INTRODUCTION
Coral reefs are ecosystems consisting of calcium carbonate (CaCO3) structures, primarily found in tropical and subtropical seas (Glynn 1997, Sheppard et al. 2010). They are characterized as feeding, reproduction, and shelter sites for many marine fish and invertebrate species and host more than 25% of total biodiversity in the oceans (Sheppard et al. 2010). Hermatypic corals are considered the main reef-building corals, which deposit CaCO3 through calcification to form their exoskeleton, thereby contributing to coral reef development and maintenance (Allemand et al. 2004, 2011). Together with other calcifying organisms, they form the structural framework of this habitat and of its underlying biological and ecological processes (Sheppard et al. 2010).
Reef accretion is antagonized by bioeroders, which remove CaCO3. Bioerosion is mediated by different factors, such as (1) environmental or physical characteristics of the site or locality (extrinsic factors) and (2) specific traits related to the composition of species, their growth strategies, and specific associations with other organisms (intrinsic factors) (Glynn 2000, Carballo et al. 2008, Tribollet and Golubic 2011). Together, these factors promote a constant remodeling of the physical structure of coral reefs through the balance between accretion and erosion (net carbonate production) (Wulff and Buss 1979, Andersson and Gledhill 2013). In turn, erosion in coral reef environments can result from different mechanisms: (1) chemical erosion involving CaCO3 dissolution, mainly caused by natural processes (metabolic and environmental dissolution) and by ocean acidification (Kleypas et al. 1999, Andersson and Gledhill 2013, Eyre et al. 2014); (2) physical erosion due to mechanical movements caused by waves, currents, storms, and other agents (Zundelevich et al. 2007); and (3) biological erosion or bioerosion, resulting from the biological activity, feeding habits, and sheltering of organisms such as fishes, sponges, echinoderms, and invertebrates associated with coral reefs (Glynn 1997, Reyes-Bonilla and Calderón-Aguilera 1999, Tribollet and Golubic 2011). Bioerosion can result directly or indirectly from the removal of CaCO3, either by the production of acidic compounds (acidification) or by the excretion of enzymes by organisms such as sponges (biocorrosion) (Neumann 1966), as well as from bioabrasion due to mechanical erosion caused by boring organisms, such as annelids, barnacles, and bivalves, that can wear away and pierce calcareous materials (Glynn 1997, Carballo et al. 2008, Maher et al. 2018). Bioabrasion is the mechanism that causes the highest production of carbonate sediments (rubble, sand, silt, and clay) in reef systems (Glynn 1997, Eyre et al. 2014). All erosion processes shape reefs and contribute to the carbonate cycle because inorganic carbonate is reintegrated into the environment and becomes available again for calcifying organisms (Carballo et al. 2008, Tribollet and Golubic 2011).
Bioerosion can be assessed by estimating the bioerosion volume (by measuring the density of calcium carbonate removed) and the bioerosion rate, which expresses the amount of CaCO3 removed in a period of time (DeCarlo et al. 2015). In turn, skeletal density can be determined using the buoyant weight, paraffin embedding (Manzello 2010), growth-band fragment freezing (Carricart-Ganivetet al. 2000), and X-ray radiography or computed tomography (Hein and Risk 1975, Prouty et al. 2017, Wizemann et al. 2018).
