Introduction
Elasmobranchs are a taxonomic group that comprises sharks and rays (Nelson et al. 2016). Around 509 sharks species and 630 ray species are currently known to exist in a wide variety of marine and freshwater ecosystems worldwide (Weigmann 2016). Sharks and rays are particularly vulnerable to overexploitation due to their K-selected life history strategy and high natural mortality rates (Stevens et al. 2000). Recent assessments indicate that approximately 24% of elasmobranchs are threatened by potentially high extinction risks (Dulvy et al. 2014), overfishing being the main cause of population biomass declines (Baum et al. 2003, Baum and Myers 2004, Clarke et al. 2007).
A wide diversity of marine elasmobranchs has been reported for Venezuela, with a total of 66 shark species and 49 ray species, which represent about 61% of all species reported for the Western Central Atlantic (Tavares 2019). However, the health status of the most important populations is unknown because quantitative stock assessments have not been carried out. This knowledge deficiency is the consequence of partly the lack of biological and fishery data such as biological parameter estimates, relative abundance indices, amount of fishing effort, and fishery production databases. On the other hand, sharks and rays constitute a traditional fishery resource used throughout most of Venezuela’s shoreline and islands, primarily via artisanal fishing activities. According to national fisheries production data, shark catches are almost entirely made by artisanal fisheries (94%) and the rest by industrial fisheries (Tavares 2019), underpinning the impact and importance of artisanal fisheries in the country.
The northeast region of Venezuela, where Sucre State is located, is one of the country’s most important fishing areas (Novoa et al. 1998). Moreover, this region is characterized by high marine productivity as a result of coastal upwelling influence (Margalef 1969, Castellanos et al. 2002). Elasmobranch landings in this region consist almost entirely (~80%) of sharks (Tavares 2019). Despite the significance of this fishery resource for the region, no inventories of the most frequently caught and marketed elasmobranch species have been made for marine and coastal areas off Sucre State. Therefore, the aim of this study was to determine species catch composition and to analyze population structure by sex for the most common species in the artisanal elasmobranch fishery.
Materials and methods
Although Venezuela’s main artisanal and industrial fleets are based in the state of Sucre (Novoa et al. 1998), most elasmobranch catches marketed in the state are made by artisanal fishing. The fishing gear that is commonly used in artisanal fisheries to capture sharks and rays are gillnets and longlines (bottom and pelagic). Data were collected between January 2016 and September 2017 during visits (n = 29) to the fishing port and the municipal market of the city of Cumaná (capital of Sucre State), both the main marketplaces for artisanal fisheries.
