Introduction
Worldwide, squids of the family Loliginidae are represented by around 47 species, which are grouped into 10 genera and 9 subgenera (Jereb et al. 2010). They are distributed in the neritic zones of tropical, subtropical, and temperate seas (Roper et al. 1984). Five Loliginidae species have been recorded for the Pacific coast of Mexico: Lolliguncula panamensis, Lolliguncula argus, Lolliguncula diomedeae, Doryteuthis opalescens, and Pickforditeuthis vossi (Fischer et al. 1995). Loliginids are caught directly or incidentally (Staudinger 2006); their catch volumes in 2002 reached 275,024 t, which represents 9% of total squid catches around the world (Rodhouse 2005). In a biological sense, they take relevance in food webs either as predators of small fishes and invertebrates or as prey to marine mammals and commercially important demersal and pelagic fishes.
Lolliguncula panamensis is distributed in the eastern Pacific, on the western coast of Baja California Sur and from the Gulf of California to Peru (Fischer et al. 1995). It can be found down to 70 m depth, although it is more abundant between 5 and 30 m, at temperatures between 21 and 27 ºC and salinities between 15 and 23 (Fischer et al. 1995, Sánchez 2003, Arizmendi-Rodríguez et al. 2012b). It exhibits sexual dimorphism, where females, larger in size, reach 110 mm mantle length (ML) and mature at 80 mm ML and males reach up to 80 mm ML and mature at 40 mm ML (Fischer et al. 1995); according to Squires and Barragán (1979), sexual dimorphism occurs before sexual maturity (Squires and Barragán 1979). On the Pacific coast of Mexico, L. panamensis is caught as part of the bycatch associated with the artisanal and deep-sea shrimp fisheries.
In Mexico, studies on the Panama brief squid have been carried out mainly in the Gulf of California and have addressed aspects related to eating habits, reproduction, abundance, and distribution (Arizmendi-Rodríguez et al. 2011, 2012a, 2012b). In the Gulf of Tehuantepec, a study on the distribution, size, and sex ratio of L. panamensis was carried out (Guzmán-Intzin et al. 2020). The studies on the biological aspects of L. panamensis have been conducted in oceanic waters and coastal fronts. Although members of the Loliginidae family can be found in bays and estuaries, their behavior in these ecosystems is unknown. The present work aims to analyze the L. panamensis length-weight relationship, reproductive aspects, distribution, and use of lagoon ecosystems and coastal fronts considering the hypothesis that the lagoon systems are used only as breeding areas.
Materials and methods
Samples were collected during 7 exploratory fishing surveys in the Gulf of California, Mexico, from 2014 to 2017. These surveys are part of the activities of the National Institute of Fisheries and Aquaculture (INAPESCA, for its acronym in Spanish) programs: Jumbo squid (1 cruise), Shrimp (1 cruise), Hake (3 cruises), and Small pelagics (2 cruises). Biological material was also obtained from sampling surveys carried out during the shrimp season closure by INAPESCA in the Agiabampo, Yavaros, Tóbari, Lobos, and Las Guásimas Bays from 2014 to 2016. For the bay surveys, Panama brief squid specimens were caught with a trawl net aboard a small fishing boat, and in the cruise surveys jigs were used for Jumbo squid, a bottom trawl for Shrimp and Hake, and a pelagic gillnet for Small pelagics. Panama brief squid individuals were separated from the target species of the cruises or bay surveys, stored in properly labeled plastic bags, and preserved on ice or frozen. They were transferred to the laboratory at the Regional Center for Aquaculture and Fisheries Research in Guaymas for processing. Surveys were carried out in coastal lagoons and fronts between 25.0º N and 31.5º N latitude (Fig. 1).
Figure 1 Study area, Gulf of California (Mexico), coastal lagoons, and geographical position of the sampling area.