The Eastern Tropical Pacific (ETP) region typically hosts coral reefs or reef patches distributed along the coast and on adjacent oceanic islands (Reyes-Bonilla and Calderon-Aguilera 1999, Glynn and Ault 2000). These reef patches are considered monospecific because they are dominated by the genus Pocillopora in shallow waters, with Porites and Pavona in deeper areas (Reyes-Bonilla 2005). In particular, the Pacific waters off the central Mexican coast (Pacific coast of central Mexico: PCM) comprise an area with high species richness of hermatypic corals. These species, through their continuous accretion, maintain the physical structure of coral communities, which can vary due to factors such as species composition, taxon morphology, sex, colony age, and local/regional conditions (Cabral-Tena et al. 2013; Norzagaray-López et al. 2014; Tortolero-Langarica et al. 2016a, 2016b, 2017). Studies have quantified the amount of CaCO3 produced by corals in this region (Norzagaray-López et al. 2014, Cabral-Tena et al. 2018, Tortolero-Langarica et al. 2019) but only a few have evaluated the rate of removal of CaCO3 and the factors that affect bioerosion in coral communities (Reyes-Bonilla and Calderón-Aguilera 1999, Herrera-Escalante et al. 2005). Both factors are crucial for understanding the functioning and conservation of these communities. Therefore, the aim of this study was to determine the specific variability of internal bioerosion and the effect of intrinsic (e.g., morphology, sex, age) and extrinsic (e.g., depth and locality) factors on massive corals growing in PCM waters. Knowing the amount of CaCO3 that remains in the system (net production), by balancing reef building (production rate) and removal (bioerosion rate, CaCO3 dissolution), enables us to evaluate the functionality and ecological resilience of coral communities (Perry et al. 2012, Alvarez-Filip et al. 2009).
MATERIALS AND METHODS
Study area
The study was conducted in 2 island areas in PCM waters: the Isla Isabel National Park (Parque Nacional Isla Isabel: PNII) and the Islas Marietas National Park (Parque Nacional Islas Marietas: PNIM) (Fig. 1). PNII is located 30 km off the coast, and the total area of the island, including the surrounding islets, is 82.16 ha (CONANP 2005). PNIM comprises an area of 1,383 ha consisting of 2 islands (Larga Island, Redonda Island), 2 volcanic islets close to the Larga Island, and a marine area (CONANP 2007). Both natural areas harbor fringing and rocky reef patches, with corals of the genera Pocillopora, Pavona, and Porites (Fig. 2). This set of islands is located between the Nearctic and Neotropical biogeographic regions of the ETP (Glynn and Ault 2000), a transition zone affected by the following: the California Current, which carries cold water from the north (18-21 ºC) from January to March (Kessler 2006, Pennington et al. 2006, Pantoja et al. 2012); the Mexican Coastal Current, which brings warm water from the south (27-30 ºC) from July to November; the Gulf of California water mass, which carries warm water (>34.9 ºC) from September to October (Kessler 2006, Pennington et al. 2006, Palacios-Hernández et al. 2010, Pantoja et al. 2012).
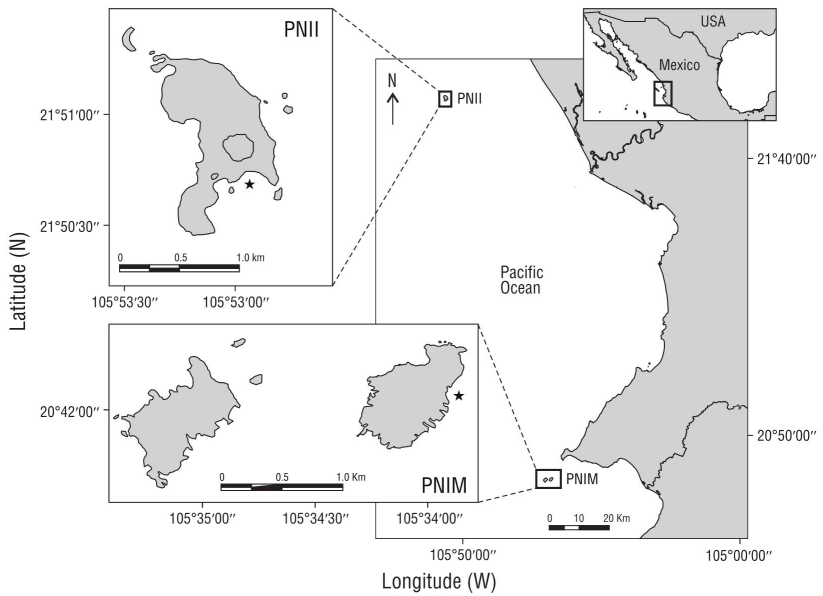
Figure 1 Study area. Parque Nacional Isla Isabel (PNII) and Parque Nacional Islas Marietas (PNIM) in Nayarit, Mexico. The star denotes the sampling sites.
