INTRODUCTION
A variety of habitats exist in coastal ecosystems that provide shelter and food to many aquatic species, and these habitats function as exchange sites between the fauna of the marine environment and that of the continental environment at different stages of their life cycle (Faunce and Serafy 2008, Kwak et al. 2015). The fish that live permanently in these habitats or that are occasional visitors have behaviors or physiological adaptations that allow them to tolerate the environmental dynamics that occur at different timescales. The distribution and abundance of these fish is determined by environmental gradients, such as salinity, temperature, and dissolved oxygen (Vega-Cendejas and Hernández-de-Santillana 2004). The marine species that use coastal lagoons as foraging, protection, and spawning areas depend on conditions imposed by temporal cycles, such as nychthemeral or seasonal cycles, where drought and precipitation patterns play a key role. Additionally, the predatory activity of solitary individuals (e.g., red snapper or common snook), the presence of species that form schools in the daytime (e.g., sardine or anchovy), and the presence of juvenile marine fish determine short-term variations in the composition of fish assemblages (Faunce and Serafy 2008, Gross et al. 2019).
The diversity of fish in coastal waters of the Gulf of Mexico has been extensively described (Arceo-Carranza and Vega-Cendejas 2009, Castillo-Rivera et al. 2011, Peralta-Meixueiro and Vega-Cendejas 2011, Bonilla-Gómez et al. 2013), but little is known regarding the variations in assemblages of juvenile marine fish and their feeding at the nychthemeral and seasonal timescales (Arceo-Carranza et al. 2010, Arceo-Carranza et al. 2013, Ayala-Pérez et al. 2014). In the Carbonera lagoon, Yucatán (Mexico), no studies on the habitat use of the fish community have been conducted on a nychthemeral scale. Thus, the objectives of the present study were to analyze the variation in fish assemblages at 2 timescales (nychthemeral and seasonal) in this coastal lagoon in southeastern Mexico and to evaluate the feeding habits of 10 species of juvenile marine fishes that inhabit this lagoon.
MATERIALS AND METHODS
Study area
The northern coast of Yucatán was declared a protected natural area in 2010 under the name of “Ciénegas y Manglares de la Costa Norte de Yucatán” [Swamps and Mangroves of the Northern Coast of Yucatán]. This area has only 3 mouths of permanent communication with the sea. One of them is the Carbonera lagoon (Fig. 1), which is a semi-closed body of water with an average depth of 30 cm (~180 cm in some water channels). The Carbonera lagoon is bordered by mangroves, primarily Rhizophora mangle and Avicennia germinans, whereas muddy plains and submerged grasses prevail at the bottom. Hydrology is influenced by semi-diurnal tidal currents and freshwater discharge through springs and seeps (Sánchez-Santillán et al. 2012).
Sampling activities
The samples were collected bimonthly from December 2010 to October 2011, which covered the 3 characteristic seasons of the region, namely dry, rainy, and “northerlies” (nortes, season characterized by cold winds) seasons. The sampling design included collection of samples every 2 h from 08:00 AM until a 24-h cycle was completed. In each collection period, the hydrological variables salinity, temperature (ºC), dissolved oxygen (mg/L), total dissolved solids (mg/L), and pH were recorded using the YSI 556 multiparameter instrument, and depth (cm) was recorded with a graduated probe. These variables were measured at 50 cm from the surface. Seine fishing was performed every 2 h in the mouth area (at a maximum depth of 1 m in the lagoon) with a 40 m-long beach seine with a half-inch mesh size, covering an area of 800 m2 (fishing permit No. DOPA/04031/310510.1940). After capture, the fish were placed on crushed ice and subsequently fixed in 10% formalin.
Laboratory procedures
At the Ecology Laboratory of the Sisal Multidisciplinary Teaching and Research Unit, National Autonomous University of Mexico (Universidad Nacional Autónoma de México), the fish were identified to the species level using specialized keys (Hoese and Moore 1998, Castro-Aguirre et al. 1999, Miller 2009). They were then counted, measured (standard length, SL; cm ± 0.1), and weighed (g ± 0.01) individually. Following identification, the specimens were deposited in the Regional Ichthyological Reference Collection of the National Autonomous University of Mexico and registered as SEMARNAT YUC-PEC-239-01-11.
