INTRODUCTION
Lutjanids are demersal teleost fish found in tropical, subtropical, and temperate waters (Allen 1985). There are 10 lutjanid species on the Pacific coast of Mexico (Zárate-Becerra et al. 2014), of which the Pacific red snapper, Lutjanus peru Nichols and Murphy, 1922, is distributed from Mexico to Peru. As juveniles, they usually form small groups that inhabit estuaries, river mouths, and shallow waters. They later undertake a short migration to rocky substrates or reefs, where they grow into the adult stage and then live in aggregations or as solitary individuals (Allen 1985, Fischer et al. 1995, Gallardo-Cabello et al. 2010). Their bathymetric distribution ranges from shallow areas to 80-m depths (Saucedo-Lozano et al. 1999, Espino-Barr et al. 2006, Gallardo-Cabello et al. 2010, Zárate-Becerra et al. 2014).
In Mexico fishing pressure on juvenile and subadult Lutjanus spp. is high (Díaz-Uribe 1994, Santamaría and Chavez 1999, Díaz-Uribe et al. 2004, Gallardo-Cabello et al. 2010). Fishery catches fluctuate according to species abundance in the environment; however, L. peru is usually fished year-round and is socioeconomically important within the scaly fish category given the high quality and nutritional value of its meat (Díaz-Uribe et al. 2004). Between 1968 and 2010 the states of Baja California Sur, Nayarit, Jalisco, Colima, Michoacán, Guerrero, and Oaxaca accounted for 90% of the fisheries production for this species (Cruz-Romero et al. 2000, Zárate-Becerra et al. 2014).
From an ecological viewpoint, the Pacific red snapper and its congeners partake in the community bioenergetic dynamics as important carnivores that regulate the density of a wide spectrum of fishes and crustaceans in the demersal environment (Allen 1985, Parrish 1987, Zárate-Becerra et al. 2014). They therefore represent the link between the low and high levels of the food chain (Acero and Garzon 1985, Arreguín-Sánchez and Manickchand-Heileman 1998, Pérez-España 2003). Identifying prey with high taxonomic resolution helps define trophic relationships, trophic levels, feeding strategies, and ontogenetic trophic changes in diet composition (Musseau et al. 2015, Morales and García-Alzate 2016).
It has been observed that the feeding habits of L. peru in the mouth of the Gulf of California and southern areas of the Pacific coast of Mexico change according to ontogeny, reproduction, and seasonality (Díaz-Uribe 1994, Santamaría-Miranda et al. 2003, Rojas-Herrera et al. 2004, Moreno Sánchez et al. 2016). These changes have been linked to different energy requirements (Hobson 1968, Abitia-Cárdenas et al. 1997), hunting ability (Hobson 1968), and prey availability (Rojas-Herrera et al. 2004). It seems that this predator does not select specific prey and that its diet is based on prey availability and abundance (Díaz-Uribe 1994, Saucedo-Lozano 1999, Rojas-Herrera et al. 2004). This fact is highly important considering the type of prey constituting its trophic spectrum in the Gulf of California and the Pacific coast of Mexico.
Using the index of relative importance (IRI, %), Moreno-Sánchez et al. (2016) reported that the diet of L. peru comprised almost exclusively benthic crustaceans on the continental coast of Sinaloa and pelagic crustaceans in La Paz Bay, Baja California Sur, on the coast of the Baja California Peninsula; both regions are located within the Gulf of California. On the other hand, Díaz-Uribe (1994) also used the IRI and described a diet comprising mainly urochordate colonies and benthic crustaceans around nearby islands in La Paz Bay. In Guerrero, outside the Gulf of California, in the Pacific off southern Mexico, Santamaría-Miranda et al. (2003) and Rojas-Herrera et al. (2004) found high percentages of pelagic coastal fish and benthic crustaceans.
Given the reported variations in the main prey items consumed by L. peru, the objective of the present study was to determine the feeding habits of the Pacific red snapper in the central portion of the Gulf of California, Santa Rosalía, Baja California Sur, and compare our results with what has been previously reported for the species. We also corroborated changes in the diet linked to sex, ontogeny, and seasonality.
MATERIALS AND METHODS
Owing to bathymetry, meridional latitude, and the upwelling system, the Gulf of California exhibits high biodiversity and primary productivity (Wilkinson et al. 2009). The bathymetry of the area consists of a wide platform and coastal lagoons to the east, contrasting the narrow platform and islands on the western side of the Baja California Peninsula (Cartron et al. 2005). Seasonal upwelling events predominate on the continental coast in the winter (December-May) and off the peninsula in the summer (July-October); June and November are transitional months (Roden 1964, Badan-Dagon et al. 1985). These events favor the entrance of nutrient-rich subsurface waters, leading to phytoplankton proliferation and thus large amounts of available food to support the trophic web (Roden 1964, Álvarez-Borrego 2008). This process regulates the abundance, composition, and distribution of costal mesopelagic and pelagic fish, among others (Avendaño-Ibarra et al. 2013).
Between 20 and 40 Pacific red snapper specimens were obtained monthly from August 2016 through October 2017 (Table S1) near the Port of Santa Rosalía (27°19ʹ45.14ʺ N, 112°15ʹ13.40ʺ W) (Fig. 1). Specimens were caught by the artisanal fishing fleet 5 to 13 nautical miles from shore at 10- to 30-m depths, using outboard motor boats and a net of 15 cm mesh size set for 10-12 h at night. Specimens were frozen and transported to the fish ecology laboratory at the Centro Interdisciplinario de Ciencias Marinas, where they were measured (total length) with a fish measuring board (±0.5 cm precision) and weighed (total weight) with a digital scale (±0.01 g precision).
