INTRODUCTION
Three Pacific thread herring species comprise a fishery resource of great commercial importance along the Pacific coast of eastern Mexico, namely, Opisthonema bulleri (Regan, 1904), Opisthonema medirastre (Berry and Barrett, 1963), and Opisthonema libertate (Günther, 1867). Their distribution extends from the western coast of the Baja California Peninsula and the Gulf of California to northern Peru (Berry and Barrett 1963).
Fisheries statistics in Mexico do not report catches by species, as identification based on morphological characteristics is difficult, and catches are therefore recorded as Pacific thread herring (Opisthonema spp.) (Jacob-Cervantes 2010). The detection of the diagnostic characteristics of these species is thus a relevant endeavor. Berry and Barrett (1963) were the first to propose a methodology for the identification of these species using the number of gill rakers on the ceratobranchial bone of the first gill arch. Torres-Ramírez (2004) proposed the use of gill raker characteristics such as the base and spicules. However, these characteristics can overlap between species, leading to errors in identification. Recently, Pérez-Quiñónez et al. (2017) used a geometric morphometric analysis and mitochondrial DNA cytochrome oxidase subunit I (COI) gene sequences to describe the particular characteristics of each Pacific thread herring species; this study validated for the first time the existence of the 3 taxonomic entities using genetic data.
Genetic techniques are very useful when morphological information alone does not provide sufficient diagnostic characteristics for species identification or when only part of the organism is recovered (García-Rodríguez et al. 2008, Lampa et al. 2015, Pérez-Quiñónez et al. 2017). The genetic information used for exact identification becomes relevant when commercially important species are evaluated or when it is necessary to verify the authenticity of species that are sold as seafood, avoiding illegal practices that replace cheap species for expensive ones (Alacs et al. 2009, Pérez-Enríquez et al. 2016).
The polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) method allows the identification of species based on products created by DNA digestion using restriction enzymes (Guan et al. 2018). This method has the advantage of being fast, simple, robust, and relatively cheaper than the sequencing method, and it has been applied for different purposes, for example, for the identification of organisms in the larval stage or for seafood traceability (García-Rodríguez et al. 2008, Ferrito et al. 2019). Recently, PCR-RFLP procedures have been incorporated into new approaches for detecting aquatic species based on environmental DNA (Clusa et al. 2017, Fernández-Fernández 2018).
In this study, we report a protocol based on the PCRRFLP technique and on the COI gene for identifying the Pacific thread herring species (Opisthonema spp.) found off the northwestern coast of Mexico. The aim was to provide a quick, effective, and cheap tool to contribute to a better evaluation of this fishery resource that has great relevance in Mexico.
MATERIALS AND METHODS
Sampling and DNA extraction
The specimens used in this study were collected in June 2015 in Mazatlán (Sinaloa, Mexico) from landings made by the small pelagic fleet operating from northern Sinaloa to northern Jalisco (Fig. 1, Table 1). All fishes measured ≥120 mm standard length and were taken for adults considering the relationship between length and sexual maturity (Berry and Barrett 1963, Pérez-Quiñónez et al. 2017). To reduce DNA degradation, specimens were collected from the small pelagic fleet as soon as the ships arrived at the harbor. Muscle tissue samples were then obtained and preserved in 95% ethanol. Specimens were initially identified using the works by Berry and Barret (1963), Torres-Ramírez (2004), and Pérez-Quiñónez et al. (2017).
Figure 1 Operation zones used by the small pelagic fishing fleet in the southern Gulf of California, Mexico (I-V).
Table 1 Sample size. The Seq1-COI column refers to the number of sequences that were amplified and digested in this study. The RFLP-COI column refers to the number of sequences obtained from Genbank to evaluate the consistency of the restriction patterns.