For the trophic analysis, the following 10 species of marine fish that use the lagoon at the juvenile stage (Froese and Pauly 2019) and had a statistically representative sample number were selected: Archosargus rhomboidalis, Mugil cephalus, Mugil trichodon, Synodus foetens, Elops saurus, Orthopristis chrysoptera, Harengula jaguana, Harengula clupeola, Opisthonema oglinum, and Sphoeroides testudineus. The trophic analysis was based on the identification of the prey found in the stomach contents. The area (mm) and weight (g) of each prey was recorded on graph paper. Prey were grouped into the following 10 main categories: (1) microcrustaceans (Amphipoda, Isopoda, and Tanaidacea), (2) crustaceans (Penaeidae and Brachyura), (3) mollusks (Gastropoda and Bivalvia), (4) fish, (5) Polychaeta, (6) insects, (7) algae (mainly benthic algae), (8) detritus, (9) plant matter (seeds and grasses), and (10) “others”.
Quantitative analysis was performed using the relative importance index (RII) described by Cortés (1997), which combines the frequency method, the gravimetric method, and the numerical method (using area for numerical analysis).
Statistical analysis
To identify differences between fish assemblages, two-way analysis of similarity (ANOSIM) was performed using time of day (grouped into 2 categories, namely daytime and nighttime) and season as factors. The data did not show a normal distribution or to comply with the homogeneity of variances. Therefore, the nonparametric Kruskal-Wallis test (KW-H) was used to identify temporal differences between seasons and light and dark cycles in community parameters (abundance, diversity, equity, and richness), by plotting the median and quartiles for each case using the software STATISTICA 10.
To analyze diet composition and prey abundance, two-way ANOSIM was performed using the fish species and their sampling times (grouped into 2 categories, namely daytime and nighttime) as factors. RII data on each prey were used to generate a similarity matrix based on the Bray-Curtis index. The similarity percentages (SIMPER) routine was used to identify the preys that accounted for more than 50% of the dissimilarity between significant groups. Cluster analysis was performed using the Bray-Curtis similarity index, wherein the clustering method was the average cluster algorithm, and the similarity profile (SIMPROF) analysis was used to statistically determine the real clusters generated by the data. Nonparametric analyses were performed using the PRIMER 6 program (Clarke and Gorley 2006). Spearman’s rank correlation coefficient was calculated to determine the effect of hydrological variables on the fish species. The level of significance was set at 5% in all analyses (Zar 1996).
RESULTS
Temporal variation
In total, 10,779 fish were caught, which corresponded to 70 species. The highest species richness and diversity was recorded at night, and the highest abundance was recorded during the day. Seasonally, more species were recorded in the rainy season, and the highest abundance occurred during the northerlies season. The range of observations was expressed as the median and quartiles for each ecological parameter (Fig. 2). However, the differences in species richness, abundance, diversity, and equity were not significant (KW-H: P > 0.05) in any time scale. The results of species composition shown by ANOSIM indicated significant differences between all seasons, albeit with no differences in species composition at the nychthemeral scale. The species that reflected these differences are outlined in Table 1. The most abundant species was H. clupeola, with 3,491 organisms at a juvenile stage, and their abundance was greater in the daytime and in the dry season. The variation between seasons revealed peak abundance of H. clupeola, S. testudineus, Eucinostomus harengulus, and Eucinostomus argenteus in the dry season. In the rainy season, the most abundant species were M. trichodon, Eucinostomus gula, E. harengulus, S. testudineus, Mugil curema, and Sphoeroides spengleri, whereas schools of juvenile (1.0-8.0 cm SL) sardines (H. jaguana, H. clupeola, and O. oglinum) were recorded in the northerlies season, as well as other fish at a juvenile stage (E. saurus and M. trichodon) (Fig. 3a).