Figure 1 Geographic location of the study area (Santa Rosalía, Baja California Sur, Mexico). The shaded area shows where Pacific red snappers, Lutjanus peru, were captured.
Pacific red snapper specimens were categorized according to sex, sexual maturity, and season. Sex was assigned using Nikolsky’s morphochromatic scale (Nikolsky 1963). Trophic categorization based on sexual maturity was explored with a cluster analysis to detect variations in the biomass of prey consumed by each size class. The data were not normal due to an excess of zeroes; the median was used as a measure of distance, and a correlation was used for grouping variables. This analysis was undertaken using the pvclust library v.2.2-0 (Suzuki et al. 2019) in the R environment (R Core Team 2020). Because no clear trophic pattern could be detected between size classes and prey biomass (Fig. S1), we decided to discriminate between mature and immature fishes, as per Díaz-Uribe et al. (2004): organisms ≥33 cm total length were classified as mature and smaller organisms were classified as immature.
To define the seasons sea surface temperature (SST) was obtained from MODIS-AQUA satellite images with 1.1 km resolution, taking into account the quadrant with influence from the artisanal fishing fleet operating in the study area from May 2016 to December 2017, which was delimited by 112.0° W to 112.4° W and 27.2° N to 27.4° N. SST data were obtained from the ERDDAP server of the US National Oceanic and Atmospheric Administration (https://coastwatch.pfeg.noaa.gov/erddap/index.html).
Monthly temperature data were taken and average SST (25.8 °C) was calculated; months above and below the average value were considered warm and cold, respectively. Two seasons were evident: a cold season (December 2016 and January, February, March, April, May, and December 2017) and a warm season (July, August, September, and October 2016 and July, August, September, and October 2017).
Prey identification was undertaken using a Zeiss Stemi 2000-C stereoscopic microscope, and specialized literature was used to determine each taxonomic group. For fish, the guides by Miller and Jorgensen (1973), Fischer et al. (1995), Díaz-Murillo (2006), and Lowry (2011) were used. For crustaceans and mollusks, the works by Brusca (1980), Morris et al. (1980), and Salgado-Barragan and Hendrickx (2010) were used.
Once prey were identified, minimum sample size was estimated to ensure that the number of analyzed stomachs was sufficient to characterize general diet, diet by sex, sexual maturity, and season. The program EstimateS (Colwell 2009) was used to graph the cumulative prey diversity curves, which are an estimate of prey variability by stomach using the Shannon-Wiener diversity index (Hʹ), considering 100 permutations without replacement. The curves gradually reached an asymptote at a specific cumulative number of stomachs, given that prey variability in stomachs was low; this coincided with the point where the coefficient of variation (CV) was 0.05 (Jiménez-Valverde and Hortal 2003, Moreno-Sánchez et al. 2016).
The quantitative importance of each prey species, considering all sampled stomachs,
was evaluated using the numerical (%N), gravimetric (%W), and frequency of
occurrence (%FO) indices (Hyslop 1980). The
same indices were calculated for each category under study (sex, sexual maturity,
and season). To avoid bias in the estimates, the indices were integrated into the
IRI, IRI = (%N + %W) × %FO, proposed by Pinkas et
al. (1971) and expressed as a percentage (Cortés 1997) to facilitate comparisons with previous L.
peru diet studies undertaken in the Gulf of California and the Pacific
coast of Mexico:
To test for differences based on the number of prey consumed by Pacific red snappers according to sex (Nikolsky’s 1963), sexual maturity (Díaz-Uribe et al. 2004), and season (SST satellite images), and to test for possible trophic differences due to interactions between these categories, a permutational multivariate analysis of variance (PERMANOVA) with 100 permutations was undertaken, based on the Bray-Curtis dissimilarity metric, applying the adonis function from the Vegan 2.2-1 library (Oksanen et al. 2016) in the R 3.0.1 environment. PERMANOVA is a useful technique for evaluating differences in data obtained from different experimental designs, including datasets having excess zeros (Anderson 2017), as is expected in stomach content analyses because different prey types are not always present in all stomachs. It also allows the use of untransformed data, and it is also quite robust when data are nonparametric, like our data (Anderson 2017). The R statistic (-1 > R < 1) resulting from this analysis describes the similarity between categories. Values close to 0 indicate no differences and values close to 1 or to -1 indicate differences between the analyzed categories. The probability values created by the R statistic were considered significant when P ≤ 0.05.
Levin’s index (Krebs 1999) was used to evaluate the trophic width of the Pacific red snapper
where B i is the trophic width of predator i, p ij is the proportion of prey j in the diet of predator i, and n is the total number of prey. Because of standardization, this index takes on values from 0 to 1 (Hurlbert 1978). Values below 0.60 are associated with specialist predators (i.e., preference for a few prey), whereas values equal to or above 0.60 indicate that the predator is a generalist and consumes a great number of prey species (Krebs 1999).
The feeding strategy was evaluated using the Costello graphs (1990) modified by Amundsen et al. (1996). These graphs detail the importance and contribution of each prey item to the trophic width on a bidimensional plane. The horizontal axis represents the frequency of occurrence of prey and the vertical axis represents the abundance by number. We deduced whether the predator consumed prey in a homogeneous manner (generalist), whether it showed preference for one or several prey (specialist), or if consumption was mixed, that is, it was characterized by individuals that consumed prey in a generalist manner and individuals that showed trophic specialization (Amundsen et al. 1996).