With the purpose of concentrating the biological information, data were grouped by source, either coastal front (Jumbo squid, Shrimp, Hake, Small Pelagic cruises) or coastal lagoon (Agiabampo, Yavaros, Tóbari, Lobos, and Las Guásimas Bays); coastal front is defined as the coastline front in the external parts of any lagoon or bay (surface, middle, and upper coastal fronts). Specimens were measured for ML (the distance between the anterior edge and the posterior apex of the mantle, ±0.1 cm) using a vernier caliper and for total weight (TW, ±0.1 g) using an Ohaus scale. Each organism was dissected ventral side up, and sex was macroscopically identified; some organisms were identified as undetermined given the poor condition of the reproductive system. The gonadal development stage (immature [I], developing [II], mature [III], and spawning [IV]) of sexed individuals was assigned according to the morphochromatic scale of Lipiński and Underhill (1995).
Size structure
Size structure was analyzed by sex after grouping ML data into 10-mm intervals. Distribution normality was assessed with the Kolmogorov-Smirnov test, and ML differences by ecosystem and sex were determined with the Kruskal-Wallis test. The R environment (R Core Team 2020) was used for both tests.
Biometric relationships
ML-TW relationships were estimated for the population and by sex by adjusting the exponential model (TW = a × ML b ), where a is the intercept (condition factor) and b is the allometric coefficient. Paired ML/TW data were transformed to natural logarithm prior to the regression analysis to identify outliers; once located, outliers were excluded from the analysis (Froese 2006). The coefficient of determination (R 2 ) was used as a measure of goodness of fit in each regression. The 95% confidence interval was estimated for parameter b, and Student’s t-test (Zar 2014) was then used to determine whether L. panamensis exhibited isometric growth (H0: b = 3, α = 0.05). After confirming homoscedasticity of slopes (parallelism) in the log-transformed data (Zar 2014), an analysis of covariance (ANCOVA) was used to determine whether the relationships between females and males showed significant differences. Statistical analyses were performed using the IBM-SPSS Statistics 20 software.
Reproductive aspects
The reproductive stage of females and males was defined after analyzing gonadal maturity data. Data drawn from developing and mature females and males were used to calculate the cumulative relative frequency by ML interval. The mean length at first maturity (ML50) was then estimated by adjusting the relative frequencies with the logistic model (Sparre and Venema 1995). The model was adjusted by maximizing the negative log likelihood (-LL):
where n is the total number of individuals in class i and m is the number of mature organisms in class i.
Confidence intervals for ML50 were estimated using the likelihood profiles and the chi-square (χ2) distribution (Venzon and Moolgavkar 1988). The confidence interval was defined as all the values of θ that satisfy the following inequality:
Where
Individuals in gonadal development stages I and II were considered immature (juvenile), and individuals in stages III and IV were considered sexually mature (adults). According to the morphochromatic maturity scale, the criterion to identify juvenile and adult females was based mainly on the color and size of the nidamental glands, as these organs grow and retain the size upon reaching sexual maturity. In males, the criterion was based on the differentiation of the reproductive system (adults), mainly the accessory sex organs (Lipiński and Underhill 1995). We determined the total sex ratio by system (coastal lagoon, CL; coastal front, CF). In each system, immature and mature organisms were contrasted by sex, and comparisons were made of mature females versus immature males and immature females versus mature males. The χ2 test was used to make comparisons and determine significant differences between sex ratios, taking as null hypothesis the sex ratio of 4 females to 1 male (4.0F:1.0M) reported by Arizmendi-Rodríguez et al. (2012a) for L. panamensis. The observed value was compared with the theoretical value of χ2 with a 95% confidence level (Zar 2014). The same statistical analysis was used to determine differences in the number of juveniles and adults in the population, by sex and by ecosystem.