Table 1 Results for the analysis of similarities showing the R statistical value, significance level (P), average similarity between samples, and the species accounting for the highest differences.
| R | P | Dissimilarity | Fish species (50%) | Cummulative % | |
| Rainy-northerlies | 0.274 | 0.001 | 79.90 | Fundulus persimilis | 20.22 |
| Floridichthys polyommus | 38.24 | ||||
| Mugil trichodon | 48.65 | ||||
| Eucinostomus gula | 56.83 | ||||
| Rainy-dry season | 0.167 | 0.001 | 80.13 | F. polyommus | 17.36 |
| Harengula clupeola | 28.69 | ||||
| F. persimilis | 38.81 | ||||
| Sphoeroides testudineus | 48.78 | ||||
| Eucinostomus harengulus | 57.19 | ||||
| Northerlies-dry season | 0.241 | 0.001 | 83.77 | F. persimilis | 19.24 |
| F. polyommus | 32.06 | ||||
| H. clupeola | 43.77 | ||||
| S. testudineus | 53.47 | ||||
| Day-night | 0.019 | 0.090 | No significant differences |

Figure 2 Nychthemeral and seasonal variations in community parameters for fish in the Carbonera lagoon. The values obtained in the Kurskal-Wallis analysis and the P values are shown.
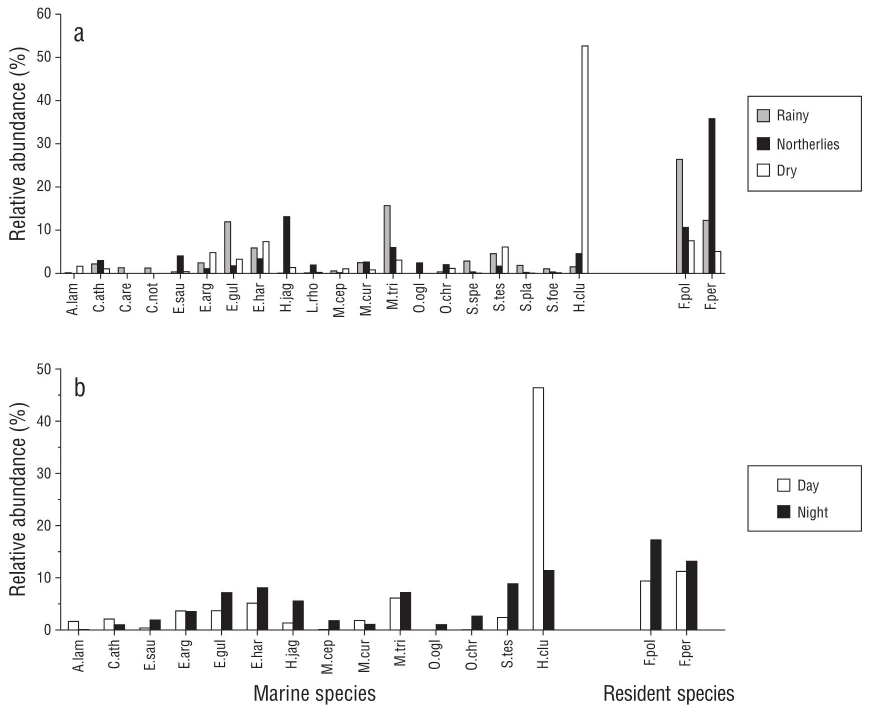
Figure 3 Seasonl (a) and nychthemeral (b) variations in resident marine fish species in the Carbonera lagoon. Total abundance is shown for every category, and only those species with more than 1% of total abundance were considered. Abbreviations show the first letter of the genus name and the first 3 letters of the species name: A.lam = Anchoa lamprotaenia, C.ath = Chriodorus atherinoides, C.are = Cynoscion arenarius, C.not = Cynoscion nothus, E.sau = Elops saurus, E.arg = Eucinostomus argenteus, E.gul = Eucinostomus gula, E.har = Eucinostomus harengulus, H.jag = Harengula jaguana, L.rho = Lagodon rhomboides, M.cep = Mugil cephalus, M.cur = Mugil curema, M.tri = Mugil trichodon, O.ogl = Opisthonema oglinum, O.chr = Orthopristis chrysoptera, S.spe = Sphoeroides spengleri, S.tes = Sphoeroides testudineus, S.pla = Symphurus plagiusa, S.foe = Synodus foetens, H.clu = Harengula clupeola, F.pol = Floridichthys polyommus, F.per = Fundulus persimilis.
The most active marine fish species in the nighttime were H. clupeola, E. saurus, E. gula, E. harengulus, H. jaguana, O. chrysoptera, S. testudineus, S. foetens, M. cephalus, and Symphurus plagiusa. Figure 3b shows the percentage of total catch per species. These organisms were mostly juveniles with maximum sizes that did not reach the reported first sexual maturity (Froese and Pauly 2019). The resident species Floridichthys polyommus and Fundulus persimilis were also recorded at higher numbers in nighttime than in daytime (Fig. 3b).