Sample processing
From February 2013 to February 2014, a total of 39 colonies were collected from the massive corals Porites lobata (n = 26), Porites panamensis (n = 5), and Pavona gigantea (n = 8). The colonies were extracted with a hammer and chisel using scuba diving equipment. They were transferred to the laboratory, where the organic material was removed with pressurized water. Subsequently, the colonies were dried and exposed to sunlight for 72 h and then placed in a conventional oven at 70 ºC for 3 h. All specimens were sectioned on the main vertical (apical) growth axis into 7-10 mm thick slabs with a diamond-bladed (QEP) tile saw using fresh water as lubricant.
Bioerosion parameters
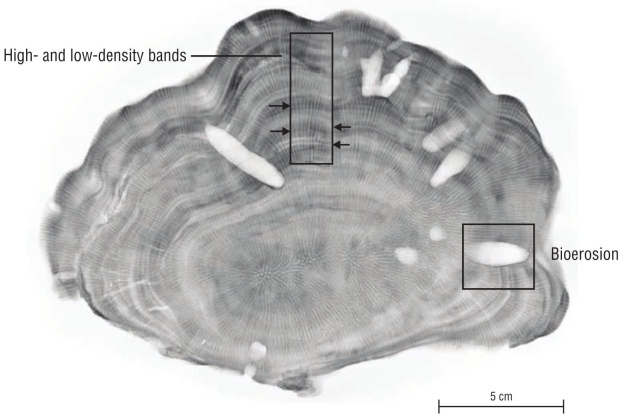
To determine the amount of CaCO3 removed internally (bioerosion volume), the Archimedes’ principle (buoyant weight, displaced volume) was applied using the method described by Bucher et al. (1998). First, the dry weight of each coral slab was assessed using a precision balance (ADAM, accuracy = ±0.01 g). Subsequently, the buoyancy or wet weight was calculated. For this purpose, the samples were previously saturated in distilled water with a density of 1 g·cm-3 at a constant temperature (20 ºC) for 12 h and then suspended in a pre-weighed plastic container with water. The samples were dried again (70 ºC, for 3 h) and saturated with low-temperature silicone (ethylene-vinyl acetate, MOD.BS7T) only at the visible bioerosion marks (cavities, Fig. 3), at a melting temperature of 84-87 ºC. After solidifying, the remaining silicone was removed just at the edge of the thickness of each coral slab. Lastly, these silicone-saturated samples were measured again to assess their saturated dry weight and saturated buoyant weight. The skeletal density was calculated with both measurements (g·cm-3), and the percentage of bioerosion (%) and bioerosion volume (cm3) of each sample were calculated using the following equations:
Where Ve is the bioerosion volume, Vs is the slab volume, Vss is the saturated slab volume, WS is the slab dry weight, WB is the slab wet weight, WSs is the saturated slab dry weight, WBs is the saturated slab wet weight, D is the skeletal density, and %e is the percentage of bioerosion.
Intrinsic factors
Morphotype
To assess the effect of morphology on bioerosion rate, the different coral colonies were grouped according to their type and growth class (massive, encrusting, columnar, and free-living), considering the morphological characteristics and skeletal structures described by Veron (2000).
Age assessment
Each coral slab was radiographed using a General Electric conventional X-ray machine (Hungay Rt. MedicalSystems), setting the exposure to 70 kv and 20 mAs from anode to cathode, at a distance of 2 m between the irradiation source and the coral slabs. The resulting radiograph images were analyzed using the image processing software ImageJ (v.1.46, http://rsb.info.nih.gov/ij/). To collect data on age, alternating high- and low-density band values were used, which together represent an annual period of growth (Lough and Cooper 2011, Tortolero-Langarica et al. 2016b) (Fig. 3). As described by Darke and Barnes (1993), and considering the half-life of Porites spp. (~5 years) polyps, the coral colonies were classified into 3 stages: juvenile (1-5 years), intermediate (6-9 years), and adult (≥10 years).