| Species | Seq1-COI | RFLP-COI | Total |
| Opisthonema bulleri | 6 | 8 | 14 |
| Opisthonema medirastre | 6 | 10 | 16 |
| Opisthonema libertate | 6 | 7 | 13 |
| Total | 18 | 25 | 43 |
PCR-RFLP analysis
The DNA of 18 individuals was obtained using a comercial kit (Qiagen; Hilden Germany). A ~464 bp fragment of COI was amplified using primers COI Olib F (5′ GAGCAGAATTAAGCCAACC) and COI Olib R (5′ GGGGTCGAAGAAAGTCGTATT). These primers were designed for this study on the basis of an O. bulleri sequence deposited in GenBank (access number KU587814-KU587838), using the program Primer3 (SDBSWeb: http://bioinfo.ut.ee/primer3-0.4.0/, accessed 20 January 2017; Koressaar and Remm 2007, Untergasser et al. 2012). PCR was performed in a total volume of 25 μL:1 μL of DNA template, 1X PCR buffer (Invitrogen), 0.2 mM dNTP mix, 0.24 μM of each primer, 2.0 mM MgCl2, and 0.025 U/μL Taq DNA polymerase (Invitrogen). A BioRAd T100 Thermal Cycler was used for the PCR; this consisted in 2 min initial denaturation at 94 ºC and 35 cycles of 30 s at 94 ºC for denaturation, 30 s at 49 ºC for annealing, and 1 min at 72 ºC for extension. A final extensión of 10 min at 72 ºC was performed. Amplifications were verified by electrophoresis in stained 1% agarose gel and visualized with a UV transilluminator. The gel was stained with GelRed. Amplified products were sequenced (Macrogen; Seoul, South Korea) in both directions with the same primers used in the amplification. Sequences were edited in the program ChromasPro 1.7.6 (Technelysium Pty. Ltd.) and alignment was performed using (Clustal W Thompson et al. 1997) in Mega 6 (Tamura et al. 2013). Sequences were deposited in GenBank (accession number MN745299-MN745316). Three sequences (one for each species) were used for the identification of diagnostic restriction enzyme recognition sites in ChromasPro software v.1.7.6 (Technelysium Pty. Ltd.). Even though several restriction enzymes revealed various cut sites in the sequences, we selected only those that digested fragments that could easily be seen on agarose gel and presented no more than 3 cut sites. To visualize the RFLP pattern, the 18 amplified products were digested using a final 7.0 μL volume with 0.7 unit/μL of the selected enzyme and 3.5 μL PCR product, considering the manufacturer´s instructions (New England Biolabs; Ipswich, MA). The digestion was directly done of unpurified PCR products, as has been done in other studies (Lin and Hwang 2007, García-Rodríguez et al. 2008, Vergara-Chen et al. 2009, Guan et al. 2018). Reaction products for each enzyme were incubated for 20 min at the optimal temperature suggested by the manufacturer. For electrophoresis, restricted products were run on 1.5% agarose gel and stained with GelRed. A molecular weight marker was added to the agarose gel to estimate fragment size. The consistency of the restriction sites was reviewed by increasing the number of sequences to 43 (Table 1): 18 sequences amplified in this study plus the 25 sequences downloaded from Genbank (access number KU587814-KU587838).
RESULTS
The COI gene fragment was correctly amplified for the 3 Pacific thread herring species, with an estimated size of 464 bp. Three restriction enzymes (HpaII, CCGG; AvaII, GGWCC; and RsaI, GTAC) were selected, as they allowed discrimination between the 3 species. Figure 2 shows the restriction sites for the 18 sequences that were amplified and digested in this study. The HpaII enzyme recognized 1 restriction site for O. libertate to generate 2 fragments (haplotype A), 3 and 2 restriction sites for O. medirastre to generate 4 and 3 fragments (haplotype B and haplotype C, respectively), and no restriction sites for O. bulleri (haplotype D). The AvaII enzyme did not recognize restriction sites for O. medirastre or O. libertate (haplotype A) and recognized only 1 restriction site for O. bulleri to generate 2 fragments (haplotype B). Finally, the enzyme RsaI recognized one restriction site for O. libertate and O. bulleri to generate 2 fragments (haplotype A) and no restriction sites for O. medirastre (haplotype B). All electrophoretic patterns obtained from the PCR-RFLP products were congruent with expected results based on sequence analysis (Fig. 3).
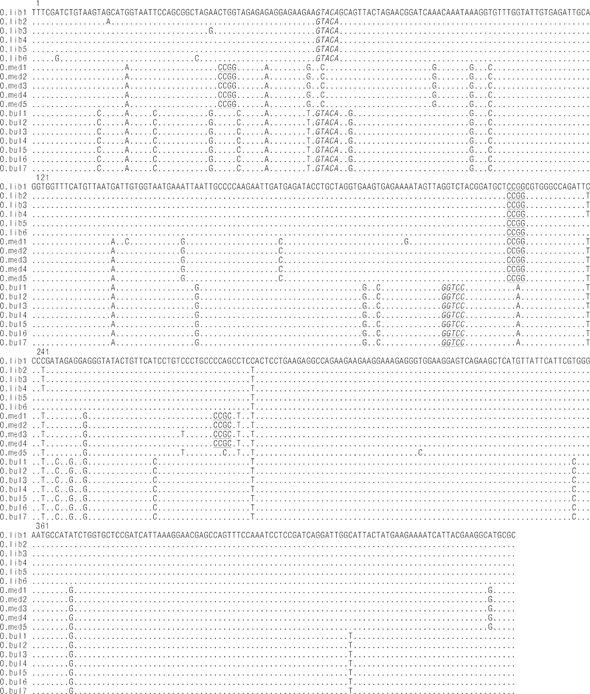
Figure 2 Nucleotide variations within and among species as obtained from the alignment of the 18 nucleotide sequences of the Pacific thread herring COI mitochondrial gene: Opisthonema bulleri (O.bul), Opisthonema libertate (O.lib), and Opisthonema medirastre (O.med). HpaII, RsaI, and AvaII recognition sites are underlined, italicized, and underlined/italicized, respectively. Dots indicate the same nucleotide as the O.lib1 sequence samples. The tag after the species abbreviation indicates the specimen code. Numbers 1, 121, 241, and 361 indicate the positions in the sequence of the first nucleotide in the row.