The equation by Christensen and Pauly (1992) was used to calculate the trophic level (TL) for the Pacific red snapper, considering the different prey found in stomach contents:
where DC ij is the proportion of prey j in the diet of predator i, TL j is the TL of prey j, and n is the number of groups in the system. The TL for each prey was obtained from Morales-Zárate et al. (2004) and Froese and Pauly (2019).
RESULTS
A total of 478 Pacific red snappers were captured, 75 (15.7%) of which had empty stomachs (specimens with empty stomachs were not taken into account for any of the analyses). The remaining 403 specimens (84.3%) contained at least 1 prey item; they measured between 21 and 60 cm total length and weighed between 195 and 1,920 g.
A total of 93% of prey comprising the trophic spectrum were identified to the level of genus and species. The diet comprised 29 prey items, including 13 fish, 11 crustaceans, 4 mollusks, and 1 tunicate. The cumulative prey diversity curve, in which all identified taxa were included, showed that the asymptote was reached at 63 stomachs; this showed that the number of analyzed samples was statistically sufficient to characterize the diet of the Pacific red snapper (CV ≤ 0.05). The minimum sample size to characterize the trophic spectrum by sex (male = 56, female = 54), sexual maturity (immature = 53, mature = 55), and season (cold = 60, warm = 43) was also met (Fig. 2).
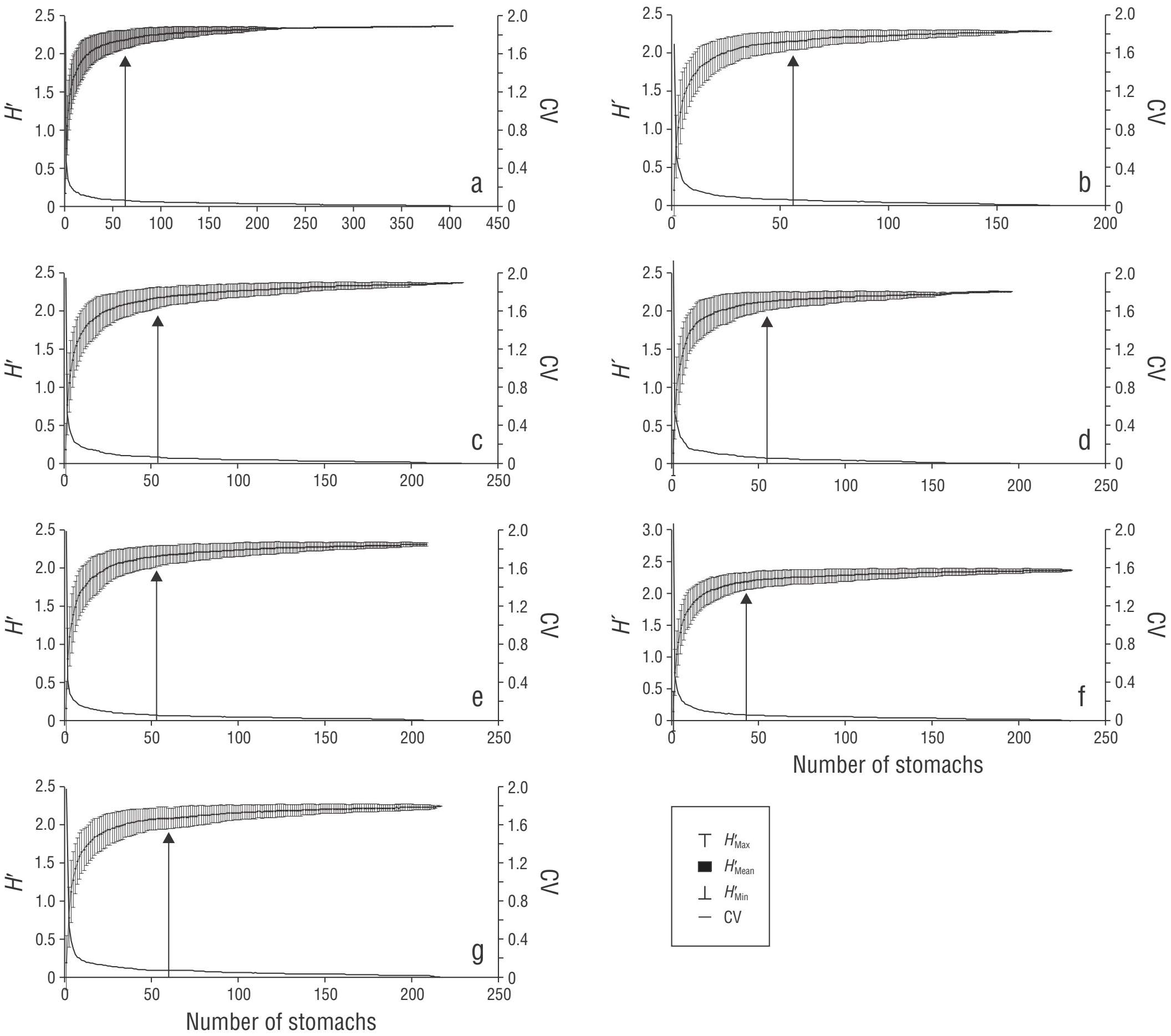
Figure 2 Number of stomachs used to characterize the feeding habits of Lutjanus peru in general (a) and by males (b), females (c), mature individuals (d), immature individuals (e), cold season (f), and warm season (g). H′, Shannon-Wiener diversity; CV, coefficient of variation. The arrow indicates the minimum number of stomachs where the curve reached the asymptote, coinciding with CV = 0.05.