Sex identification
When sampling each colony, a fragment of approximately 1 cm2 was collected. This fragment was then fixed in 10% formaldehyde in sea water and stored at room temperature until histological processing. Each fragment was individually decalcified with 10% HCl mixed with a buffer (0.7 g EDTA, 0.14 g sodium tartrate, 0.008 g potassium sodium tetrahydrate); the resulting tissue was rinsed under running water and preserved in 70% ethanol. Subsequently, the tissue samples were dehydrated using a series of 10 ethanol solutions (70% to 100% v/v ethanol), with 2 xylene steps. After embedding them in blocks with Paraplast X-tra, they were cut into 8-µm-thick sections with a semi-automatic Leica microtome. These preparations were then stained using Masson’s trichrome staining protocol (Humanson 1967). Sex was determined based on evidence of oocytes (female), sperm (male), or both gametes (hermaphrodites) using a LABO JAZANZ compound microscope, as described by Rodríguez-Troncoso et al. (2011).
Extrinsic factors
Depth and locality
To assess the effect of depth on bioerosion volume and percentage in coral species, colony depth was determined and classified according to the vertical distribution gradient (5-15 m). For this purpose, colonies at depths of 5-10 m were considered shallow water colonies, and those found at depths of 11-15 m were considered deep-water colonies. All samples were grouped by depth and sampling site (PNII and PNIM).
Statistical analysis
The mean (±standard deviation) of each bioerosion and skeletal density parameter was calculated using the means for each colony (~1-2 slabs per colony), along with the assessment of normality (Shapiro-Wilk test, P < 0.05) and homoscedasticity (Levene’s test, P < 0.05) of each dataset (i.e., bioerosion parameters). Bioerosion values were compared between treatment levels (species, morphology, and age) by fixed-effects 3-way analysis of variance (ANOVA) (without interaction). In addition, 2-way ANOVA was used to determine differences in bioerosion between extrinsic factors (depth and locality). Each dataset with significant differences was evaluated a posteriori using the Dunn-Bonferroni multiple comparison test (P < 0.05). Furthermore, Student’s t-test was performed to determine bioerosion differences between males and females, in addition to simple linear regression analysis, for assessing the correlation (Pearson product-moment correlation coefficient: Pearson’s R) and determination (R 2) coefficients to evaluate the relationship between bioerosion and skeletal density values. All statistical tests were evaluated at 95% minimum confidence interval (α = 0.05) using RStudio (×64, v.3.3.2) and the software InfoStat/L.
RESULTS
A total of 55 coral slabs were analyzed, yielding a bioerosion volume of 36.10 ± 30.83 cm3 and a percentage bioerosion of 20.86 ± 18.96%. At the species level, P. gigantea had the highest bioerosion at 71.31 ± 32.35 cm3 (27.28 ± 18.05%), with significant differences (P < 0.001, Table 1) in bioerosion volume between species (Fig. 4). By morphotype (columnar, free-living, massive, and encrusting), massive corals had the highest bioerosion volume (64.02 ± 31.66 cm3), whereas the columnar corals had the highest mean percentage of bioerosion (44.11 ± 13.30%) (Table 1). Comparison analysis showed significant (P < 0.001, Table 2) differences in both volume and percentage between morphotypes, mainly resulting from massive colonies (Dunn-Bonferroni, P < 0.001).
Table 1 Descriptive bioerosion parameters by species and morphology: volume, percentage, and skeletal density. The number of colonies (n) and mean values (± standard deviation) are shown.