Feeding
In general, based on the RII (Table 2), the prey most consumed by marine fish were microcrustaceans (amphipods), fish, and microalgae. This trend of the most important preys was consistently observed between the different components (area, weight, and frequency) of the RII (Fig. 4). Dietary differences in prey composition and abundance were significant between nychthemeral cycles (R global = 0.17; P < 0.01), between species (R global = 0.308; P < 0.01), and even between species of the same genus (Mugil) and species of the same trophic guild. The feeding activity of piscivorous (E. saurus and S. foetens) and zoobenthivorous (O. chrysoptera) species primarily occurred at night.
Table 2 Percent weight (%W), area (%A), and frequency (%FO) and relative importance index (%RII) for the prey groups consumed by the fish species in the Cabonera lagoon, Yucatán.
| Archosargus rhomboidalis | Mugil cephalus | Mugil trichodon | ||||||||||
| %W | %A | %FO | %RII | %W | %A | %FO | %RII | %W | %A | %FO | %RII | |
| Microcrustaceans | 41.96 | 41.52 | 33.33 | 58.90 | ||||||||
| Crustaceans | 3.62 | 3.62 | 7.69 | 1.18 | ||||||||
| Mollusk | 3.49 | 3.56 | 12.82 | 1.91 | ||||||||
| Algae | 18.01 | 18.38 | 20.51 | 15.79 | 30.32 | 31.25 | 27.27 | 22.66 | 100 | 100 | 100 | 100 |
| Detritus | 27.92 | 27.92 | 17.95 | 21.21 | 44.68 | 43.75 | 54.55 | 65.08 | ||||
| Plants | 4.23 | 4.23 | 5.13 | 0.92 | 25.00 | 25.00 | 18.18 | 12.27 | ||||
| Fishes | ||||||||||||
| Polychaeta | ||||||||||||
| Insects | ||||||||||||
| Other | 0.77 | 0.77 | 2.56 | 0.08 | ||||||||
| Synodus foetens | Elops saurus | Orthopristis chrysoptera | ||||||||||
| %W | %A | %FO | %RII | %W | %A | %FO | %RII | %W | %A | %FO | %RII | |
| Microcrustaceans | 5.56 | 6.98 | 7.89 | 0.74 | 39.21 | 43.51 | 34.60 | 62.30 | ||||
| Crustaceans | 10.00 | 7.00 | 4.50 | 0.44 | 5.56 | 5.40 | 5.26 | 0.43 | 1.41 | 2.90 | 8.21 | 0.77 |
| Mollusk | 19.49 | 17.53 | 21.41 | 17.25 | ||||||||
| Algae | 0.03 | 0.18 | 3.23 | 0.01 | ||||||||
| Detritus | 8.09 | 8.84 | 2.35 | 0.85 | ||||||||
| Plants | 2.73 | 4.15 | 11.44 | 1.71 | ||||||||
| Fishes | 89.00 | 92.00 | 95.00 | 99.55 | 84.21 | 83.71 | 78.95 | 98.52 | 0.04 | 0.19 | 0.88 | 0.01 |
| Polychaeta | 28.95 | 22.53 | 15.25 | 17.07 | ||||||||
| Insects | 0.48 | 0.66 | 2.63 | 0.02 | 0.02 | 0.07 | 0.88 | 0.01 | ||||
| Other | 1.00 | 1.00 | 0.50 | 0.01 | 4.20 | 3.25 | 5.26 | 0.29 | 0.03 | 0.10 | 1.76 | 0.01 |
| Harengula jaguana | Harengula clupeola | Opisthonema oglinum | ||||||||||
| %W | %A | %FO | %RII | %W | %A | %FO | %RII | %W | %A | %FO | %RII | |
| Microcrustaceans | 85.74 | 87.12 | 74.28 | 98.58 | 92.81 | 91.10 | 68.39 | 99.08 | 90.88 | 88.74 | 83.34 | 98.24 |