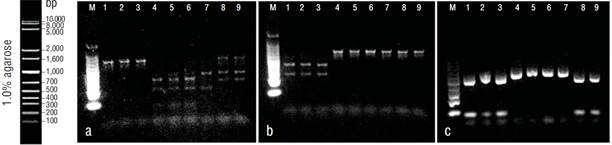
Figure 3 RFLP validation. Agarose gels (1%) showing digestion results with HpaII (a), AvaII (b), and RsaI (c). In all gels, lanes 1-3 correspond to Opisthonema bulleri, 4-7 to Opisthonema medirastre, and 8-9 to Opisthonema libertate.
The consistency of the restriction patterns considering also the 25 sequences downloaded from Genbank was high in the 3 species with the HpaII and AvaII enzymes, since no new haplotype was found. However, the RsaI enzyme presented additional restriction patterns for O. libertate, characterized for the absence of restriction sites (haplotype B), and for O. medirastre, characterized for the recognition of one restriction sites (haplotype C). Considering these results, the HpaII enzyme allowed discrimination between the 3 species, the AvaII enzyme distinguished O. bulleri from the other 2 species, and the RsaI enzyme discriminated O. medirastre from the other 2 species only when haplotype C was present. The composed haplotypes obtained directly of the revisión of the 43 sequences considering the 3 enzymes were AAA and AAB for O. libertate; BAB, BAC, and CAB for O. medirastre; and DBA for O. bulleri (Table 2).
Table 2 COI gene (464 bp) fragment sizes of the 43 sequences obtained from the HpaII, RsaI, and AvaII restriction enzyme recognition sites for the 3 Opisthonema species. Hap = haplotypes, % = percentage of haplotype occurrence in all analyzed sequences. Haplotypes in bold and italicized font were found in the sequences that were downloaded from Genbank. The composite haplotypes were found in the revision of the 43 sequences considering the 3 enzymes.
| Species | Enzyme | Fragment size (bp) |
Hap | % | Composite Hap (%) |
| Opisthonema libertate | HpaII | 223, 241 | A | 100 | AAA (94.0) |
| AvaII | 464 | A | 100 | AAB (6.0) | |
| RsaI | 62, 402 | A | 94 | ||
| 464 | B | 6 | |||
| Opisthonema medirastre | HpaII | 41, 182, 57, 184 | B | 92 | BAB (75.0) |
| 41, 182, 241 | C | 8 | BAC (16.7) | ||
| AvaII | 464 | A | 100 | CAB (8.3) | |
| RsaI | 464 | B | 83 | ||
| 104, 360 | C | 17 | |||
| Opisthonema bulleri | HpaII | 464 | D | 100 | DBA (100) |
| AvaII | 210, 254 | B | 100 | ||
| RsaI | 62, 402 | A | 100 |
DISCISSION
Pacific thread herring identification has been done using meristic, morphometric, and genetic criteria (Pérez-Quiñónez et al. 2017). The use of meristic and morphometric criterio requires that organisms be in good shape so that diagnostic characteristics can be observed clearly. Genetic análisis requires only a tissue sample to carry out successful identifications, but because molecular protocols are complex, the search for and design of strategies to simplify these protocols are important.
The PCR-RFLP technique applied in this study to Pacific thread herring identification was highly efficient, and we thus considered it relevant for supporting other identification procedures based on non-genetic variables (Berry and Barret 1963, Torres-Ramírez 2004, Pérez-Quiñónez et al. 2017). The 43 sequences that were reviewed to detect differences in restriction patterns revealed restriction sites that allow to discriminate the 3 species. Our analysis demonstrated that identification based on meristic and morphological characteristics can be biased, even if the person carrying out the identification is experienced in the use of specialized keys. In the present study, preliminary identification using the proposed keys for the Opisthonema genus resulted in erroneous results in some cases. After analyzing the restriction patterns and reviewing the sequences, individuals were correctly relocated (Fig. 4).
