INTRODUCTION
The morphological variability in hydrozoan populations is a common phenomenon that should not go unnoticed (Ralph 1956; Wyttenbach et al. 1973; Kosevich 2008; Cunha et al. 2015, 2016, 2020). The lack of knowledge on this issue has resulted in numerous misidentifications of species over history. An example of this are the species of the genus CladonemaDujardin, 1843, which has a worldwide distribution and a semi-epibenthic mode of life (Schuchert 2006, Gershwin and Zeidler 2008, Schuchert 2012, Cedeño-Posso 2014). The taxonomy of Cladonema has been subject of discussion over the years because of the lack of research on its morphological variability (Schuchert 2006). Currently, the genus Cladonema includes 8 valid species: Cladonema californicumHyman, 1947; Cladonema myersiRees, 1949; Cladonema novaezelandiaeRalph, 1953; Cladonema pacificum Naumov, 1955; Cladonema radiatum; Cladonema timmsiiGershwin and Zeidler, 2008; Cladonema multiramosumZhou, Gu, Wang & Chen, 2022; and Cladonema digitatumFang et al., 2022 (Schuchert 2022). However, few works have focused on delimiting the diagnostic morphological features, whereby the taxonomic boundaries of the genus remain uncertain. Some examples of these works are those of Billard (1905), Mayer (1910), Russell (1953), Brinckmann-Voss (1970), Bouillon et al. (2004), and Schuchert (1996, 2006, 2012). Most of these works emphasize the development and morphology of the medusa, leaving important diagnostic features of the hydroid and some possible morphological variations understudied.
Cladonema radiatumDujardin, 1843 is the most studied species of the genus and probably of the Cladonematidae family. Most of the studies on this species are from the Mediterranean Sea (Brinckmann-Voss 1970, Bouillon et al. 2004, Schuchert 2006, Gravili et al. 2015). In Mexico, C. radiatum has been recorded several times in the Caribbean Sea (Segura-Puertas and Damas-Romero 1997, Segura-Puertas et al. 2003); however, only one work on its taxonomy in this region is available from the southern Gulf of Mexico (Ahuatzin-Hernández et al. 2020). This species is mainly distributed in the Atlantic Ocean, in the Mediterranean Sea, along the coasts of Europe, in the Caribbean Sea, and in the Gulf of Mexico, although it is also distributed in some localities of the Indo-Pacific (Gershwin and Zeidler 2008, Gravili et al. 2015, Ahuatzin-Hernández et al. 2020). Cladonema radiatum presents numerous morphologies, which have generated synonyms and confusion within the group over history (see Schuchert 2006 for a complete synonymy). Currently, the location of the gonads, the features in the branching patterns and number of radial canals, and the number of marginal tentacles are recognized as the most reliable taxonomic characters to identify the species (Bouillon et al. 2004, Schuchert 2006). However, the studies focused on the morphological variability of C. radiatum are scarce and old or represent populations from Europe, so its taxonomy continues to be understudied in some locations of the Atlantic Ocean (Billard 1905, Mayer 1910, Russell 1953).
Throughout the years, the main research on the morphological variability in populations of hydrozoans has been mainly focused on hydroid colonies (e.g., Ralph 1956; Cunha et al. 2015, 2020), but research on the morphological variability in hydromedusae is scarce (e.g., Russell 1953, Álvarez-Silva et al. 2003, Ocaña-Luna et al. 2021). This bias has generated a fragmentary knowledge of taxonomy and the natural history of several hydrozoan groups, which has led to misidentifications, synonyms, and doubtful species (Hirai 1958, Schuchert 2006). Recent techniques based on molecular biology have been useful in solving some of these problems, with many cryptic speciations in hydrozoans being reported (Nawrocki et al. 2010, Miglietta and Cunningham 2012, Miglietta et al. 2019); however, taxonomic descriptions based on morphology remain fundamental for the understanding of the systematics and evolution of taxonomic groups (Cunha et al. 2016).
Studies that provide information regarding the morphology and taxonomy of hydromedusae in Mexico are scarce (e.g., Álvarez-Silva et al. 2003, Cortés-Lacomba et al. 2013, Ahuatzin-Hernández et al. 2020, Mendoza-Becerril et al. 2020, Ocaña-Luna et al. 2021). In this work, the morphological variations in a population of C. radiatum are analyzed. New features in the bulbs and the arrangement of nematocyst in the marginal tentacles are described for the first time in the species, providing an enriched taxonomic description and improving the knowledge of the taxonomy of hydrozoan populations in Mexico.
MATERIALS AND METHODS
Study area
The coastal lagoon of Chelem is located in the southern Gulf of Mexico, between the Port of Progreso and the town of Chelem, on the Yucatán Peninsula (Herrera-Silveira and Morales-Ojeda 2010). It is surrounded mainly by Rhizophora mangle, and its salinity levels are usually euhaline. The depth of Chelem ranges from 0.5 to 2.5 m. The lagoon has been impacted by the construction of roads and bridges, dredging, filling, and the creation of artificial connections with the sea, which has changed the ecosystem’s hydrological conditions, depth, and biological composition (Herrera-Silveira 2006). The bottom is covered principally by Halodule wrightii and Thalassia testudinum; however, green and red macroalgae are also present in the area (Herrera-Silveira 2006, Herrera-Silveira and Morales-Ojeda 2010).