| Crustaceans | 6.18 | 4.34 | 11.45 | 0.92 | 0.50 | 0.51 | 3.97 | 0.03 | 0.02 | 2.56 | 1.85 | 0.03 |
| Mollusk | 9.10 | 8.70 | 14.81 | 1.73 | ||||||||
| Algae | ||||||||||||
| Detritus | ||||||||||||
| Plants | ||||||||||||
| Fishes | 6.66 | 7.90 | 2.85 | 0.32 | 4.00 | 4.30 | 9.21 | 0.60 | ||||
| Polychaeta | 2.45 | 3.10 | 5.70 | 0.25 | ||||||||
| Insects | 1.42 | 0.64 | 11.42 | 0.18 | 0.08 | 0.34 | 10.53 | 0.03 | ||||
| Other | 0.09 | 0.12 | 1.32 | 0.01 | ||||||||
| Sphoeroides testudineus | ||||||||||||
| %W | %A | %FO | %RII | |||||||||
| Microcrustaceans | 9.17 | 10.70 | 8.04 | 1.20 | ||||||||
| Crustaceans | 10.59 | 13.25 | 8.62 | 1.54 | ||||||||
| Mollusk | 80.14 | 75.95 | 83.14 | 97.25 | ||||||||
| Algae | ||||||||||||
| Detritus | ||||||||||||
| Plants | ||||||||||||
| Fishes | ||||||||||||
| Polychaeta | ||||||||||||
| Insects | ||||||||||||
| Other | 0.10 | 0.10 | 0.20 | 0.01 | ||||||||
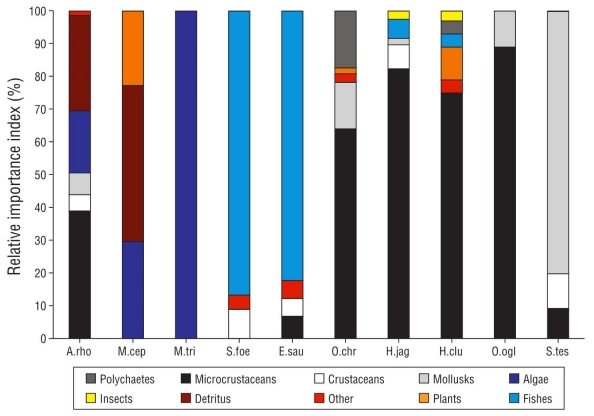
Figure 4 Relative importance index for prey species making up the diets of juvenile marine fish collected in the Carbonera lagoon (A.rho = Archosargus rhomboidalis, M.cep = Mugil cephalus, M.tri = Mugil trichodon, S.foe = Synodus foetens, E.sau = Elops saurus, O.chr = Orthopristis chrysoptera, H.jag = Harengula jaguana, H.clu = Harengula clupeola, O.ogl = Opisthonema oglinum, S.tes = Sphoeroides testudineus).
The results from the classification analysis confirmed the formation of 4 trophic guilds, clustering species with dietary similarities greater than 20% (Fig. 5). Synodus foetens and E. saurus consumed fish. Mugil cephalus, M. trichodon, and A. rhomboidalis fed on detritus and benthic algae. Sphoeroides testudineus fed almost exclusively on mollusks. Sardines (H. jaguana, H. clupeola, and O. oglinum) and pigfish (O. chrysoptera) mainly fed on amphipods.

Figure 5 Dietary similarities in juvenile marine fish from the Carbonera lagoon, Yucatán (S.foe = Synodus foetens, E.sau = Elops saurus, M.tri = Mugil trichodon, A.rho = Archosargus rhomboidalis, M.cep = Mugil cephalus, S.tes = Sphoeroides testudineus, O.chr = Orthopristis chrysoptera, H.clu = Harengula clupeola, H.jag = Harengula jaguana, O.ogl = Opisthonema oglinum). Trophic guilds were clustered at 20% similarity (Bray-Curtis).
Hydrological variables
No significant differences (KW-H, P > 0.05) in hydrological variables were found throughout the day-night cycles, except for dissolved oxygen (KW-H, P < 0.05), which reached the highest level during the day (between 2:00 PM and 4:00 PM) and the lowest at dawn (4:00 AM). The mean and standard deviation values of the time of day (day and night) and season clusters are outlined in Table 3.