Overall, the most important prey by weight were the herrings Sardinops sagax (Jenyns, 1842) (%W = 53.32) and Harengula thrissina (Valenciennes, 1847) (%W = 18.59), whereas the most important prey by number were the decapod Nyctiphanes simplex (Hansen, 1911) (%N = 86.39) and S. sagax (%N = 3.68) (Table 1). The most important prey by frequency of occurrence were the herrings S. sagax (%FO = 41.68) and H. thrissina (%FO = 26.79), and the crustacean N. simplex (%FO = 21.09) (Table 1).
Table 1 Lutjanus peru prey in Santa Rosalía, Mexico. The values contributed by each prey by number (N), weight (W), and frequency of occurrence (FO) are expressed as absolute values and as percentages. The results of these methods were integrated into the absolute index of relative importance (IRI) and percent index of relative importance (%IRI). The trophic level of the prey (TL) is shown at the end.
| Species | N | W | FO | %N | %W | %FO | IRI | %IRI | TL |
| Mollusca | |||||||||
| Cephalopoda | |||||||||
| Loliginidae | |||||||||
| Loligo spp. | 12 | 39.0 | 9 | 0.23 | 2.08 | 2.23 | 5.16 | 0.10 | 2.9 |
| Octopodidae | |||||||||
| Octopus spp. | 1 | 1.0 | 1 | 0.02 | 0.05 | 0.25 | 0.02 | 0.00 | 3.8 |
| Bivalvia | |||||||||
| Veneridae | |||||||||
| Chione spp. | 1 | 0.5 | 1 | 0.02 | 0.03 | 0.25 | 0.01 | 0.00 | 2.4 |
| Gastropoda | |||||||||
| Columbellidae | |||||||||
| Parvanachis spp. | 1 | 0.5 | 1 | 0.02 | 0.03 | 0.25 | 0.01 | 0.00 | 2.0 |
| Crustacea | |||||||||
| Malacostraca | |||||||||
| Euphausiidae | |||||||||
| Nyctiphanes simplex | 4,463 | 87.0 | 85 | 86.39 | 4.64 | 21.09 | 1,919.92 | 38.50 | 2.2 |
| Munididae | |||||||||
| Munida tenella | 37 | 67.5 | 24 | 0.72 | 3.60 | 5.96 | 25.68 | 0.52 | 2.5 |
| Portunidae | |||||||||
| Portunus xantusii | 45 | 38.0 | 29 | 0.87 | 2.02 | 7.20 | 20.84 | 0.42 | 2.6 |
| Penaeidae | |||||||||
| Penaeus spp. (1) | 117 | 14.5 | 10 | 2.26 | 0.77 | 2.48 | 7.54 | 0.15 | 2.6 |
| Penaeus spp. (2) | 2 | 5.5 | 2 | 0.04 | 0.29 | 0.50 | 0.16 | 0.00 | 2.4 |
| Squillidae | |||||||||
| Squilla bigelowi | 2 | 6.0 | 2 | 0.04 | 0.32 | 0.50 | 0.18 | 0.00 | 3.3 |
| Squilla tiburonensis | 1 | 2.0 | 1 | 0.02 | 0.11 | 0.25 | 0.03 | 0.00 | 3.3 |
| Sicyoniidae | |||||||||
| Sicyonia disedwardsi | 2 | 5.0 | 2 | 0.04 | 0.27 | 0.50 | 0.15 | 0.00 | 2.5 |
| Munididae | |||||||||
| Pleuroncodes planipes | 1 | 0.5 | 1 | 0.02 | 0.03 | 0.25 | 0.01 | 0.00 | 2.6 |
| Cymothoidae | |||||||||
| Cymothoa exigua | 1 | 0.5 | 1 | 0.02 | 0.03 | 0.25 | 0.01 | 0.00 | |
| Amphipoda | 13 | 14.0 | 3 | 0.25 | 0.75 | 0.74 | 0.74 | 0.01 | 2.4 |
| Urochordata | |||||||||
| Thaliacea | |||||||||
| Salpidae | |||||||||
| Salpa spp. | 10 | 1.5 | 2 | 0.19 | 0.08 | 0.50 | 0.14 | 0.00 | 2.0 |
| Vertebrata | |||||||||
| Actinopterygii | |||||||||
| Clupeidae | |||||||||
| Sardinops sagax | 190 | 1,001.0 | 168 | 3.68 | 53.33 | 41.69 | 2,376.50 | 47.66 | 2.4 |
| Harengula thrissina | 117 | 349.0 | 108 | 2.26 | 18.59 | 26.80 | 558.98 | 11.21 | 2.9 |
| Opisthonema libertate | 5 | 6.0 | 1 | 0.10 | 0.32 | 0.25 | 0.10 | 0.00 | 2.9 |
| Myctophidae | 63 | 73.5 | 27 | 1.22 | 3.92 | 6.70 | 34.41 | 0.69 | 3.0 |
| Benthosema panamense | 46 | 82.0 | 20 | 0.89 | 4.37 | 4.96 | 26.10 | 0.52 | 3.0 |
| Triphoturus spp. | 24 | 31.0 | 13 | 0.46 | 1.65 | 3.23 | 6.83 | 0.14 | 3.3 |
| Diaphus spp. | 1 | 0.5 | 1 | 0.02 | 0.03 | 0.25 | 0.01 | 0.00 | 3.0 |
| Engraulidae | |||||||||
| Engraulis mordax | 3 | 15.0 | 3 | 0.06 | 0.80 | 0.74 | 0.64 | 0.01 | 2.7 |
| Mugilidae | |||||||||