| Species/Morphotype | n | Volume eroded (cm3) |
Percent eroded (%) |
Skeletal density (g∙cm-3) |
| Pavona gigantea | 8 | 71.33 ± 32.35 | 27.45 ± 18.05 | 1.61 ± 0.22 |
| Columnar | 2 | 89.30 ± 0.01 | 31.08 ± 0.01 | 1.27 ± 0.02 |
| Massive | 6 | 68.74 ± 34.05 | 26.94 ± 19.43 | 1.65 ± 0.21 |
| Porites lobata | 26 | 26.60 ± 24.87 | 16.87 ± 16.31 | 1.32 ± 0.10 |
| Columnar | 4 | 34.35 ± 13.49 | 47.37 ± 12.84 | 1.29 ± 0.11 |
| Massive | 4 | 55.75 ± 29.66 | 20.83 ± 11.31 | 1.42 ± 0.13 |
| Free-living | 17 | 18.41 ± 20.90 | 9.21 ± 7.39 | 1.32 ± 0.09 |
| Porites panamensis | 5 | 29.60 ± 14.61 | 31.13 ± 29.43 | 1.43 ± 0.80 |
| Encrusting | 5 | 29.60 ± 14.61 | 31.13 ± 29.43 | 1.43 ± 0.80 |
Table 2 Results of the 3- and 2-way analyses of variance for the parameters bioerosion and skeletal density of massive corals, at the intrinsic and extrinsic levels. Values in bold indicate significant differences.
| Volume eroded | Percent eroded | Skeletal density | ||||||||||||
| Source | d.f. | MS | F | P | d.f. | MS | F | P | d.f. | MS | F | P | ||
| Intrinsic | ||||||||||||||
| Species | 2 | 9,364.400 | 18.022 | <0.001 | 2 | 1,308.528 | 5.634 | 0.008 | 2 | 13.012 | 469.800 | <0.001 | ||
| Morphotype | 3 | 16,294.570 | 31.358 | <0.001 | 3 | 6,738.094 | 29.014 | <0.001 | 3 | 16.128 | 582.316 | <0.001 | ||
| Age | 2 | 1,432.840 | 2.757 | 0.079 | 2 | 201.201 | 0.866 | 0.430 | 2 | 0.017 | 0.615 | 0.547 | ||
| Extrinsic | ||||||||||||||
| Depth | 1 | 152.816 | 0.065 | 0.800 | 1 | 3,868.128 | 18.410 | <0.001 | 1 | 5.629 | 3.070 | 0.088 | ||
| Locality | 1 | 22.404 | 0.010 | 0.923 | 1 | 141.701 | 0.674 | 0.417 | 1 | 1.862 | 1.016 | 0.320 | ||
MS, mean square.
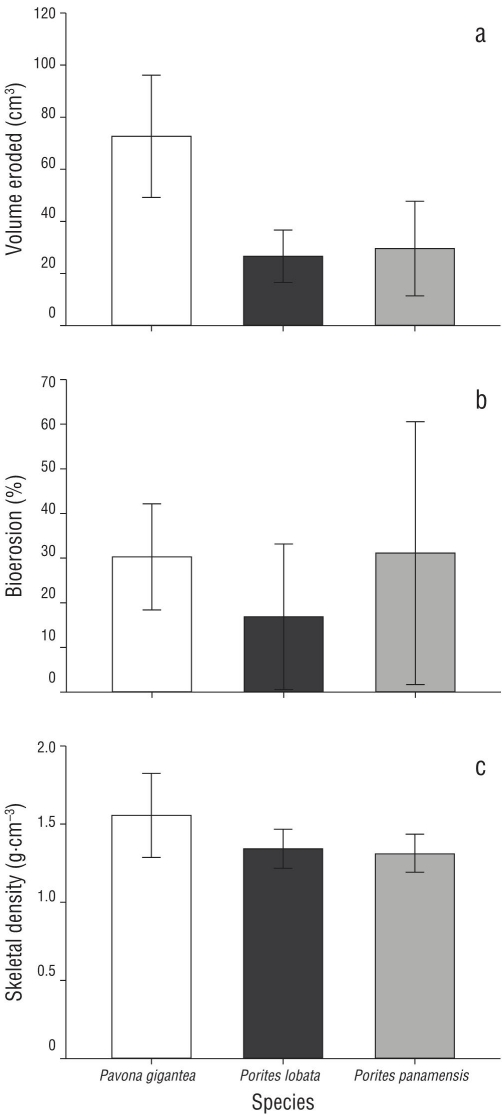
Figure 4 Parameters of bioerosion in massive corals (mean ± standard deviation). (a) Volume of CaCO3 removed (cm3), (b) percentage of bioerosion (%), and (c) skeletal density (g·cm-3) in Porites lobata, Pavona gigantea, and Porites panamensis.