Results
Size structure
A total of 2,354 individuals were captured, of which 1,144 (48.6%) were females, 677 (28.8%) were males, and 533 (22.6%) were undetermined. In general, ML sizes ranged from 9 to 125 mm (Fig. 2a). Both sexes were found across the length range; however, females were dominant at sizes >60 mm ML (80.0%) and males at sizes <60 mm ML (62.0%)
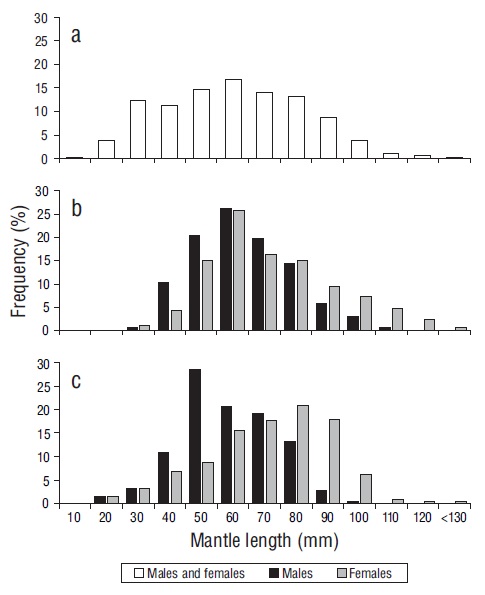
Figure 2 Mantle length frequency distribution for the Panama brief squid (Lolliguncula panamensis): (a) both sexes from coastal lagoons and coastal fronts, (b) males and females from coastal lagoons, and (c) males and females from coastal fronts.
In the lagoon systems, 667 individuals were quantified, of which 398 (59.7%) were females, 245 (36.7%) were males, and 24 (3.6%) were undetermined. Males showed ML sizes of 29 to 101 mm and a mode of 60 mm, whereas females showed ML sizes from 28 to 123 mm and a mode of 60 mm (Fig. 2b). In total, the 7 cruises (CF) caught 1,687 squid, of which 746 (44.0%) were females, 433 (26.0%) were males, and 508 (30.0%) were undetermined. In CFs, males showed ML sizes from 11 to 94 mm of and a mode of 50 mm, whereas females showed ML sizes from 11 to 125 mm and a mode of 80 mm (Fig. 2c). The Kruskal-Wallis analysis showed that there were no differences in male (H = 0.1, P = 0.6) or female (H = 2.5, P = 0.1) MLs between ecosystems.
Biometric relationships
The ML/TW relationship for both sexes showed an exponential relationship (R 2 > 0.95) (Table 1, Fig. 3). The values of slope b were in the range of 2.50 to 2.59, which indicates negative allometric growth in the 3 cases (P < 0.05, Table 1). The ANCOVA showed significant differences in the ML/TW relationship between females and males (P < 0.05) (Table 1).
Table 1 Summary of the mantle length vs total weight regression values for females, males, and both sexes of Lolliguncula panamensis. The F and P values from the analysis of covariance (ANCOVA) are shown. CI, confidence interval.
| Sex | a | b ± CI 95% | R2 | P for t test (b) | F (ANCOVA) | P (ANCOVA) |
| Females | 0.000473 | 2.59 ± 0.07 | 0.984 | 0.002 | ||
| Males | 0.000690 | 2.50 ± 0.20 | 0.951 | 0.002 | ||
| Both | 0.000464 | 2.59 ± 0.08 | 0.967 | 0.001 | 4.050 | 0.006 |
Reproductive aspects
Of the 2,354 L. panamensis individuals analyzed, 750 were mature. With the model, a population ML50 of 68.3 mm ML was estimated (Fig. 4a), and the likelihood profiles indicated that individuals attain sexual maturity between 64.6 and 72.2 mm ML (Fig. 4b). Males attain ML50 at 64.0 mm ML (Fig. 4c), and the profiles showed that they can mature at ML sizes between 62.8 and 64.6 mm (Fig. 4d). Females mature at 71.4 mm ML (Fig. 4e), and the profiles indicated that they can mature at ML sizes between 70.4 and 72.4 mm (Fig. 4f).

Figure 4 Mean size at first maturity (ML50) and likelihood profiles for Lolliguncula panamensis: (a, b) population, (c, d) males (♂), and (e, f) females (♀).
Gonad stage frequencies showed that, in CLs the immature stages (I and II) were the most frequent in both sexes (males, 44.5% and 41.6%; females, 61.3% and 20.9%, respectively), with a smaller proportion of males and females in the mature stages (III and IV) (Fig. 5a, Table 2). In CF both sexes had the smallest proportion of immature organisms, with a large proportion of males and females (49.0% and 58.0%, respectively) in the mature stage (III) (Fig. 5b, Table 2).