| Mugil spp. | 2 | 19.5 | 2 | 0.04 | 1.04 | 0.50 | 0.53 | 0.01 | 2.0 |
| Cirrhitidae | |||||||||
| Cirrhitichthys spp. | 2 | 2.5 | 2 | 0.04 | 0.13 | 0.50 | 0.09 | 0.00 | 4.0 |
| Triglidae | |||||||||
| Prionotus spp. | 2 | 1.0 | 2 | 0.04 | 0.05 | 0.50 | 0.05 | 0.00 | 3.5 |
| Scombridae | |||||||||
| Scomber japonicus | 1 | 0.5 | 1 | 0.02 | 0.03 | 0.25 | 0.01 | 0.00 | 3.0 |
| Pomacentridae | |||||||||
| Chromis atrilobata | 1 | 0.5 | 1 | 0.02 | 0.03 | 0.25 | 0.01 | 0.00 | 3.4 |
| Crustacean remains | 0 | 10.5 | 11 | 0.00 | 0.56 | 2.73 | 1.53 | 0.03 | 2.5 |
| Fish remains | 0 | 1.5 | 3 | 0.00 | 0.08 | 0.74 | 0.06 | 0.00 | 3.0 |
| Total | 5,166 | 1,877.0 | 403 | 100.00 | 100.00 | 4,986.44 | 100.00 | 3.4 |
Regarding the %IRI, the prey that contributed over 95% to the diet were S. sagax (47.65%), N. simplex (38.50%), H. thrissina (11.21%), fish from the Myctophidae family (0.68%), and Benthosema panamense (Tåning, 1932) (0.52%). The remaining items (24) contributed <5.00% to the diet (Fig. 3).
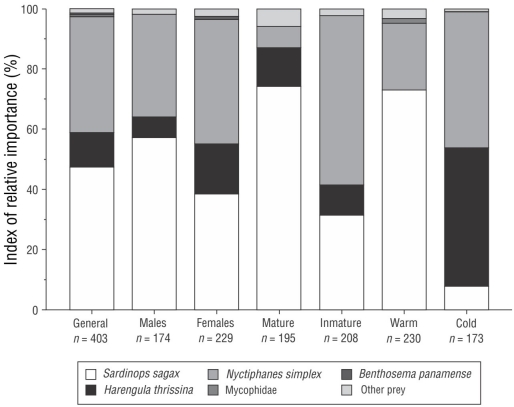
Figure 3 Index of relative importance of the main prey consumed by Lutjanus peru in general and as a function of sex (male or female), sexual maturity (mature or immature), and season (warm or cold) in Santa Rosalía, Mexico.
A total of 174 stomachs from males and 229 stomachs from females were analyzed. The trophic spectrum comprised 22 items, which had different proportional importance in the stomachs of the 2 sexes. In males the most important prey, according to the %IRI, were S. sagax (57.27%), N. simplex (34.26%), and H. thrissina (6.61%), and the secondarily important prey included Myctophidae (0.61%) and Munida tenella (Benedict, 1902) (0.35%). In females the most important prey were N. simplex (41.63%), S. sagax (38.59%), and H. thrissina (16.24%), followed by B. panamense (0.92%) and Myctophidae (Gill, 1893) (2.62%) (Fig. 3). According to the PERMANOVA, there were significant differences in the diet between males and females (PERMANOVA F = 2.01, P < 0.049) (Table 2).
Table 2 Results of the permutational multivariate analysis of variance (PERMANOVA) obtained from permutations carried out according to categories and their interactions, to test for significant differences in the diet of the Pacific red snapper, Lutjanus peru, in Santa Rosalía (Baja California Sur, Mexico). F = PERMANOVA test, r = similarity between groups, P = probability values.
| Categories | F | r | P | Significance at 95% confidence |
| Sex | 2.01 | 0.004 | 0.049 | yes |
| Season | 45.52 | 0.100 | 0.001 | yes |
| Maturity | 4.99 | 0.011 | 0.001 | yes |
| Sex:season | 1.03 | 0.002 | 0.399 | no |
| Sex:maturity | 0.79 | 0.001 | 0.596 | no |
| Season:maturity | 1.65 | 0.003 | 0.099 | no |
| sex:season:maturity | 0.59 | 0.001 | 0.800 | no |
Of all analyzed stomachs, 195 belonged to mature specimens. According to the %IRI, the trophic spectrum comprised 17 prey items; the most important prey species were S. sagax (74.16%), H. thrissina (12.85%), N. simplex (7.14%), Myctophidae (2.71%), and B. panamense (1.34%). A total of 208 immature organisms were analyzed and 22 prey items were identified. The most important prey according to the %IRI were N. simplex (56.36%), S. sagax (31.44%), H. thrissina (9.97%), Portunus xantusii (Stimpson, 1860) (0.53%), and Triphoturus spp. (Paxton 1972) (0.37%) (Fig. 3). The PERMANOVA showed that there were significant differences in the trophic spectrum between mature and immature organisms (F = 4.99, P < 0.001) (Table 2).