A total of 12 males and 5 females were analyzed histologically. The analysis of bioerosion parameters between sexes showed no significant differences in bioerosion volume (t = -0.044, P = 0.483) or percentage (t = 0.076, P = 0.530). By age, adult colonies (n = 5) had higher bioerosion values (61.56 ± 35.38 cm3) than the juvenile (n = 16, 20.54 ± 16.54 cm3) and intermediate (n = 17, 44.82 ± 34.80 cm3) age groups, albeit with no significant differences between age groups (P > 0.05, Table 2).
The data grouped by depth level showed significant differences in the percentage of bioerosion (P < 0.001, Table 2) between deep- (31.10 ± 23.29%) and shallow-water (16.84 ± 15.81%) colonies. Conversely, the site (PNII and PNIM) had no significant effect on the volume or percentage of bioerosion in massive corals (P > 0.05, Table 2).
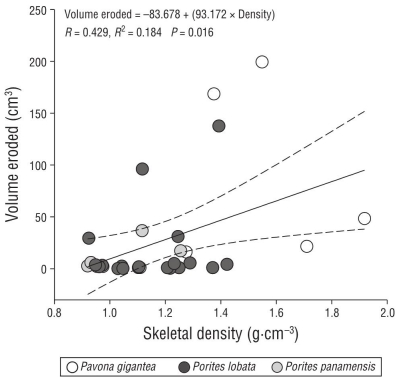
In contrast to the bioerosion results, significant differences in density were found between species and between morphologies (P < 0.05, Table 2). At the species level, P. gigantea had a higher density (1.61 ± 0.22 g·cm-3) than Po. lobata (1.32 ± 0.10 g·cm-3) and Po. panamensis (1.43 ± 0.80 g·cm-3). Considering the type of growth, the massive colonies had higher densities (1.54 ± 0.24 g·cm-3) than the other morphotypes (Table 1). The results showed a positive association between the volume of CaCO3 removed and the skeletal density of the massive coral species (Fig. 5).
DISCUSSION
Reef communities in the eastern Pacific have been historically affected by various stressors, both natural and anthropic, which have decreased their growth rate (Manzello et al. 2008, Manzello 2010, Tortolero-Langarica et al. 2017) and CaCO3 production, thereby causing a loss of the community structure (Glynn 2001, Cabral-Tena et al. 2018). Similarly, given the presence of internal bioerosion, this study reveals the high susceptibility of the massive coral species of this region, such as Po. lobata, Po. panamensis, and P. gigantea, which show high variability in CaCO3 loss by bioerosion, primarily due to intrinsic (e.g., species and morphology) and extrinsic (e.g., depth) factors (Table 1).
In a global context, bioerosion rates of ~25-40 kg CaCO3·m-2·y-1 have been reported for coral reefs and are mainly caused by epilithic organisms (Glynn 1997, Pari et al. 1998), and 7% to 28% carbonate has been removed by endolithic species in massive corals (Hein and Risk 1975, Scott and Risk 1988, Hernández-Ballesteros et al. 2013). In this context, the results of this study show high percentages of CaCO3 (24%-44%) having been removed from massive coral species (Fig. 4), which indicates that the massive coral communities of the ETP show a higher level of bioerosion than other coral communities worldwide (Hein and Risk 1975, Scott and Risk 1988). Several studies relate the high bioerosion observed in the ETP with mesoscale and regional processes (e.g., upwellings, El Niño-Southern Oscillation events) that generate more acidic conditions (low pH values) (Manzello 2010, Wizemann et al. 2018). This correlation could be a proxy for future climate change scenarios and the possible volumes of bioerosion expected for coral reefs worldwide (Wizemann et al. 2018). However, this correlation remains a hypothesis requiring further research.