Sampling
The zooplanktonic material was collected through circular trawls with a zooplankton net of 60 cm in diameter and 333-μm mesh size in the coastal lagoon of Chelem (21°17ʹN, 89°40ʹ W). The samplings were carried out for 3 months (July, September, and November) in 2017. Due to the shallow depth of the lagoon, it was possible to collect polyps belonging to C. radiatum (21°16ʹ33ʺ N, 89°40ʹ06ʺW; 0.5-1.5 m depth), which allowed corroborating its identification. The polyps were collected incidentally through the zooplanktonic trawls and through fouling structures. Temperature, dissolved oxygen, and salinity were recorded at each sampling site with a multi-parametric YSI model 550 (Table 1). The collected samples were fixed in a 4% formalin solution with sea water and the best-preserved specimens of hydromedusae were deposited in the Regional Collection of Cnidarians of the Yucatán Peninsula based at the Universidad Nacional Autónoma de México: Facultad de Ciencias, Unidad Multidisciplinaria de Docencia e Investigación-Sisal, Yucatán (catalogue numbers: YUC-CC-254-11-001544, YUC-CC-254-11-001545).
Table 1 The hydromedusa abundance, salinity, dissolve oxygen (DO, mg·L-1), and temperature (Temp, °C) values recorded during the 3 sampling months in each station.
| Stations | |||||||||||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | ||||
| July | Medusae | 28 | 11 | 7 | 3 | 1 | 5 | 0 | 0 | 1 | 0 | 1 | 13 | ||
| Temp | 30.0 | 29.6 | 29.3 | 29.5 | 29.6 | 29.7 | 29.5 | 29.5 | 29.9 | 29.8 | 30.2 | 29.7 | |||
| DO | 6.0 | 4.4 | 3.7 | 4.0 | 2.9 | 1.3 | 1.5 | 1.7 | 1.8 | 1.9 | 2.3 | 2.4 | |||
| Salinity | 37.3 | 39.6 | 40.2 | 40.3 | 40.7 | 41.1 | 39.6 | 38.3 | 37.5 | 37.5 | 37.4 | 38.4 | |||
| September | Medusae | 3 | 0 | 5 | 0 | 0 | 0 | 6 | 13 | 7 | 5 | 0 | 0 | ||
| Temp | 31.6 | 31.0 | 30.4 | 30.8 | 30.3 | 30.7 | 30.6 | 30.4 | 29.8 | 29.9 | 30.4 | 30.4 | |||
| DO | 1.4 | 2.6 | 2.9 | 3.9 | 3.3 | 3.4 | 5.3 | 5.4 | 2.9 | 3.4 | 3.4 | 3.6 | |||
| Salinity | 40.9 | 45.6 | 46.4 | 40.3 | 41.1 | 39.9 | 41.1 | 39.8 | 36.9 | 36.3 | 36.8 | 33.3 | |||
| November | Medusae | 16 | 1 | 1 | 0 | 0 | 2 | 0 | 0 | 3 | 2 | 0 | 0 | ||
| Temp | 27.7 | 27.6 | 27.8 | 27.4 | 27.3 | 26.7 | 27.6 | 27 | 26.2 | 26.3 | 26.4 | 26.5 | |||
| DO | 7.4 | 6.8 | 6.1 | 6.5 | 6.6 | 6.4 | 6.8 | 6.2 | 5.9 | 5.9 | 5.8 | 4.7 | |||
| Salinity | 42.3 | 45.1 | 45.5 | 42.0 | 41.8 | 41.7 | 39.9 | 38.9 | 38.1 | 38.0 | 38.1 | 38.2 | |||
| Long | 89°46'8.36'' | 89°47'19.1'' | 89°47'7.1'' | 89°44'48.2'' | 89°44'39.5'' | 89°44'27.5'' | 89°44' 4.7'' | 89°43'41.4'' | 89°42'47.3'' | 89°42'15.4'' | 89°42'17.3'' | 89°41'42.7'' | |||
| Lat | 21°14'48.8'' | 21°14'36.9'' | 21°14'58.0'' | 21°15'21'' | 21°14'51.7'' | 21°15'43.7'' | 21°15'12.1'' | 21°15'39.6'' | 21°15'49'' | 21°15'34.1'' | 21°15'55'' | 21°16'03'' | |||
The zooplanktonic material was examined with a stereomicroscope, and 134 specimens belonging to C. radiatum were sorted for this study. Drawings and photographs of the features were made using a digital camera mounted on an optical microscope. Morphological features (i.e., radial canals, oral tentacles, marginal tentacles, branches of marginal tentacles, and nematocyst arrangement; characteristics of the manubrium; the form, width, and height of the umbrella; and the presence of apical projection) were observed and recorded from preserved specimens. The morphologies of C. radiatum were established considering the number and branching patterns of radial canals and the number of oral tentacles and marginal bulbs since the major variability in the species occurs in these features (Gravili et al. 2015). In addition, these features prevail during the ontogenetic development (Brinckmann-Voss 1970) and are not affected by the sampling method as may happen with the branches of marginal tentacles or the shape of the umbrella.
RESULTS
In general, the specimens had an umbrella slightly wider (0.82 mm [0.3 ± 2.2]) than high (0.67 mm [0.2 ± 1.8]). Cladonema radiatum was more abundant during July, and on average, the specimens collected in this month had the bigger umbrella sizes. In addition, July recorded the lowest levels of salinity and dissolved oxygen (39.0 and 2.8 mg·L-1, respectively). The temperature during this month showed intermediate values between September and November (29.7 °C) (Table 2).