Table 2 Sex ratio for the Panama brief squid by combination of gonadal stages: general (G), coastal front (CF), coastal lagoon (CL), CF and immature individuals (CFINM), CF and mature individuals (CFMAT), CF and mature females and immature males (CFMFIM), CF and immature females and mature males (CFIFMM), CL and immature individuals (CLINM), CL and mature individuals (CLMAT), CL and mature females and immature males (CLMFIM), and CL and immature females and mature males (CLIFMM).
| Comparison | Females | Males | Probability | Ratio (F:M) |
| G | 1,144 | 677 | <0.05* | 1.7 |
| CF | 746 | 433 | <0.05* | 1.7 |
| CL | 398 | 245 | <0.05* | 1.6 |
| CFINM | 308 | 221 | <0.05* | 1.4 |
| CFMAT | 438 | 212 | <0.05* | 2.1 |
| CFMFIM | 438 | 221 | <0.05* | 2.0 |
| CFIFMM | 308 | 212 | <0.05* | 1.5 |
| CLINM | 327 | 211 | <0.05* | 1.5 |
| CLMAT | 71 | 34 | <0.05* | 2.1 |
| CLMFIM | 71 | 211 | <0.05* | 0.3 |
| CLIFMM | 327 | 34 | <0.05* | 9.6 |
*Significant difference
Sex ratio, juveniles, and adults
The sex ratio observed during the study period was 1.7F:1.0M, which is significantly different (χ2 = 319.6, P < 0.05) when compared with the hypothetical value of 4.0F:1.0M (Arizmendi-Rodríguez et al. 2012b). Comparisons by system, sex, and gonadal stages showed differences in most of the combinations (P < 0.05). In general, females were more abundant than males, but when comparing mature females against immature males in CLs (0.3F:1.0M), immature males were more abundant (Table 2). Abundances of juveniles and adults by system are shown in Table 3. The χ2 test showed that juveniles were dominant in relation to adults in CLs, both for the population as a whole (χ2 = 39.3, P < 0.05) and for females (χ2 = 28.2, P < 0.05) and males (χ2 = 11.5, P < 0.05) separately. In CF, differences were observed for the population (χ2 = 44.9, P < 0.05), females (χ2 = 6.9, P < 0.05), and males (χ2 = 57.8, P < 0.05). Therefore, the number of juveniles in both ecosystems is significantly higher than the number of adults.
Discussion
Squid of the family Loliginidae are found in tropical, temperate, and subpolar waters, mainly on the continental margins (Brakoniecki 1986, Vecchione and Young 1998, Anderson 2000, Jereb et al. 2010, Alejo-Plata et al. 2015). There are 2 species of loliginids reported to inhabit estuaries, coastal lagoons, and bays: Lolliguncula brevis and Uroteuthis noctiluca (Jereb et al. 2010). The present study reports the first record of L. panamensis in the lagoon systems of the Gulf of California (GC). In its known distribution range, L. panamensis has been documented in marine waters between 7 and 180 m depth (Fischer et al. 1995, Arizmendi-Rodríguez et al. 2012b). Considering that L. panamensis is a resident species in the GC, it is pertinent to analyze its importance in the different systems within the gulf, such as CFs and CLs, by defining and understanding its biological processes.
In the present study, the number of L. panamensis individuals found in CFs (n = 1,687) was higher than that found in CLs (n = 667). This difference can be attributed to a higher sampling effort, and comparing abundances between systems is therefore meaningless. In the CFs, where the number of sets was higher, surveys were carried out in August 2014; May, November, and December 2015; May, June, and October 2016; and May 2017. On the other hand, in CLs, the sets were made in May 2014; March, April, and July 2015; and April, May, and June 2016. Three cruises used trawling nets and 1 cruise used both beach seine nets and jigs. The Kruskal-Wallis analysis indicated that there were no differences in MLs between fishing gears. Different fishing gears allowed us to record ML sizes from 9 to 125 mm; for the Panama brief squid, previous works had reported individuals with ML sizes from 29 to 120 mm (Arizmendi-Rodríguez et al. 2012b). If we were to make comparisons between the fishing gears used in this work, we would detect a bias in the sizes of the organisms, the number of individuals by sex, and the gonadal maturity stages because of gear selectivity.