Table 3 Mean and standard deviation values of the hydrological variables at the nycthemeral (day and night) and seasonal scales (rainy, northerlies and dry).
| Day | Night | Rainy | Nortes | Dry | |
| Temperature (ºC) | 27.88 ± 3.89 | 26.30 ± 3.64 | 31.66 ± 2.45 | 23.25 ± 2.09 | 31.11 ± 2.43 |
| Total dissolved solids (ppm) | 34.48 ± 3.75 | 36.14 ± 3.63 | 36.33 ± 3.25 | 34.04 ± 1.55 | 41.92 ± 3.04 |
| Salinity | 34.97 ± 4.14 | 37.04 ± 4.29 | 37.10 ± 4.15 | 34.51 ± 1.74 | 42.92 ± 3.55 |
| Dissolved oxygen (mg/L) | 6.99 ± 1.47 | 4.97 ± 1.41 | 6.47 ± 1.96 | 5.57 ± 1.86 | 6.61 ± 1.72 |
| Depth (cm) | 78.30 ± 16.45 | 69.38 ± 17.74 | 85.41 ± 20.83 | 65.58 ± 19.97 | 81.95 ± 12.85 |
Significant differences in temperature (KW-H = 44.10, P < 0.01), total dissolved solids (KW-H = 38.90, P < 0.01), salinity (KW-H = 34.57, P < 0.01), pH (KW-H = 15.30, P < 0.01), and depth (KW-H = 6.19, P < 0.05) were identified between seasons. Average temperatures reached maximum values in the dry and rainy months, particularly in June (29.9 ºC) and August (30.5 ºC), and minimum values during the northerlies season (21.6 ºC). The mean salinity was 36.0 (25.0-46.5). The results showed significant temporal variations in total dissolved solids (from 25.2 to 44.52 mg/L) and in pH (from 7.41 to 10.73). Seasonal differences in depth were related to precipitation (112 cm in the rainy season and 76 cm in the dry season). The mean and standard deviation values at the nychthemeral and seasonal scales are outlined in Table 3.
Fish and hydrology analysis mainly showed negative relationships (Table 4), with the relationships between salinity and suspended solids and marine species such as Sciaenidae (C. nebulosus and Cynoscion arenarius) showing prominent results.
Table 4 Spearman correlation index values for the correlations between fish species and hydrological variables. Only the species that showed at least one significant correlation (P < 0.05, bold font) are shown.
| Temperature | Total dissolved solids | Salinity | Dissolved oxygen | Depth | |
| Chriodorus atherinoides | -0.13 | 0.01 | -0.007 | -0.06 | -0.25 |
| Cynoscion arenarius | 0.04 | -0.32 | -0.34 | -0.14 | 0.07 |
| Cynoscion nothus | 0.02 | -0.39 | -0.41 | -0.005 | 0.05 |
| Elops saurus | -0.36 | 0.10 | 0.09 | -0.26 | -0.24 |
| Lagodon rhomboides | -0.14 | 0.16 | 0.14 | -0.12 | -0.27 |
| Opisthonema oglinum | -0.27 | -0.14 | -0.14 | -0.09 | -0.006 |
| Orthopristis chrysoptera | -0.16 | 0.26 | 0.23 | -0.36 | 0.02 |
| Sphoeroides spengleri | -0.07 | -0.47 | -0.42 | -0.03 | 0.05 |
| Sphoeroides testudineus | 0.22 | 0.24 | 0.20 | 0.006 | -0.06 |
| Symphurus plagiusa | 0.05 | -0.45 | -0.40 | 0.01 | 0.19 |
| Synodus foetens | 0.007 | -0.35 | -0.29 | -0.03 | 0.14 |
| Fundulus persimilis | -0.36 | -0.30 | -0.32 | -0.18 | -0.02 |
DISCUSSION
Temporal variation
The studied fish species showed a differential use of the lagoon because the species composition, abundance, and feeding behavior differed during nychthemeral and annual cycles. The abundance of marine species peaked in the nighttime, when the juvenile stages were dominant (Ayala-Pérez et al. 2014, Gross et al. 2019). This pattern in size composition is common in tropical coastal lagoons that function as nursery areas (Pattrick and Strydom 2014, Enchelmaier et al. 2020).