Table 2 Average measurements of the specimens and physicochemical parameters for each month
| Width (mm) | Height (mm) | Temperature (°C) | Dissolved oxygen (mg·L-1) | Salinity | ||
| Jul | Average | 0.86 | 0.73 | 29.7 | 2.8 | 39.0 |
| Jul | Min | 0.40 | 0.20 | 29.3 | 1.3 | 37.2 |
| Jul | Max | 1.40 | 1.40 | 30.2 | 6.0 | 41.0 |
| Jul | n | 70.00 | 70.0 | 8.0 | 8.0 | 8.0 |
| Sep | Average | 0.85 | 0.64 | 30.4 | 3.5 | 39.9 |
| Sep | Min | 0.30 | 0.20 | 29.8 | 1.3 | 36.2 |
| Sep | Max | 2.20 | 1.80 | 31.6 | 5.3 | 46.4 |
| Sep | n | 39.00 | 39.00 | 6.0 | 6.0 | 6.0 |
| Nov | Average | 0.68 | 0.56 | 27.0 | 6.4 | 40.8 |
| Nov | Min | 0.30 | 0.20 | 26.2 | 4.7 | 38.0 |
| Nov | Max | 1.80 | 1.50 | 27.7 | 7.4 | 45.4 |
| Nov | n | 25.00 | 25.00 | 6.0 | 6.0 | 6.0 |
Six different morphological configurations (A-F) were identified (Fig. 1). The radial canals and oral tentacles were the most variable features. The total number of radial canals and marginal bulbs showed linked patterns. Likewise, the branching patterns of the radial canals are related to the oral tentacles and the edges that conform to the base of the manubrium since these are the same in number and the radial canals arise from these edges, so the distribution of the branching patterns depends on this feature. The D morphology was the most numerous in this study (n = 121), while the B, C, and F morphologies were represented by only one specimen (Table 3). A possible seventh morphology represented by one specimen with 9 marginal tentacles, 5 oral tentacles, and 3 straight and 3 branched radial canals was recognized. However, this morphology was not considered since the state of preservation was poor and the features mentioned above could have turned out uncertain. The presence of papillae in the abaxial surface of the bulbs and an abaxial nematocyst arrangement are features observed in the species for the first time.
Table 3 Morphological configurations (A-F) recorded in a population of Cladonema radiatumDujardin, 1843 from the southern Gulf of Mexico.
| A | B | C | D | E | F | |
| Total radial canals (n) | 9 | 8 | 7 | 9 | 8 | 10 |
| Straight canals (n) | 5 | 4 | 3 | 3 | 2 | 2 |
| Branched canals (n) | 2 | 2 | 2 | 3 | 3 | 4 |
| Marginal bulbs (n) | 9 | 8 | 7 | 9 | 8 | 10 |
| Oral tentacles (n) | 7 | 6 | 5 | 6 | 5 | 6 |
| Width average (mm) | 0.95 (0.9-1.0) | 0.80 | 0.40 | 0.81 (0.3-2.2) | 0.92 (0.3-1.2) | 0.60 |
| Height average (mm) | 0.85 (0.6-1.10) | 0.90 | 0.40 | 0.67 (0.2-1.8) | 0.71 (0.4-1.0) | 0.20 |
| Pouches | Present | Absent | Absent | Present | Present | Absent |
| Black marks | Present | Present | Absent | Present | Absent | Absent |
| Total specimens (n) | 2 | 1 | 1 | 121 | 7 | 1 |
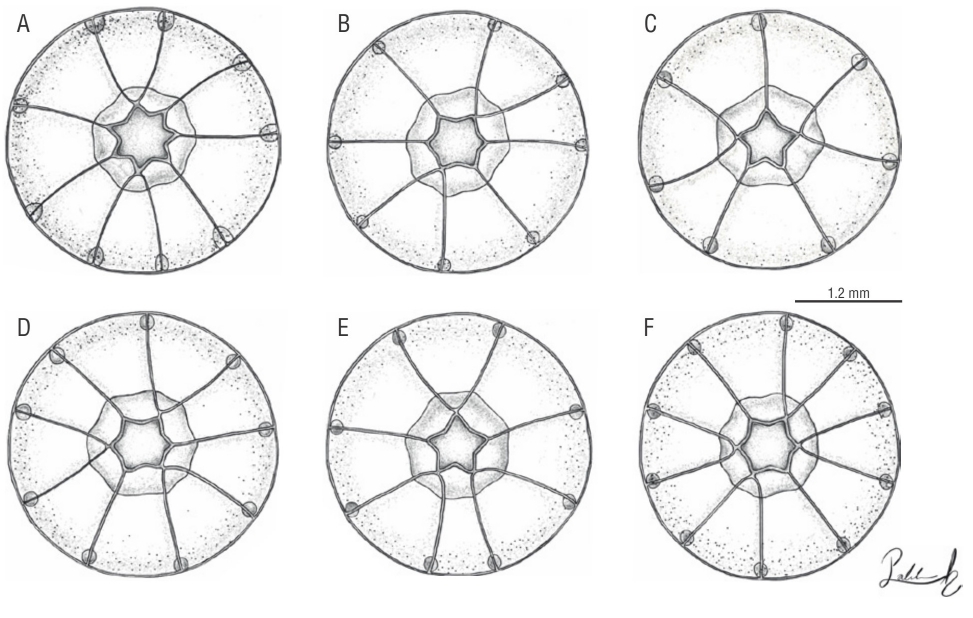
Figure 1 Patterns recorded in the radial canals of the different morphologies (A-F) found in Cladonema radiatumDujardin, 1843.
Taxonomy
The classification for C. radiatum is as follows.