The presence of L. panamensis in CLs suggests that the species is a plastic organism, meaning that it has the ability to adapt to environmental changes, which has been reported for other squids (Pecl et al. 2004, Nevárez-Martínez et al. 2006). In the lagoon systems where the squid were collected, temperature ranges between 14.2 and 33.9 ºC and salinity ranges between 26.8 and 42.3 (Romero-Sedano et al. 2004, Padilla-Serrato et al. 2017, Ruiz-Ruiz 2017). Therefore, we can ponder that the temperature and salinity ranges tolerated by L. panamensis are broader than those previously reported (temperatures of 21-27 ºC and salinities of 15-23) (Jereb et al. 2010, Arizmendi-Rodríguez et al. 2012b). In general salinity, temperature, and depth are associated with squid migratory processes (Herke and Foltz 2002). However, their abundance and spatial distribution in estuaries (coastal lagoons) are strongly related to high salinities and habitat structure (Rodrigues and Gasalla 2008). This would explain the presence of L. panamensis in Sonora lagoons, where high salinities, reported most of the year, create suitable conditions for this species to inhabit these ecosystems.
Size structure
In loliginid squids like Loligo gahi (d’Orbigny, 1835), Loligo sanpaulensis (Brakoniecki, 1984), and Loligo plei (Blainville, 1823), size structure differences between females and males have been considered a form of sexual dimorphism (Pineda et al. 1998b, Alvarez-Perez et al. 2002), with males reaching larger sizes than females. In our study we found that females are the ones that reach the larger sizes. This difference is attributed to the fact that females maximize their reproductive success (ability to develop and store oocytes) at larger sizes, whereas males obtain success at shorter lengths, since they can transfer spermatophores to several females (Emery et al. 2001).
Boyle and Rodhouse (2005) attributed size differences between sexes to the fact that females need to be larger for ovary development during sexual maturity. The present study confirms the same behavior in L. panamensis, where females reached maturity at 71.4 mm ML, and males at 64.0 mm ML. Pineda et al. (1998a) observed that for Loligo sanpaulensis gonadal maturity in males increases as they grow but maturation in females is slow and changes abruptly with respect to growth upon reaching 60 mm ML. Collins et al. (1995) associated size differences between sexes in loliginid squid with a differential distribution during the reproductive period or with the mortality of females after spawning and of males after copulation.
Biometric relationships
The ML-TW relationship between L. panamenis females and males was significantly different. This difference is a response to the smaller sizes of males, the low number of individuals measuring >60 mm ML, and the heavier weight of females as their ovaries make up 50% of their weight (Arizmendi-Rodríguez 2010). The negative allometry observed in L. panamenis indicates that it first increases its size in length and then in weight. Squires and Barragan (1979) observed this type of growth in L. panamenis on the Pacific coast of Colombia, with values of b = 2.72 for females and b = 2.69 for males, and they emphasized that this coefficient is higher in females because of the larger size of its reproductive apparatus compared with that of males. In the present study females showed a value of b = 2.59 and males a value of b = 2.50; allometric coefficient values under 2.70 are characteristic of neritic squids of the family Lolliginade and are associated with their migratory patterns in the water column (Flores and Garland 2002). Differences in b values between sexes are associated with size at maturity and the maturation process, which was observed for L. panamenis on the Pacific coast of southern Mexico (Guzmán-Intzin et al. 2020).
Reproductive aspects
The pattern in ML50 by sex observed in the present study indicated that females reach maturity at larger lengths (71.4 mm ML) than males (64.0 mm ML), which is consistent with that reported by Arizmendi-Rodríguez et al. (2012a). Alejo-Plata et al. (2016) also reported that mature L. argus males were smaller than females. The larger the ML50, the greater the capacity of females to store oocytes (Arizmendi-Rodríguez et al. 2012a); in addition, the ability of females to store spermatophores at immature stages increases their reproductive success (Hanlon and Messenger 1996), and this has a positive impact on the reproductive potential of the population (Arizmendi-Rodríguez et al. 2012a).
