where a thermohaline front occurred, is correlated with temperature. The different stages of copepodites showed a differential distribution in the cyclonic gyre in Bay of La Paz, with the highest concentration of C-I at the center, where low temperature and the maximum concentration of nitrates, phosphates, and chlorophyll a occurred; as their development progressed, they were distributed radially towards the periphery. The difference in population density was caused by the presence of different cohorts during this study. Therefore, we can conclude that the area of the front is a reproductive zone for the species that maintains a sustained population density for adults and results in a lower ratio of females to males (2:1). Inside the bay, the progressive decrease in population density is influenced by low temperature, the speed of the gyre, and natural mortality, which produced a greater ratio of females to males (4:1).











 texto em
texto em 