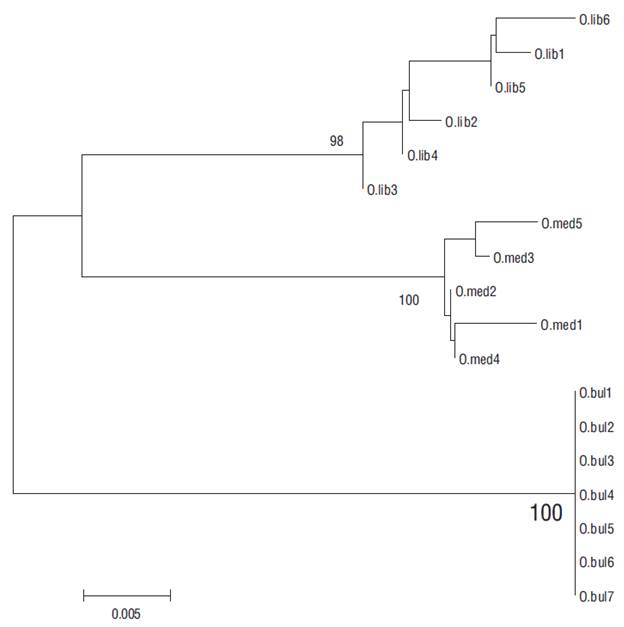
Figure 4 Neighbor-joining phylogenetic tree based on the mitochondrial COI gene haplotypes from a total of 18 adults of 3 Pacific thread herring species: Opisthonema libertate (O.lib), Opisthonema medirastre (O.med), and Opisthonema bulleri (O.bul). Tree reconstruction was based on Kimura’s 2-parameter distance (K2P) model with 1,000 replications. Numbers on the nodes are bootstrap values. The branch length is measured as the number of nucleotide substitutions. O.lib1, O. med5, and O. bul1 represent the organisms that were correctly relocated using the PCR-RFLP technique.
The PCR-RFLP technique was successful in discriminating adult Opisthonema species because, in large part, of the relatively simple procedure for amplifying the COI gene. Specific primers were designed in this study, resulting in the high success of the described protocol, as has also been reported in other studies (Vergara-Chen et al. 2009, Clusa et al. 2017, Guan et al. 2018, Pérez-Quiñonez et al. 2019). Compared with other PCR methods, the combination of specific primers and RFLP has advantages in terms of simplicity, specificity, and sensibility, and it has been used to identify food and species (Lin and Hwang 2007, García-Rodríguez et al. 2008, Vergara-Chen et al. 2009, Huang et al. 2013, Erwanto et al. 2014, Clusa et al. 2017). Thus, comercial samples with different degrees of processing can be analyzed, provided that the DNA has not been overly degraded (Lin and Hwang 2007).
PCR-RFLP can detect a large number of animal species and differentiate between animal species that are taxonomically close (Guan et al. 2018). Since the Opisthonema fishery focuses only on the capture of O. bulleri, O. medirastre, and O. libertate, there is no other Opisthonema species on the Pacific coast of Mexico, and the Opisthonema genus can be morphologically distinguished from other genera, confusion of digestion profiles of other species is absent. However, the more complex use of PCR-RFLP could lie in the identification of incomplete organisms from a mixed sample. To explore this situation, we analyzed, in-silico, sequences available in Genbank for clupeids distributed in the region (Sardipos sagax: FJ165121-FJ165130, JF494407-JF494411; Etrumeus teres: MH378518, MH378548, MH378591, MH378495, HQ945957, JF493477-JF493481, JF952732, JF952733, GU440512; Dorosoma petenense: HQ573343-HQ573347; and Harengula thrissina: HQ010050), and we found that the digestion profiles were different among these species. Therefore, the set of enzymes that we have studied could (potentially) work for other commercially important species in the region that are phylogenetically close. A stronger effort should be done to explore the effectiveness of our primers and define in the best manner the digestion profiles for each species.
The protocol developed in this study allowed the correct discrimination of the 3 Pacific thread herring species inhabiting the central and southern Gulf of California. It also allowed the simple detection of inexact species allocations based on morphological characteristics. In this sense, if there were any doubt in the identification of these 3 species regarding morphological or meristic characteristics, the PCR-RFLP of the COI gene protocol could be applied successfully. In case a new restriction pattern is found that leads to uncertainty, the analysis of the sequences could be considered as a last resort to support correct identifications and finds of new digestion profiles.
Adequate methods for the identification of species are chosen for different reasons, such as sensibility, specificity, precision, discrimination ability, reproducibility, cost, speed, equipment availability, and availability of duly trained personnel (Guan et al. 2018). We applied a protocol that consumes around 5 h (after DNA extraction), has a relatively low cost, and requires equipment that is found in any molecular ecology laboratory. The information provided in this study on the genetic identification of adults in the Opisthonema spp. Pacific thread herring complex constitutes a valuable tool because studies centered on the taxonomic or ecological revision or on the fishery resource management of this complex require precise identification of species.











 nueva página del texto (beta)
nueva página del texto (beta)