The difference in ML50 between sexes allows males to copulate with several females for longer periods (Boyle and Rodhouse 2005). In this study, the smallest mature male measured 40 mm ML and 64 mm ML50, indicating that males well below the ML50 were already copulating. Copulation leads to physiological wear, which causes males to die because they are unable to hunt their prey (Arizmendi-Rodríguez et al. 2012b).
In CLs, individuals of both sexes were immature. In CFs, mature individuals were predominant and females were the most abundant. Our results suggest that individuals carry on their reproductive events (i.e., courtship, mating, or spawning) mainly in CFs, whereas they possibly shelter and grow in CLs. This behavior is consistent with that reported by Roper et al. (1995), who mentioned that mature Ommastrephes bartramii females migrate to coastal waters for reproduction. Giese and Pearse (1974) mentioned that when choosing the spawning season, organisms consider food availability and favorable abiotic conditions (light, temperature). Mature Panama brief squid individuals have been documented to make latitudinal migrations in the GC based on food availability (Arizmendi-Rodríguez et al. 2011). Furthermore, it is known that CFs provide protection from predators and are suitable for the fixing of egg capsules released by females when spawning (Sevilla 1977). On the other hand, Loligo sanpaulensis has been reported to make northward migrations along the Brazilian shelf in search of spawning, breeding, and feeding areas, which are influenced by the Brazil and Malvinas Currents (Andriguetto and Haimovici 1996). Also, reports on the presence of Loligo forbesi egg masses at 507 m depth suggests that the species makes sporadic migrations to the oceanic zone to spawn or that currents detach and drag its eggs to such depths (Lordan and Cassey 1999).
The sex ratio found in the present study (1.7F:1.0M) is similar to that reported for Loligo plei by Alvarez-Perez et al. (2002), who attributed the ratio to a copulation event at the time females were spawning or to female migration too. Considering the hypothesis made by these authors, female Panama brief squid not only migrate to copulate, but they also move to other areas for protection, growth, and feeding, events in which the sex ratio is close to 1.0F:1.0 M. This migratory behavior is related to the reproductive potential at the population level, which includes aspects such as ovarian development, maturity size, and sex ratio (Saborido 2004). Arizmendi-Rodríguez et al. (2012a) reported predominance of females over males (4.0F:1.0M) and associated this with the fact that a single male can transfer spermatophores to up to 4 females; however, the results in our study show that the sex ratio in the Panama brief squid inhabiting the CFs and CLs in the GC may vary depending on the event performed by the squid (feeding, growing, seeking shelter, and reproducing) in such a way that it optimizes success in its life cycle.
In our study, a series of combinations were analyzed, and in no case was the ratio 1.0F:1.0M. Females always predominated over males except in the combination of mature females vs immature males in CLs (0.3F:1.0M). The low presence of mature females suggests that females do not inhabit CLs when they are sexually mature; they are found spawning in CFs. In Loligo vulgaris reynauddi, females have been observed to migrate to specific areas in CFs before and after spawning (Sauer et al. 1992). On the other hand, with the combination of immature females vs mature males in CLs, the sex ratio was 9.6F:1.0M, which would indicate that mature males in CLs can transfer spermatophores to up to 9 immature females. This reproductive tactic has been reported by Emery et al. (2001), who performed genetic tests on Loligo forbesi and found that females had been implanted with spermatophores by up to 4 males; this allowed the authors to prove that the female-biased sex ratio is normal reproductive behavior and that the reproductive success is defined by females.
The presence of mature males and immature females in CLs indicates that males only transfer sperm packets to females at these sites and that females later migrate to CFs to mature and spawn as they are capable of storing sperm when they are still immature, a strategy for the reproductive success of the population (Hanlon and Messenger 1996, Pineda et al. 1998a, Boyle and Rodhouse 2005). According to our results, juveniles predominated in CLs and adults in CFs, so we believe that L. panamensis can migrate between both ecosystems as juveniles and adults. This is the first study that documents the presence of L. panamensis mainly in the CLs of the GC continental margin, with predominance of juvenile males and females.











 text in
text in 