The presence and abundance of marine fish species in the lagoon changed at the nychthemeral scale. Variations in fish assemblages at the nychthemeral scale are markedly affected by a subset of marine species that form schools and enter lagoon and estuarine systems for short periods for feeding and avoidance of predators (Castillo-Rivera et al. 2011, Zárate-Hernández et al. 2012, Kruse et al. 2016). Nychthemeral variations in mullet (M. trichodon) and sardine (Clupeidae) abundance account for changes in the dominant species of the lagoon. Accordingly, variations in H. jaguana, O. oglinum, and M. trichodon abundance at an annual scale could be related to their reproductive cycles and to the use of the lagoon for juvenile fish growth (García-Abad et al. 1999, Ibáñez and Gutiérrez-Benítez 2004).
Feeding
The fish present at this site fed on different resources and occupied various trophic levels. According to the classification reported by Elliott et al. (2007), zoobenthivorous fish form one of the most abundant groups in coastal ecosystems, as in the Carbonera lagoon (Arceo-Carranza et al. 2013, Enchelmaier et al. 2020). The substantial presence of marine juvenile species, such as the piscivorous S. foetens, indicates that the lagoon is used as a feeding ground (Arceo-Carranza and Chiappa-Carrara 2015). The species Elops saurus and H. jaguana depend on the lagoon; they spawn on the coast, and their larvae and juveniles seek estuarine waters for growth and feeding (Santos-Martínez and Arboleda 1993, McBride et al. 2001). Sphoeroides testudineus is a species with high trophic plasticity with a diet that changes according to the availability of prey in other coastal lagoons of the Gulf of Mexico (Arceo-Carranza et al. 2013, Chi-Espinola and Vega-Cendejas 2013). This fish feeds on amphipods, macrophytes, and detritus; however, mollusks are its most important prey in the Carbonera lagoon (Palacios-Sánchez and Vega Cendejas 2010). The pigfish (O. chrysoptera) is a commercially important species found in the lagoon throughout the year; this species, which was only caught at the juvenile stage, feeds on benthic prey, which suggests that the lagoon is a suitable habitat for its development, as reported by other authors (Elliott et al. 2007). Other commercially important marine species are the mullets M. cephalus and M. trichodon; these fish feed mainly on detritus and benthic algae and have been recognized as key elements in energy transfer within the system (Franco and Bashirullah 1992), as a link between the base of the trophic web and the predators of the lagoon.
These species support local fishing (Salas et al. 2006). Therefore, this lagoon is a breeding habitat that helps maintain the artisanal fisheries of the region. Selectivity and differential resource use result in diets specific to each species. Fish size, the time of day, and season can contribute to dietary differences. These changes in diet can be interpreted as a strategy to reduce competition when prey abundance decreases (Dias et al. 2017). In this case, the difference in predation intensity is one of the mechanisms that reduce dietary overlap between species (Ramírez-Luna et al. 2008).
Fish and hydrological variables
Variations in salinity, temperature, dissolved oxygen concentrations, and turbidity regulate the distribution and abundance of fish in coastal systems during temporal cycles. (Carpentieri et al. 2005). These changes are mainly due to the dry and rainy seasons, which determine the extent to which the habitat is available to fish along the shallow and karst wetlands of the Yucatan coast (Bonilla-Gómez et al. 2013); similarly, the presence of cold fronts gradually decreases the temperature during the northerlies season, which increases the vertical mixing of the water column through turbulence. These changes in hydrology affect the distribution of fish, as in other coastal systems on the northeast coast of the Yucatán Peninsula (Arceo-Carranza and Vega-Cendejas 2009, Peralta-Meixueiro and Vega-Cendejas 2011).
The Carbonera lagoon is an environmentally dynamic site, which is geographically important for marine species due to its distance from other coastal lagoons in the region. The early life stages in many species are replaced at different timescales (nychthemeral and seasonal) because the habitat can be used as a nursery, feeding site, or shelter from predators. The lagoon is important for interactions with neighboring systems such as the sea, the petenes, and the swamp, allowing for considerable richness in freshwater and marine fish, which exploit food resources occupying various guilds and levels in the trophic web of the system. This study is the first analysis of habitat use by juvenile marine species. Further studies on this subject should be conducted (Beck et al. 2001, Able 2005) to determine whether in fact this lagoon can function as a nursery habitat.











 texto en
texto en