Elasmobranchs were quantified and identified to the species taxonomic level using the taxonomic procedures available in the literature (Cervigón and Alcalá 1999, Compagno 2002, McEachran and de Carvalho 2002). When possible, the size (total length [TL, cm] for sharks and disc diameter [DD, cm] for rays) and sex of each examined animal were recorded. Because most sharks were eviscerated and beheaded before arriving at the fishing ports, additional length measurements were recorded when complete animals were found: modified interdorsal length (first dorsal fin [1D] to second dorsal fin [2D], 1D-2D) and length from the pectoral fin (PC) (Fig. 1). This allowed reconstructing TLs for incomplete sharks and for the 2 most important species with the following conversion equations:
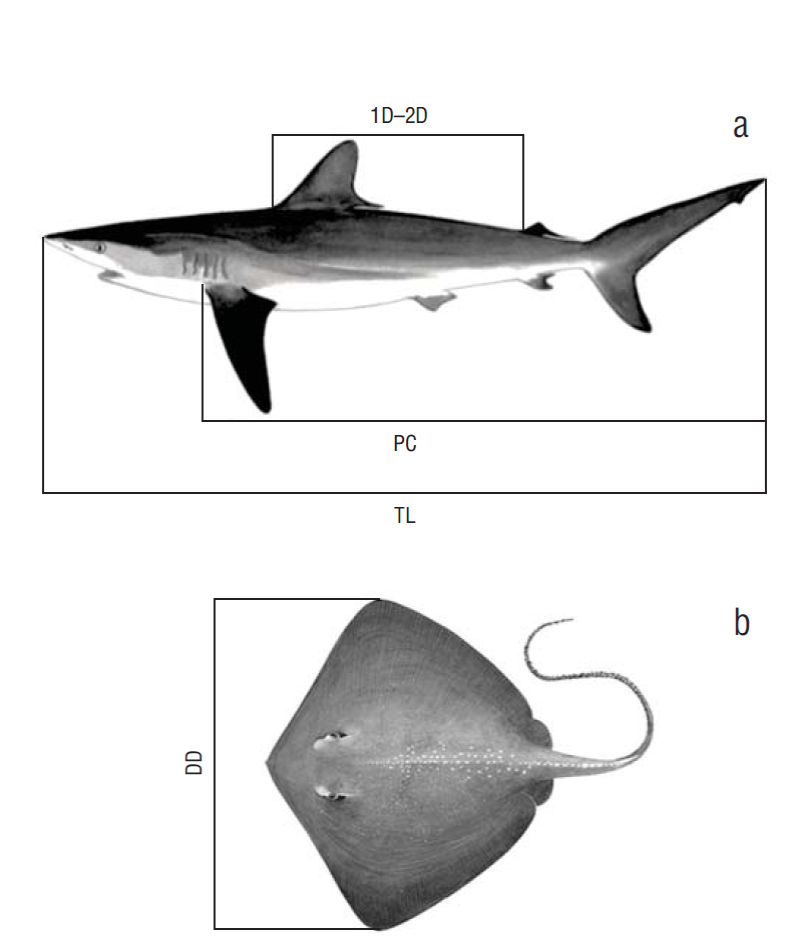
Figure 1 Morphometric measurements used for sharks (a) and rays (b) captured in commercial fisheries of the state of Sucre, Venezuela. 1D-2D, modified interdorsal length; PC, length from the pectoral fin; TL, total length; DD, disc diameter.
Species composition of shark catches was given as a numerical percentage of the total number of species recorded. Sex and size data were analyzed in detail for the 4 elasmobranch species that were most frequently captured in the study area. A chi-square (χ2) test was used to evaluate sex ratio (M:F) and to detect whether there were statistical differences in male and female abundances (Zar 1996). Size structure was represented in frequency histograms by grouping individuals into 10-cm (TL/DD) size classes. In addition, the categorical description of each species in the International Union for Conservation of Nature (IUCN) Red List of Threatened Species (IUCN 2019) and in the Libro Rojo de la Fauna Venezolana (Red Book of Venezuelan Fauna) (Tavares 2015) was used to determine the global and national risk levels for the species reported in the study area.
Results
During the study period, a total of 2,167 elasmobranchs were quantified and grouped into 24 species, 15 of which were sharks and 9 rays (Table 1). Analysis of fishery data indicated that the most important species in the catch composition were the sharks M. higmani (36.2%) and M. canis (18.0%), followed by the rays Hypanus guttatus (15.1%) and Hypanus americanus (7.1%). Other relatively important species in the elasmobranch fishery were Rhizoprionodon lalandii (6.5%), Fontitrygon geijskesi (4.0%), and Carcharhinus porosus (3.1%).
Table 1 Species composition (%) of elasmobranchs captured in commercial fisheries of the state of Sucre, Venezuela. Global (IUCN 2019) and national (Tavares 2015) conservation status: endangered (EN), vulnerable (VU), near threatened (NT), least concern (LC), data deficient (DD), and not evaluated (NE).