Class: Hydrozoa Owen, 1843
Subclass: Hydroidolina Collins, 2000
Superorder: Anthoathecata Cornelius, 1992
Order: Capitata Kühn, 1913
Family: Cladonematidae Gegenbaur, 1857
Species: Cladonema radiatumDujardin, 1843
(Fig. 2 a-g, see Schuchert 2006 for a complete synonymy).
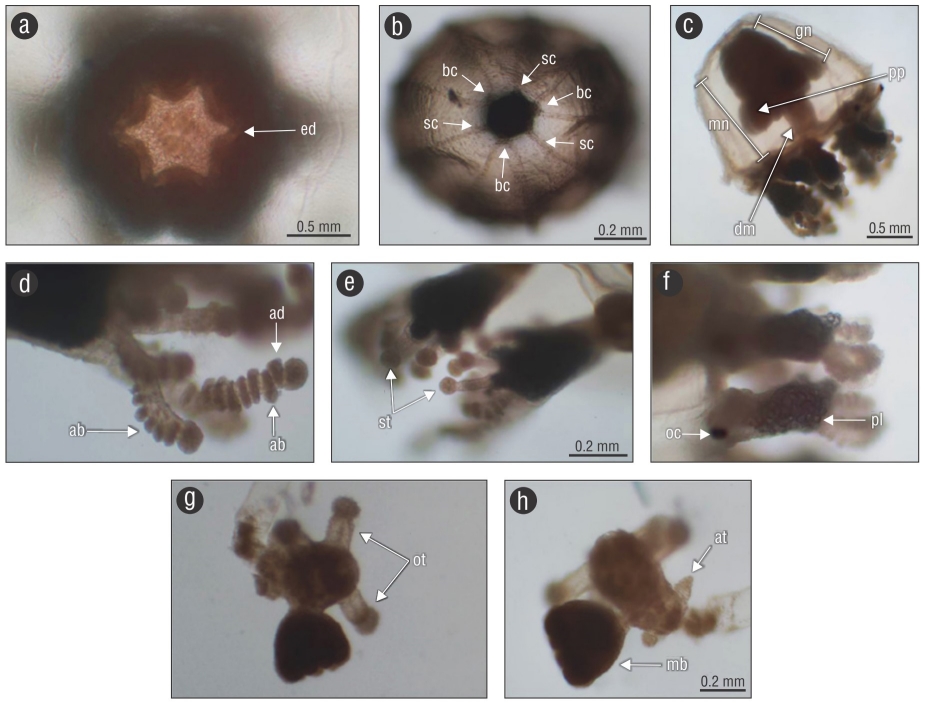
Figure 2 Diagnostic features of Cladonema radiatumDujardin, 1843. (a-b) Aboral view of the medusa: ed = edges that make up the base of the manubrium, bc = branched canals, and sc = straight canals. (c) Mature specimen of C. radiatum: mn = manubrium, gn = gonads, pp = perradial pouch-like protuberances, and dm = distal part of the manubrium. (d) Stinging tentacles: ab = abaxial nematocyst rows and ad = adaxial nematocyst rows. (e) Secondary tentacles: st = suctorial tentacles. (f) Marginal bulb: oc = ocellus and pl = papillae. (g-h) Hydroid: ot = oral tentacles, at = aboral tentacles, and mb = medusa bud.
Diagnosis
The specimens showed the following characteristics: bell-shaped umbrella; 7-10 radial canals, some of them branched; manubrium cylindrical, with 5-7 oral tentacles with a terminal nematocyst knob. The gonads surround the upper 2/3 of the manubrium. The number of marginal tentacles corresponds to the number of total radial canals. The marginal bulbs have a black and rounded abaxial ocellus at their base and numerous gelatinous papillae over the abaxial surface. The marginal tentacles are branched and have an elongated thickened base and numerous nematocyst clusters, with a long main branch from behind of which grow numerous short adhesive tentacles. The nematocysts are arranged in alternate positions, abaxial- adaxial along the entire main tentacle and exclusively abaxial in the shortest lateral branches. A terminal knob is evident at the end of each branch. One to 6 single tentacles arise from behind the marginal bulbs; these bear only a terminal knob.
Description of the hydromedusae
Medusae have a bell-shaped umbrella, with or without an apical projection and when present, it is rounded and slightly perceptible. The manubrium is cylindrical, lacks a peduncle, and has a hexagonal, heptagonal, or pentagonal base, depending on the number of edges that conform to its base, which is the same in number as the oral tentacles. Five to 7 (usually 6) oral tentacles are present and bear a terminal knob. Rounded marks constituted of black pigments located near the base of the manubrium are present, as are other elongated ones, in stripe-form, near the distal part; the marks are more evident in mature specimens, and both types of marks (rounded and stripes) correspond in number with the oral tentacles and edges at the base of the manubrium. The manubrium is present with or without perradial pouch-like protuberances. In this study, 18 specimens showed pouches, and 116 lacked them. When pouch-like protuberances are present, they corresponded in number with the marks in the manubrium and oral tentacles. The gonads surround the upper 2/3 of the manubrium. Seven to 10 (usually 9) radial canals are present and some (usually 3) are branched; the branching can be observed at different distances from the radial canal base. The number of radial canals corresponds with the number of marginal tentacles, which arise from elongated tentacular bulbs. Each marginal bulb has a black and rounded abaxial ocellus at its base and numerous gelatinous papillae on the abaxial surface. The marginal tentacles branch up to 8 times in the most mature specimens. The tentacles present a long main branch from which other short tentacles branch with numerous nematocyst clusters, and from behind of which grow 1-6 short suctorial tentacles, which only bear a terminal knob for attachment in some substrate. The nematocyst clusters in the branches show 2 arrangements. The nematocysts in the main branch are arranged in an alternate abaxial-adaxial way or randomly scattered, while those in the short branches have an exclusively abaxial arrangement. Each branch has an evident terminal knob.