The percentages of internal bioerosion found in this study (20.86 ± 18.96%) are similar to those reported by Herrera-Escalante et al. (2005), with external erosion rates caused by Diadema mexicanum of 16%-27% in Mexican Pacific waters, albeit slightly higher than those observed by Norzagaray-López (2010) in the Marietas Islands (13.9%) and in Cabo Pulmo (15.5%). The variation between localities could be due to variations in specific environmental conditions and in the species composition and abundance at each site because Po. lobata and P. gigantea have the highest living coral cover in PNII and PNIM, respectively (Cupul-Magaña and Rodríguez-Troncoso 2017). Similarly, at the species level, P. gigantea colonies have a higher bioerosion volume (71.33 cm3) than Po. lobata (26.60 cm3) and Po. panamensis (29.60 cm3) colonies. Concurrently, P. gigantea had a higher skeletal density than the other species (Medellín-Maldonado et al. 2016, Tortolero-Langarica et al. 2017). Linear regression analysis showed the strong association between density and volume of CaCO3 removed (Fig. 5) and could explain the higher abundance of endolithic bioeroders in corals with higher skeletal density, as well as the consequent higher bioerosion rate, which supports the hypothesis that denser massive corals provide a more resilient shelter to bioeroders (Highsmith et al. 1983, Hernández-Ballesteros et al. 2013). Therefore, bioerosion variability between species is related to their structural and morphological characteristics (Table 1) as a growth strategy because they can help mitigate the erosion rate (Carricart-Ganivet 2007, Hernández-Ballesteros et al. 2013, Tortolero-Langarica et al. 2016a).
By morphology, massive corals have greater bioerosion in both volume (64.02 cm3) and percentage (44.11%) of CaCO3 removed than corals with other morphologies (Table 1). The bioerosion and calcification rates in hermatypic corals have been reported to differ with their growth strategy (Norzagaray-López et al. 2014, Tortolero-Langarica et al. 2016a) because different morphologies (massive, encrusting, columnar, and free-living) of colonies of Pavona and Porites are differentially prone to colonization by endolithic bioeroders. Encrusting colonies of Porites grow more “planarly” than columnar or massive species, which have a greater exposed basal surface (Fig. 2). Conversely, free-living colonies periodically expose the calcium skeleton, thus allowing the colonization of erosive organisms, which cause lesions that allow endolithic bioeroders to settle. These phenomena explain the high bioerosion observed in the basal section of P. gigantea colonies and the effect of bioeroders on their weakening, which increases the likelihood of coral framework collapse (Fang et al. 2013, Manzello et al. 2014). Age data show that adult colonies have a higher volume of bioerosion than juvenile or intermediate-aged colonies given the time of exposure to bioerosion activity. By sex, the results of this study did not show significant differences in bioerosion volume between male and female colonies, unlike the results for growth and calcification (Cabral-Tena et al. 2013; Tortolero-Langarica et al. 2016a, 2017). On the other hand, depth was an intrinsic factor determining differences in CaCO3 removal, in both volume and percentage, for Porites and Pavona, as removal was higher in deep-water colonies. The combined effect of increased nutrients and lower temperatures produced by seasonal upwelling prompts favorable conditions for the settlement and development of bioeroders in the ETP (Wizemann et al. 2018).
The bioerosion values found in this study suggest that under high acidification scenarios, erosion could exceed the potential calcification ranges reported for coral reefs in PCM waters (Cabral-Tena et al. 2018). Therefore, if bioerosion continues on the rise, it could pose a risk to the formation of coral reef communities in the ETP (Glynn 1997) and, as a result, threaten their ecological function. Bioerosion estimates serve as a tool and valuable indicators for assessing community health status. We recommend carrying out more studies that collectively take into account different aspects of net erosion (physical erosion, bioerosion, metabolic and environmental dissolution) (Andersson and Gledhill 2013) and their variability under climate change scenarios in order to integrate information aiming to improve management strategies for coral reef communities in the region.











 texto en
texto en