| Species | Common name in English | Common name in Spanish | Number (n) | Percentage (%) | Conservation status | |
|---|---|---|---|---|---|---|
| Global | National | |||||
| Mustelus higmani | Smalleye smoothhound | Viuda virma amarilla | 784 | 36.2 | LC | VU |
| Mustelus canis | Dusky smoothhound | Viuda amarilla | 390 | 18.0 | NT | NE |
| Hypanus guttatus | Longnose stingray | Raya látigo hocicona | 328 | 15.1 | DD | NE |
| Hypanus americanus | Southern stingray | Raya americana | 154 | 7.1 | DD | NE |
| Rhizoprionodon lalandii | Brazilian sharpnose shark | Cazón chino | 141 | 6.5 | DD | NE |
| Fontitrygon geijskesi | Sharpsnout stingray | Raya látigo picúa | 86 | 4.0 | NT | NE |
| Carcharhinus porosus | Smalltail shark | Cazón poroso | 68 | 3.1 | DD | NE |
| Myliobatis goodei | Southern eagle ray | Chucho amarillo | 52 | 2.4 | DD | NE |
| Myliobatis freminvillei | Bullnose eagle ray | Chucho blanco | 51 | 2.4 | DD | NE |
| Rhizoprionodon porosus | Caribbean sharpnose shark | Cazón playón | 50 | 2.3 | LC | NE |
| Pteroplatytrygon violacea | Pelagic stingray | Raya látigo voladora | 15 | 0.7 | LC | NE |
| Squatina dumeril | Atlantic angelshark | Pez ángel | 14 | 0.6 | LC | NE |
| Carcharhinus limbatus | Blacktip shark | Macuira | 8 | 0.4 | NT | VU |
| Prionace glauca | Blue shark | Tiburón Azul | 6 | 0.3 | NT | VU |
| Aetobatus narinari | Spotted eagle ray | Chucho pintado | 6 | 0.3 | NT | VU |
| Galeocerdo cuvier | Tiger shark | Tiburón tigre | 4 | 0.2 | NT | NE |
| Carcharhinus perezii | Caribbean reef shark | Tiburón piedrero | 2 | 0.1 | NT | NE |
| Gynglimostoma cirratum | Nurse shark | Tiburón gata | 2 | 0.1 | DD | NE |
| Alopias superciliosus | Bigeye thresher | Tiburón zorro ojón | 1 | 0.0 | VU | VU |
| Carcharhinus falciformis | Silky shark | Tiburón bobo | 1 | 0.0 | VU | VU |
| Heptranchias perlo | Sharpnose sevengill shark | Tiburón de fondo | 1 | 0.0 | NT | NE |
| Isurus oxyrinchus | Shortfin mako | Tiburón carite | 1 | 0.0 | EN | VU |
| Pseudobatos percellens | Southern guitarfish | Chola | 1 | 0.0 | NT | NE |
| Rhinoptera bonasus | American cownose ray | Mancha | 1 | 0.0 | NT | NE |
The sex ratio observed in M. higmani catches (1.0:0.2) showed a highly significant predominance of males compared to females (χ2 = 46.1; P < 0.000). Male sizes ranged from 31.9 to 61.2 cm TL, and female sizes ranged from 29.3 to 73.7 cm TL (Fig. 2). For M. canis, the sex ratio (1.0:0.5) indicated that males were also significantly predominant in the catches (χ2 = 10.1; P = 0.002). Regarding sizes for this species, males measured between 36.4 and 86.0 cm TL, and females measured between 34.0 and 66.8 cm TL (Fig. 2).

Figure 2 Sex-based size structure for the 4 most common elasmobranch species captured in commercial fisheries of the state of Sucre, Venezuela. Mustelus higmani (a), Mustelus canis (b), Hypanus guttatus (c), and Hypanus americanus (d).
The sex ratio for H. guttatus (0.9:1.0) showed no significant difference between males and females (χ2 = 0.1, P = 0.762). Male sizes ranged from 42.0 to 138.0 cm DD, and female sizes ranged from 17.0 to 118.0 cm DD (Fig. 2). For H. americanus, the sex ratio (0.9:1.0) also revealed no statistical difference between males and females (χ2 = 0.1; P = 0.787). Sizes for this species ranged from 49.0 to 100.0 cm DD in males and from 48.0 to 108.0 cm DD in females (Fig. 2).