Description of the hydroid
The hydroid shows stolonal growth, is slender, and has a pedicel greater in length than the hydranth, with a smooth and thin perisarc that ends just below the hydranth. The hydranth shows a rounded hypostome and 2 whorls of tentacles; the first whorl is a row of 4 oral capitate tentacles and the second is a row of 4 aboral filiform tentacles, arising below the oral ones. The medusa buds are very evident on the hydranth, arising just above the aboral tentacles.
Remarks
The presence of perradial pouch-like protuberances in the manubrium was not considered when diagnosing the morphologies in this study. The pouches were present throughout the different morphologies and were not useful to recognize any additional pattern (Fig. 3).
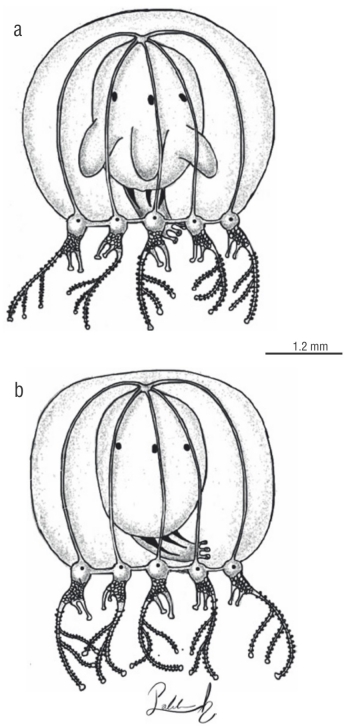
Figure 3 Mature specimens of Cladonema radiatumDujardin, 1843. (a) Medusa with perradial pouch-like protuberances in the manubrium. (b) Medusa without pouches.
Cladonema radiatum was first described in the Mediterranean, St. Malo and Lorient (France) (Dujardin 1843). Since then, it has been recorded in many locations of the world, mainly throughout the Atlantic and Pacific oceans. Some records of the species are as follow: Norway, the British Isles, Ireland, Sweden, Denmark, Helgoland, Holland, Belgium, and France (Mayer 1910, Russell 1953, Schuchert 1996, Schuchert 2006); Italy (Brinckmann-Voss 1970); and the Black Sea and Atlantic coast of the Iberian Peninsula (Medel and López-González 1996). It is also known from the coasts of Brazil (Migotto 1996, Bittencourt-Farias et al. 2020), the Arabian Sea (Daly-Yahia et al. 2003, Ghory et al. 2020), the Colombian and Mexican Caribbean seas (Segura-Puertas and Damas-Romero 1997, Cedeño-Posso 2014), the southern Gulf of Mexico (Ahuatzin-Hernández et al. 2020), Florida (Kramp 1959, Vervoort 1968), Bermuda (Calder 1988), Belize (Calder 1991), and the Bahamas (Mayer 1910, Kramp 1970).
DISCUSSION
Cladonema radiatum was more abundant in July, when salinity showed the lowest values and temperature ranged from 29.3 to 30.2 °C. The temperature values recorded during July in Chelem match with those recorded by Bittencourt-Farias et al. (2020) in the South Atlantic Ocean. However, temperature in Chelem was lower than that recorded by Ghory et al. (2020) in the Arabian Sea. Likewise, the salinity of Chelem is higher than that in other ecosystems where this species has been recorded (Bittencourt-Farias et al. 2020, Ghory et al. 2020,). In general, the ecological studies on C. radiatum are scarce; however, much information on its artificial culture is available since it is a species that is easy to maintain in the laboratory. In this sense, Brinckmann-Voss (1970) pointed out that the hydroid and medusa are easy to keep in dishes at 18 °C, a significantly lower temperature than that recorded in this study. Schuchert (2006) reported that the medusa buds from populations in the Mediterranean died at temperatures lower than 14 °C but grew well in temperatures ranging from 22 to 30 °C, which are temperature values similar to those recorded in Chelem. Likewise, Ranjith et al. (2021) maintained a C. radiatum population from the southeast coast of India at temperatures and salinities of 30 °C and 35, respectively, and these values fit in the ranges recorded during the 3 months of the present study. The variability in the physicochemical conditions where C. radiatum develops suggests a wide range of tolerance to environmental heterogeneity, one of the keys to the success of this taxa.
The hydroid of C. radiatum can be hard to distinguish between some members of the Corynidae family (Schuchert 2006, 2012); however, the medusa buds, the stolonal growth, the features of the perisarc and tentacles, and the patterns of the radial canals, marginal tentacles, and gonads of the medusae (Gershwin and Zeidler 2008, Schuchert 2012, Cedeño-Posso 2014, Ghory et al. 2020) allowed determining C. radiatum as the species under study.
The hydroid of C. pacificum can be distinguished from that of C. radiatum by the lack of filiform tentacles (Rees 1979, Cedeño-Posso 2014, Ghory et al. 2020). Likewise, the gonads are the only feature that allows differentiating these species during their medusa stage (Schuchert 2006). Studies based on the taxonomy of C. pacificum are scarce, which has generated various synonyms with the species (Rees 1982). Cladonema pacificum is restricted to the Pacific Ocean, near the seas of Japan, Russia, and the United Stated of America (Gershwin and Zeidler 2008), while C. radiatum is a common species in the Atlantic Ocean, mainly on the coasts of Europe (Schuchert 2006).