A total of 230 stomachs were obtained during the warm season and 22 prey items were identified. The most important items according to the %IRI were S. sagax (72.91%), N. simplex (22.29%), Myctophidae (1.50%), B. panamense (1.17%), and M. tenella (0.84%). A total of 173 stomachs were obtained during the cold season and 16 items were identified. According to the %IRI, the most important prey items were H. thrissina (45.95%), N. simplex (45.24%), S. sagax (7.74%), Penaeus spp. (1) (Burukovsky, 1997) (0.40%), and Triphoturus spp. (0.26%) (Fig. 3). The Pacific red snapper fed on significantly different prey in both seasons (F = 45.52, P < 0.001). There were no significant differences in the diet resulting from the interaction sex-season (F = 1.03, P > 0.399), sex-maturity (F = 0.79, P > 0.596), season-maturity (F = 1.65, P > 0.099), or sex-season-maturity (F = 0.59, P > 0.800) (Table 2).
According to Levin’s index, the Pacific red snapper had a narrow trophic niche width (B i = 0.16). This behavior was seen in males (B i = 0.14), females (B i = 0.17), mature organisms (B i = 0.12), and immature organisms (B i = 0.17), and in the cold season (B i = 0.08) and the warm season (B i = 0.11). These results were consistent with the feeding strategy represented on a bidimensional plane, which showed that there was alternate consumption of reduced number of prey in general (Fig. 4a) and by category (Fig. 4b-g), mainly pelagic-coastal prey such as S. sagax, H. thrissina, and N. simplex, and mesopelagic prey such as Myctophidae and B. panamense.
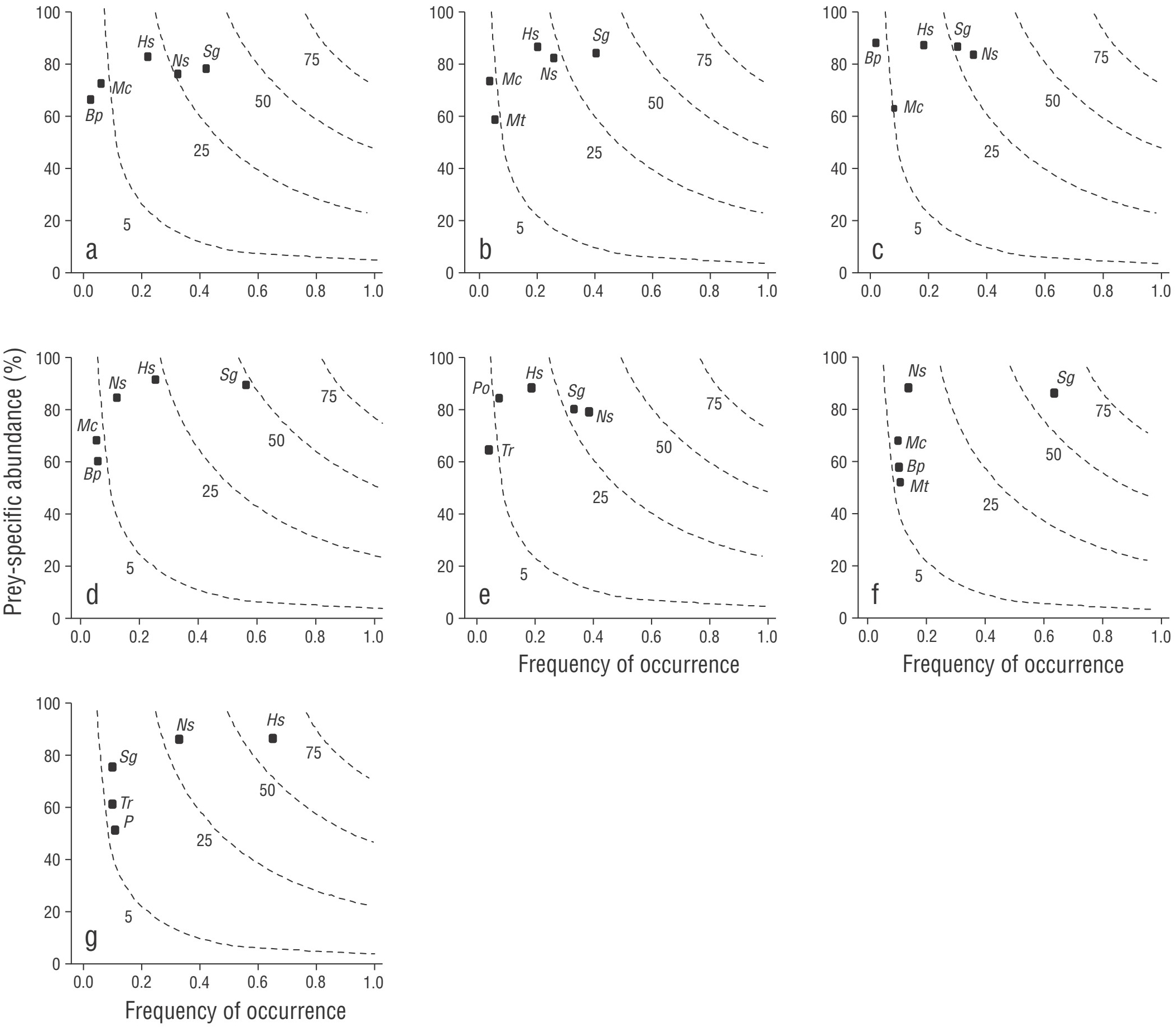
Figure 4 Costello graphs, which are 2-dimensional representations of food resources, in general (a) and by males (b), females (c), mature individuals (d), immature individuals (e), cold season (f), and warm season (g). Abbreviations denote the first 5 and most important food items: (Sg) Sardinops sagax, (Ns) Nyctiphanes simplex, (Hs) Harengula thrissina, (Mc) Myctophidae, (Bp) Benthosema panamense, (Mt) Munida tenella, (Po) Portunus xantusii, (P) Penaeus spp. (1), and (Tr) Triphoturus spp.