Following IUCN criteria, global conservation assessments indicate that 3 of the species recorded in this study are threatened to some level (Table 1). Isurus oxyrinchus is listed as endangered and Alopias superciliosus and Carcharhinus falciformis are listed as vulnerable. The rest of the species are listed as near threatened (n = 10), data deficient (n = 7), and least concern (n = 4). National conservation assessments indicate that 7 of the species caught in the study area (M. higmani, Carcharhinus limbatus, C. falciformis, I. oxyrinchus, A. superciliosus, Prionace glauca, and Aetobatus narinari) are categorized as vulnerable (Table 1). The rest of the species (n = 17) have not been evaluated at the national level because the technical and scientific information needed to adjust their conservation status is missing; therefore, the population health status of these species is currently unknown.
Discussion
The most common elasmobranchs in the study area were the sharks M. higmani and M. canis and the rays H. guttatus and H. americanus. For the elasmobranch fishery in Margarita Island, adjacent to Sucre State, Tavares et al. (2010) reported that catches consisted mainly of M. higmani (40.8%), Rhizoprionodon porosus (12.6%), and R. lalandii (9.4%). In a different study carried out off Cubagua, also located in the northeastern region, Cordovés et al. (2010) found that the most frequently captured ray species were H. guttatus (48.9%), Myliobatis freminvillei (18.0%), and H. americanus (15.7%). However, further to the east, between Trinidad and Tobago and Guyana, the most important species in commercial landings were R. lalandii and C. porosus (Shing 2006, Kolmann et al. 2017). Altogether, these results show that sharks belonging to the genus Mustelus and rays belonging to the genus Hypanus are important elasmobranch groups in the northeastern region of Venezuela.
Results showed that for M. higmani and M. canis, male specimens were largely more dominant than females and that there were sex-related differences in their size structures. Discrepancies between sexes may be related to differences in certain biological variables, such as growth parameters (Cortés et al. 2012). Previous studies for these 2 species indicate that growth rates and maximum sizes are different between sexes (Conrath et al. 2002, Carrillo 2019). Moreover, spatial and sexual segregation in elasmobranchs could be the result of the philopatric behavior exhibited by females, which tend to move less distances compared to males (Heist 2012).
With respect to the conservation assessments, 7 of the species recorded in the study area (M. higmani, C. limbatus, C. falciformis, I. oxyrinchus, A. superciliosus, P. glauca, and A. narinari) are listed as vulnerable at the national level. Tavares (2015) stated that the culprits of this risk include long-term and uncontrolled intensive fishing, capturing high proportions of sexually immature specimens, and degrading essential habitats. In a global context, different elasmobranch conservation strategies have been proposed, such as implementing fishing bans for certain fishing areas, regulating fishing gear, and establishing fishing quotas (Shiffman and Hammerschlag 2016); however, these measures have not been fully effective in most regions. More recently, the creation of marine protected areas (MPAs) to preserve elasmobranchs and their habitats has increasingly attracted the attention of researchers and conservationists. Several examples of this conservation measure have been described and discussed in several studies (Garla et al. 2006, Knip et al. 2012).
In Venezuela, there is only one national fishing resolution to prohibit the fishing and marketing of C. falciformis, A. superciliosus, Carcharhinus longimanus, and species of the family Sphyrnidae (Gaceta Oficial de la República Bolivariana de Venezuela, 19 June 2012, no. 39.947). Nevertheless, most fishing regulations in the resolution are not being followed in most Venezuelan insular and coastal regions. The reasons for the neglect are diverse, but the main reason is the collapse of national institutions in charge of regulating and managing fishery resources that came after the country’s economic, social, and political crisis in recent years (Tavares 2019). For now, an effort should be made to continue generating and supplementing information for the most important fishery resources; however, future advances in scientific research and conservation will depend, to a large extent, on the recovery of the country’s political and economic stability.











 nueva página del texto (beta)
nueva página del texto (beta)