Some authors have considered that there is not enough material to differentiate C. myersi from C. radiatum (Rees 1979, Stepanjants et al. 1993). However, the former can be distinguished by the features of the hydroid, which lacks filiform aboral tentacles (Rees 1949). Likewise, some differences in the medusa can be highlighted, such as the straight radial canals (maximum 7, rarely 5 or 6), the location of gonads (Schuchert 2006, Cedeño-Posso 2014), and the number of branches in the primary tentacles (Rees 1949). Cladonema myersi is very similar to C. pacificum during the polyp stage. However, the main difference between these species can be pointed out in the radial canals of the medusa (Gershwin and Zeidler 2008, Cedeño-Posso 2014).
The diagnostic features of C. timmsii are the straight radial canals, dimorphic gonads, the presence of papillae on the bulbs, and a characteristic arrangement of the nematocysts in the marginal tentacles (Gershwin and Zeidler 2008). Considering the above, C. radiatum could be distinguished from C. timmsii by the branched radial canals and the nematocyst arrangement since papillae were recorded in the specimens of this study. Some specimens of C. radiatum with 8-10 straight radial canals have been reported previously in the literature (Mayer 1910, Russell 1953); however, these are uncommon, so until more research is done on this issue, this feature can be considered diagnostic for C. timmsii. Unfortunately, the hydroid of C. timmsii is unknown, so complete and integrative morphological comparisons are not possible.
The original description of C. novaezelandiae is limited (Ralph 1953, Schuchert 1996). This species had been synonymized with C. radiatum by Schuchert (1996) due to the poor state of preservation of the original material. Nevertheless, C. novaezelandiae is currently a valid species (Schuchert 2022). Records after the first description of C. novaezelandiae are null, and the morphological features mentioned in recent works are based on the original description (Gershwin and Zeidler 2008, Cedeño-Posso 2014, Bittencourt-Farias et al. 2020, Ranjith et al. 2021). In addition, the features of the manubrium and ocelli are still undescribed, and those in the branching canals and stinging tentacles are unclear (Gershwin and Zeidler 2008, Ranjith et al. 2021). After analyzing the features of C. novaezelandiae, it is remarkable that these coincide with those recorded for the B, C, and D morphologies of this work, except for the number of branches in the primary tentacles. However, the branching of the tentacles is related to the development of the organisms, so it is not a completely reliable diagnostic feature to delimit a species (Fujiki et al. 2019). Unfortunately, the hydroid of C. novaezelandiae is unknown. Therefore, a complete differentiation of the species is not possible, and synonymy with C. radiatum is not sufficiently established, as its features may turn out to be different from those of C. radiatum (Schuchert 2006). The taxonomic identity of C. novaezelandiae is uncertain, so it would be desirable to focus more research on the Cladonema populations from New Zealand to clarify their identity through integrative works, describe their polyp stage and morphological varieties, and decode their molecular identity.
Cladonema californicum is probably the easiest medusa to distinguish within the genus. It has unbranched radial canals and up to 9 marginal bulbs, from which a bifurcated primary tentacle arises. Cladonema californicum presents only a secondary tentacle with a terminal knob for adhesive function (Hyman 1947). The rest of the morphological features in the medusa and hydroid are similar to those in C. radiatum and other species of Cladonema (Rees 1979, Gershwin and Zeidler 2008, Bittencourt-Farias et al. 2020). In addition, C. californicum is the only species in which the black stripes reported on the manubrium of the specimens in this work have been documented (Hyman 1947). It is hard to attribute a function to this feature; however, it can be considered a useful pattern to distinguish further morphologies in the species.
Cladonema digitatum can be distinguished from its congeners by the presence of finger-like protuberances on the manubrium, radial canals with Y-shaped bifurcations, tentacles with 3-11 adhesive branches, and 3-7 stinging branches growing from the main branch as side branches (Fang et al. 2022). The shape of the protuberances and the branching pattern of the marginal tentacles of C. digitatum are similar to the characteristics observed in the specimens of this study. However, C. digitatum can be distinguished from C. radiatum by the Y-shaped branching of the radial canals, which is different from that observed in the specimens in this work. The characteristics described for the hydroid of C. digitatum are similar to those reported in C. radiatum (Fang et al. 2022).
Cladonema multiramosum can be distinguished from its congeners by having a greater number of adhesive tentacles (8-24, rarely 5-7) and by having tiny irregular side branches on the upper radial canals (Zhou et al. 2022). The hydroid of C. multiramosum is similar to that of C. radiatum; however, both can be distinguished by the number of medusa buds, which is higher in C. multiramosum. Furthermore, the arrangement of the medusa buds reported for C. multiramosum can be considered a diagnostic feature since it has not been reported in other species (Zhou et al. 2022).