The graphs also showed the important contribution of other prey to the diet, such as the myctophid fishes Triphoturus spp. and the crustaceans M. tenella, Penaeus spp. (1), and P. xantusii. There was little variation in the TL estimated for the Pacific red snapper (3.4); this value was consistent by sex (males = 3.5, females = 3.4), sexual maturity (immature = 3.4, mature = 3.5), and season (cold = 3.4, warm = 3.5).
DISCUSSION
The level of detail obtained here on Pacific red snapper (L. peru) prey in Santa Rosalía constitutes a necessary baseline for detecting changes in trophic composition. It also provides supporting information regarding dietary changes linked to ontogeny, sex, or seasonality (Musseau et al. 2015, Morales and García-Alzate 2016).
The main prey items identified in the trophic spectrum (90% IRI) were pelagic coastal fish such as the pilchard S. sagax, the herring H. thrissina, the euphausiid N. simplex, and mesopelagic fish of the family Myctophidae, particularly B. panamense. In Santa Rosalía, some of these prey have comprised an important part of the stomach contents of the leopard grouper Mycteroperca rosacea (Moreno-Sánchez et al. 2019a). The distribution and abundance of these prey is affected throughout the year by seawater temperature and mesoscale oceanographic processes in the Gulf of California (Gómez-Gutiérrez et al. 2010, Avendaño-Ibarra et al. 2013, Inda-Díaz et al. 2014, Contreras-Catala et al. 2015). These parameters can thus affect the trophic spectrum composition of the Pacific red snapper and other demersal predators in Santa Rosalía (Moreno-Sánchez et al. 2019a).
The stomach contents of the Pacific red snapper could provide information on the abundance and/or presence of prey in a given habitat (Santamaría-Miranda et al. 2003). Previous studies have shown that L. peru selects the most abundant prey (Díaz-Uribe 1994, Saucedo-Lozano 1999, Rojas-Herrera et al. 2004). This type of behavior means that opportunistic fish (Gerking 1994) are different from predators that select prey based on energy optimization, regardless of abundance (Spitz et al. 2010). In the case of the Pacific red snapper, consumption of abundant prey that are mostly gregarious, such as S. sagax, H. thrissina, B. panamense, and N. simplex (Ladrón-de-Guevara et al. 2015, Moreno-Sánchez et al. 2019a), would provide important amounts of trophic resources with minimum energy expense for capture and consumption (Stephens and Krebs 1986, Moreno-Sánchez et al. 2016).
According to several reports, fish and crustaceans are the main food source for the red snapper in the Gulf of California and other areas on the Pacific coast of Mexico. The prey species that dominate the red snapper diet vary according to geographic location. The western Pacific coast of Mexico is characterized by a wide platform and rocky reefs (Santamaría and Chavez 1999). Santamaría-Miranda et al. (2003) and Rojas Herrera et al. (2004) showed that off the coast of Guerrero, this predator fed on prey that were particularly abundant, such as the pelagic anchovy Anchoa ischana and Anchoa lucida, and amphipods of the genus Lembas spp. These coastal pelagic prey are different from those found near the peninsula in the present study, possibly due to differences in microhabitats (Moreno et al. 1979, Saucedo-Lozano et al. 1999, Acevedo-Cervantes et al. 2009) and to the biogeographic affinity of the species (Avendaño-Ibarra et al. 2013).
Within the Gulf of California, especially around the islands near Baja California Sur and in the coastal area adjacent to La Paz Bay, Díaz-Uribe (1994) reported that the Pacific red snapper fed on large amounts of urochordates (Doliolum spp. and Salpa spp.) and crustaceans (Squilla spp., Penaeus spp., and Callinectes spp.). In general, rocky substrate and coral reefs increase abundance and diversity around the islands of the Gulf of California (Alvarez-Filip et al. 2006, Barjau et al. 2012). Urochordates seem to be important prey for several predators at these sites (Díaz-Uribe 1994, Aguilar-Mora 2009).
Moreno-Sánchez et al. (2016) reported that there was a large proportion of pelagic crustaceans (Penaeus californiensis, Pleuroncodes planipes, Myodocopida gen spp.) and a smaller proportion of cephalopods (Loligo spp.) in the diet of L. peru in a coastal area of La Paz Bay. These authors also observed that the diet of this predator differed by habitat between La Paz (peninsula) and Sinaloa (continental coast), because in Sinaloa there is a wide platform, sandy substrate, mangroves, and coastal lagoons, which are crucial for the presence of benthic prey, whereas in the area near the peninsula the platform is narrow, the substrate is rocky, and there is seasonal upwelling, which favors pelagic prey. This coincides with the results of the present study, as the most important prey in the trophic spectrum were pelagic.
Significant variations were also detected when taking into account sex, ontogeny, and seasonality in Santa Rosalía. Considering only the main prey, females consumed different percentages (%IRI) of crustaceans (41.63%) and fish (56.57%) compared to males (crustaceans = 34.62%, fish = 64.49%). The caloric content per unit of wet weight is lower in crustaceans than in fish; however, the energetic cost due to capture and ingestion of crustaceans is lower. If crustaceans were consumed in large amounts, this would compensate for their low caloric content (Díaz-Uribe 1994). In general, the stomachs of Pacific red snapper females (6,089 kg, 95 g) were heavier and contained greater crustacean biomass than those of males (4,806 kg, 74 g). The higher energy demand of snapper females is apparently linked to reproduction (Schwartzkopf and Cowan 2017).