Some morphologies reported in this work have been documented in other localities of the world. Specimens from Salento, Italy (Gravili et al. 2015), coincide with those of the E morphology in the present study. The number of bulbs of the specimens from the Arabian Sea (Ghory et al. 2020) is the same as that recorded for the A and D morphologies. Specimens from the coast of Brazil (southern Atlantic) and the southern Gulf of Mexico (in a different coastal lagoon to that in this study) (Ahuatzin-Hernández et al. 2020, Bittencourt-Farias et al. 2020) coincide with the D morphology, the most numerous in this work. Likewise, the morphologies recorded by Billard (1905), Mayer (1910), and Russell (1953) are consistent with the B and E morphologies of this study. The morphological configuration recorded on the coasts of India (Ranjith et al. 2021) coincides with the seventh morphology ruled out in this work (4-5 oral tentacles and 9 tentacular bulbs). However, only 1 specimen with these features was found in this study, and the state of preservation was poor. This encourages more systematic research on hydrozoans in the coastal lagoons of the southern Gulf of Mexico since other morphologies could remain undescribed. These facts suggest that prevalence in the different morphologies of C. radiatum could depend on the geographical distribution and environmental heterogeneity (Mayer 1910; Russell 1953; Schuchert 1996, 2006; Ahuatzin-Hernández et al. 2020; Bittencourt-Farias et al. 2020; Ghory et al. 2020).
Intraspecific morphological variation in hydrozoans can be related to diverse environmental cues such as temperature, food availability, predation pressure, and hydrodynamics (Cunha et al. 2016). Nevertheless, the variation in the branching patterns of the canals of the Cladonema species is not associated with any specific environmental factor, and it is not completely clear if the branching occurs before or after the liberation of the medusa (Brinckmann-Voss 1970). In some species such as C. pacificum, the branching pattern is present since an early development stage (Fujiki et al. 2019), so it is likely that this feature corresponds to an ontogenetic characteristic. On the other hand, Schierwater and Hadrys (1998) studied the relation between the metagenesis and environmental factors in other species of the Cladonematidae family and showed that the size of the umbrella in Eleutheria dichotoma is affected by temperature. They also concluded that the development of the species is strongly genetically predetermined; however, environmental factors can have an important effect on the initiation of sexual reproduction. Lack of knowledge on the ontogenetic features of Cladonema encourages more efforts in this field since it is an important issue to understand the biology and evolution of the group.
Morphological features such as the perradial pouch-like protuberances and rounded and striped black marks in the manubrium have been reported previously in Cladonema species (Mayer 1910, Hyman 1947, Russell 1953, Kramp 1959, Schuchert 2006, Gershwin and Zeidler 2008). However, their functions remain uncertain. Gershwin and Zeidler (2008) attributed the presence of pouches to sexual dimorphism; Mayer (1910) mentioned that these pouches are confined to the gonads, having a reproductive function, while Russell (1953) and Schuchert (2006) mentioned that the pouches are part of the organism’s development, being present only in sexually mature specimens. In the present study, juvenile specimens (small umbrella sizes, few branches of the marginal tentacles, little development in the gonads) were observed with the presence of pouches, so the idea that the pouches are part of the ontological development is ruled out, at least for C. radiatum. This idea is a contrast to that pointed out by Ranjith et al. (2021) since pouches were absent in smaller specimens of their study. The sexual dimorphism proposed by Gershwin and Zeidler (2008) must be confirmed for the rest of Cladonema species through specific histological studies. More detailed information on these features is needed, together with molecular and histological analyses, to define their function. Cladonema radiatum has a variable morphology, making it difficult to identify its sexual dimorphism using the literature (Ranjith et al. 2021).
This work analyzed the morphological differences among the congeners of C. radiatum, which allowed recognizing the morphological boundaries of the genus (Table 4). This is the first time that gelatinous papillae in the bulbs of C. radiatum were recorded, as these were considered diagnostic features for C. timmsii (Gershwin and Zeidler 2008). The arrangement of the nematocysts in the marginal tentacles of C. radiatum coincides with that previously recorded for the main stinging tentacles (Schuchert 2006, 2012). However, the pattern of the short branches for the Cladonema populations from the southern Gulf of Mexico adds a new consideration since it had not been recorded previously. This evidence shows the need for more research on this group because other important features may remain undescribed.
Table 4 Comparison of the morphology of the valid species within CladonemaDujardin, 1843.
| Cladonema radiatum Dujardin, 1843 | Cladonema californicum Hyman, 1947 | Cladonema timmsii Gershwin & Zeidler, 2008 | Cladonema pacificum Naumov, 1955 | Cladonema myersi Rees, 1949 | Cladonema novaezelandiae Ralph, 1953 | Cladonema multiramosum Zhou, Gu, Wang & Chen, 2022 | Cladonema digitatum Fang et al., 2022 | |
| Medusa stage | ||||||||
| Bell size | Up to 4.00 mm high and 3.00 mm wide | Up to 3.00 mm wide and shorter than wide | About 2.00 mm high and wide | 2.00-3.50 mm high and 2.2 mm wide | Up to 0.80 mm wide | About 3.00 mm wide | 1.36-1.95 mm high and 1.57-2.26 mm wide | 1.62-3.17 mm high and 2.22-3.75 mm wide |
| Radial canals | 7-11 | 9 (rarely 11) | 9 | 9 (rarely 6) | 7 (rarely 5 or 6) | 7-8 | 8-11 | 7-9 (usually 9) |
| Straight canals | 3-5 | 9 (rarely 11) | 9 | 3 | 7 (rarely 5 or 6) | 7-8 | 8-11 | 5 |
| Branched canals | 2-4 | Absent | Absent | 6 | Absent | 1 | Some radial canals bifurcate close to the manubrium, and some with several tiny branches on the top | 1-4 Y-shaped |
| Manubrium | Not extending beyond bell margin | Beyond bell margin | Not extending beyond bell margin | Not available | Half the height of bell cavity | Not available | Manubrium spindle-shaped, able to extend beyond the umbrella margin | Extending beyond bell margin |
| Gonads | Surrounding the upper 2/3 of manubrium | Surrounding almost the whole manubrium | Surrounding stomach in upper half, female without pouches; male with 6 radially arranged pouches | Surrounding almost the whole manubrium | Not available | Surrounding the manubrium | Around upper 1/2-1 (mean 4/5) of the manubrium | Around upper 1/2-8/9 of the manubrium |
| Pouches | Present: 4-7 perradial pouch-like protuberances | Present: 6, rarely 7 elongated rounded protrusions | Present | Absent | Not available | Present: 6 pouches | Present: 5-8 pouches (usually 6) | Present: 4-7 finger-like pouches |
| Oral tentacles | 4-7 | 6 | 6 | 6 | 6 | 6 | 5-8 | 3-6 (usually 5 or 6) |
| Tentacle branches | 4-10 | 1-2 | 6-8 | 6-12 | 7 | Up to 10 | 3-6 | 3-7 growing from the main branch as side branches |
| Nematocyst arrangement | Abaxial-adaxial in the main branch and only abaxial in the shorter branches | Scattered | The main median branch with 2 rows of alternate clusters, side branches with 1 row of abaxial warts | Abaxial-adaxial in the main branch, according to figures provided in Fujiki et al. (2019) | Scattered | Not available | Not available | Not available |
| Suctorial tentacles | 1-6 | 1 | 5-7 | Up to 12 | 3 | Up to 7 | 8-24 | 3-11 (usually >4) |
| Ocelli | Rounded and black, with ectodermal cuticular lens | Elongated and red | Dark red; cup-like, with lens | Rounded and dark, according to figures provided in Fujiki et al. (2019) | Reddish | Not available | Black | Black |
| Cnidoma (µm) | Desmonemes (9.00-12.00 × 3.50-5.00) and stenoteles (13.00-16.00 × 9.00-10.00, 9.50-11.00 × 5.00-8.50) | Desmonemes (8.50-10.00 × 4.00-5.00, 9.00-11.00 × 4.50-5.00) and stenoteles (10.00 × 16.00, 8.00-8.50 × 12.00, 21.00-23.00 × 14.00-16.00,14.00-18.00 × 9.50-11.00) | Not available | Not available | Not available | Not available | Stenoteles (7.50-18.50) × (4.60-11.90), desmonemes (6.20-9.00) × (3.30-5.10), and mastigophores (11.10-14.30) × (3.30-4.70) | Mastigophores (12.16-18.45) × (3.12-7.57), stenoteles (6.03-22.38) × (3.16-13.83) and desmonemes (5.58-8.88) × (3.09-4.93) |
| Polyp stage | ||||||||
| Filiform tentacles | Present | Present | Unknown | Absent | Absent | Unknown | Present | Present |
| Cnidoma (µm) | Stenoteles (11.00-17.00 × 8.00-10.00) and mastigophores (present only in stolons 10.00-12.00 × 3.50-4.00) | Stenoteles (14.00-18.50 × 10.00-12.00, 18.00 × 28.00) | Not available | Not available | Not available | Not available | Stenoteles (10.20-18.60) × (6.50-10.90) | Stenoteles (10.55-25.00) × (6.23-14.83) |
| Distribution | Atlantic, Indian, and Pacific Oceans | Pacific Ocean, Northern California, USA | Indian Ocean, South Australia | Pacific Ocean, East Asia, North of Japan, Russia, San Francisco Bay? | Pacific Ocean, Southern California | Pacific Ocean, New Zealand | Fuzhou, China | Fuzhou, China |
| References | Schuchert (1996, 2006), Gershwin and Zeidler (2008), Cedeño-Posso (2014), Ranjith et al. (2021), present study | Hyman (1947), Rees (1979), Schuchert (2006), Gershwin and Zeidler (2008), Cedeño-Posso (2014), Ranjith et al. (2021) | Gershwin and Zeidler (2008), Ranjith et al. (2021) | Rees (1982), Gershwin and Zeidler (2008), Fujiki et al. (2019), Ranjith et al. (2021) | Schuchert (2006), Gershwin and Zeidler (2008), Cedeño-Posso (2014), Ranjith et al. (2021) | Gershwin and Zeidler (2008), Cedeño-Posso (2014), Ranjith et al. (2021) | Zhou et al. (2022) | Fang et al. (2022) |
The taxonomy of Cladonema continues to be confusing because of its morphological variability and the lack of research on its taxonomy. Only integrative studies considering the morphology, molecular analyses, the complete life cycle, and the different type localities would be able to decode the cryptic nature of the genus (Ranjith et al. 2021). The study of the development of the life cycle from hydroid to medusa considering their morphological variations is one of the main keys to understanding the real identity and taxonomic boundaries of Cladonema, together with molecular analyses of species from around the world, so more studies on these issues are needed (Brinckmann-Voss 1970, Schuchert 2006, Nawrocki et al. 2010, Miglietta and Cunningham 2012, Miglietta et al. 2019). Though this work dealt with only the morphological variability of C. radiatum, it set a base for further integrative studies. Unfortunately, taxonomic studies of Hydrozoa based on morphology are scarce, especially in Mexico, where the lack of research on this issue is notable (e.g., Cortés-Lacomba et al. 2013, Ahuatzin-Hernández et al. 2020, Mendoza-Becerril et al. 2020). This work contributes to the knowledge of this subject and encourages more researchers to be interested in it because it is relevant not only to understand the taxonomy and evolution of a group but to understand its diversity and ecology as well.











 texto en
texto en 