There was an important change in diet with sexual maturity (Table 2). These differences were not evident in the cluster analysis (Fig. S1), but were observed with the PERMANOVA. This is because the PERMANOVA was more efficient and robust in detecting differences between data matrices with many zeroes (Anderson 2017) and in detecting small differences in the proportion of prey biomass between Pacific red snapper size classes. Although we should have observed a pattern of greater prey biomass at larger snapper sizes, it was not the case in this study (Fig. S1), possibly because the degree of prey digestion (moderate to high) was similar in all size categories. This has also been reported for other carnivorous predators (Lagler et al. 1984, Moreno-Sánchez et al. 2019b).
Trophic differences between maturity stages have been previously reported for the Pacific red snapper. This predator has shown higher consumption of small and abundant crustaceans in its earlier life stages, and of fish as it increases in size (Saucedo-Lozano et al. 1999, Rojas-Herrera and Chiappa-Carrara 2002, Santamaría et al. 2003, Moreno-Sánchez et al. 2016). This difference in the diet has been observed for the 2 maturity stages, and for other predatory fish during these life stages. They direct their efforts to catching specific prey depending on their mandibular morphology, hunting abilities, energetic requirements, and/or habitat changes (Hobson 1968, Gerking 1994, Abitia-Cárdenas et al. 1997, Musseau et al. 2015).
There were also significant differences in feeding between seasons. In particular, summer upwelling (Cotero-Altamirano et al. 2015) and the closeness of Santa Rosalía to important productivity and prey dispersion centers, such as the large islands in the Gulf of California (Martínez-Zavala et al. 2010), could influence the abundance of gregarious species such as S. sagax and B. panamense (Avendaño-Ibarra et al. 2013, Inda-Díaz et al. 2014, Cotero-Altamirano et al. 2015). The presence of these prey in the stomach contents of Pacific red snappers during the warm season coincides with the greatest commercial catches (259,791 t for S. sagax) (Lanz et al. 2008, Martínez-Zavala et al. 2010, López-Serrano 2018) and maximum abundances (20,954 individuals per 1,000 m3 for B. panamense) of these species (Santana-Iturrios et al. 2013). In the present study, the weight in grams of Pacific red snapper prey was greater in the summer, coinciding with what was found by Díaz-Uribe (1994) for the same species in La Paz Bay.
During the cold season, primary productivity levels in the Gulf of California are high (Lavín and Marinone 2003, Álvarez-Borrego 2008), and there are vertical migratory movements of euphausiids to the surface of the water column, where they form dense aggregations (Gómez-Gutiérrez et al. 2010). In particular, N. simplex plays an important trophodynamic role. Its calculated abundance is >1,000 individuals per 1,000 m3 in the productive areas of the Gulf of California (Martínez-Gómez 2009), with maximum values in winter (Mendoza-Portillo 2013). This could explain the high presence of this type of prey in Pacific red snapper stomachs in the cold season. During the same season, H. thrissina was also an important trophic resource, as was Lutjanus argentiventris and M. rosacea in the southern portion of the peninsula (Vázquez-Sánchez 2005, Moreno-Sánchez et al. 2019a).
Regarding trophic width, 29 prey items were identified in the stomach contents of the Pacific red snapper. Parrish (1987) described the Pacific red snapper as a generalist predator having a wider trophic spectrum than other demersal fish families (e.g., serranids). However, although numerous prey could be identified in the trophic spectrum of this predator, only a reduced number of prey were consumed in large amounts in the Gulf of California and the Pacific coast of Mexico (Díaz-Uribe 1994, Santamaría-Miranda et al. 2003, Moreno-Sánchez et al. 2016). This pattern was also observed in this study, with low values of Levin’s index; it was also reflected in the bidimensional representation of the feeding strategy (Costello graph), where 5 prey species were the main items in the diet of the Pacific red snapper and provided over 90% (%IRI) to the diet in general, by sex, by sexual maturity, and by season. All this evidence suggests an opportunistic pattern in the diet of the Pacific red snapper, which changes to take advantage of the most abundant and available resources in a given time and habitat. This behavior has already been reported in other studies (Yáñez-Arancibia and Nugent 1977, Moreno-Sánchez et al. 2016).
According to the TL obtained for the Pacific red snapper in Santa Rosalía (TL = 3.4), this species could be classified as a tertiary consumer, as has been reported for other snapper species in the Gulf of Mexico and the Caribbean Sea off Colombia (TL = 3.4-4.6). If the populations of these ecologically important predators were affected, there could be immediate impacts on the ecosystem, depending on the TL they occupy in different habitats (Acero and Garzon 1985, Arreguín-Sánchez and Manickchand-Heileman 1998, Pérez-España 2003, García and Contreras 2011).
In general, we conclude that the Pacific red snapper was an opportunist demersal carnivorous predator in Santa Rosalía, whose trophic spectrum comprised fish and crustaceans that were abundant in the area, particularly coastal pelagic species (S. sagax, H. thrissina, N. simplex) and mesopelagic species (B. panamense and other myctophids). This trophic spectrum was different in composition when compared to snappers in other areas of the Gulf of California and the Pacific coast of Mexico.











 texto en
texto en 



