Introduction
Pinnipeds are marine mammals that live partly in the ocean (e.g., for feeding, resting, migrating) and partly on land (e.g., for maternal care, resting, and mating) (King 1983). In general, polygynous pinnipeds (ottarids and some phocids) are highly philopatric (tendency to return to the natal site) (Pomeroy et al. 2000, Chilvers and Wilkinson 2008). Females continuously return to the colonies where they gave birth to nurse their offspring during the lactation period (Peterson and Bartholomew 1967, Melin et al. 2000, Wolf and Trillmich 2007, Hoffman and Forcada 2012). Conversely, most adult and subadult males migrate and abandon the colonies at the end of the breeding season and return before the next one begins (Peterson and Bartholomew 1967, Elorriaga-Verplancken et al. 2018).
Philopatric behavior can influence not only social structure but also evolutionary processes and gene flow patterns. This behavior promotes inbreeding, which reduces genetic variability in populations given the presence of identical alleles. Reduced genetic variability increases the presence of homozygous individuals, which is counterproductive for life history traits such as growth, reproduction, survival, and the potential for evolution, adaption, and speciation, up to the extent of driving some populations to extinction (Greenwood 1980, Matthiopoulos et al. 2005, Campbell et al. 2007, Chilvers and Wilkinson 2008, Serna-Lagunes and Díaz-Rivera 2011). Nonetheless, philopatry allows individuals to adapt to the conditions in their local environment, thus balancing intraspecific competition for resources (González-Forero 1998, Schramm et al. 2009).
In addition to being philopatric, some pinnipeds exhibit intracolonial site fidelity or fine-scale site fidelity, where an individual prefers a site or specific territory within a colony throughout its lifetime (Lunn and Boyd 1991, Cassini 2000, Pomeroy et al. 2000, Wolf et al. 2005, Cameron et al. 2007, Wolf and Trillmich 2007). When fine-scale site fidelity is persistent, aggregations of related individuals are formed, which increase access to limited resources or to the best nursing sites, decrease the frequency of male agonistic encounters and harassment, and promote altruistic behavior; this creates social stability within the colony and increases reproductive success in gregarious species (Cassini 2000, Pomeroy et al. 2000, Cameron et al. 2007, Wolf and Trillmich 2007).
Studies on fine-scale site fidelity in pinniped colonies are scarce because these studies require long-term databases of the geographic locations of individuals in a colony. For this, animals must be permanently branded at birth and tracked throughout their lifetime. Some of these studies have found correlations of age, sex, and reproductive success with fine-scale site fidelity. In the Galápagos sea lion, Zalophus wollebaeki, males were found to use sites that were not frequently used by females (Wolf et al. 2005, Wolf and Trillmich 2007). Moreover, females with pups used different sites from females without pups or with older pups. In the Antarctic fur seal, Arctocephalus gazella, reproductive success was reported to be a better predictor of the ability of females to occupy preferred sites in a colony (Hoffman and Forcada 2012). Likewise, studies on Weddell seals, Leptonychotes weddellii, showed that females having more pups within a certain period of time were more likely to visit the same sites than females having less pups (Cameron et al. 2007).
California sea lion colonies in Mexico are in a critical state given the alarming declines in past decades (Szteren et al. 2006). Los Islotes is one of the few colonies where California sea lion populations have increased, and it is thus a very important site for the conservation of this species. Understanding life history traits, such as fine-scale site fidelity, can help us elucidate the social and genetic structure of California sea lions and infer the recolonization rate in rookeries (Greenwood 1980, Matthiopoulos et al. 2005).
In the present study we estimated the degree of fine-scale site fidelity in female California sea lions (Zalophus californianus) and its relationship with reproductive success (number of pups a female had during the study period) at Los Islotes rookery, La Paz Bay, Mexico.
Materials and methods
Study area
Los Islotes is a small rocky island located on the northeastern boundary of La Paz Bay, Gulf of California (Fig. 1), and it is formed by 2 volcanogenic sedimentary rock islets that together extend out to a total surface area of 0.046 km2 (Labrada-Martagón et al. 2005, Young and Gerber 2008). It is inhabited by a breeding colony of ~600 sea lions.

Figure 1 Los Islotes rookery, Gulf of California, Mexico. Sites where most individuals gather during the mating season are indicated as A, B, and C.
The rookery is divided into 3 main breeding sites: A, B, and C. The first 2 host 32% ±4 and 15% ±3 of the total population size, respectively. Surface areas for sites A, B, and C are 802 m2, 122 m2, and 254 m2 (Young and Gerber 2008), and current sea lion densities are 0.20 ind·m-2, 0.48 ind·m-2, and 0.65 ind·m-2, respectively. Site C was a “bachelor zone” occupied by non-territorial males, but territorial males began to use it approximately 10 years ago. At present, site C is the breeding site with the most individuals in the colony (33% ± 2) (Hernández-Camacho 2001, Labrada-Martagón et al. 2005); the rest of the population (~20%) is distributed in the intermediate zones of the island.
Branding and resighting of individuals
The analysis was done using the presence-absence database pertaining to 5 generations (1980-1984) of California sea lion females that were hot-iron branded as pups at Los Islotes (Hernández-Camacho et al. 2008a , b). Pups were captured and branded in the first week of July at sites A and B; site C was a bachelor zone and there were no pups there. When branding was carried out, the study sites in the colony had not been defined yet. Pups were manually held and branded by 4 people. Markings were composed of 1, 2, or 3 digits and were applied on the dorsal side; digits measured 8 cm in length and were placed 2.5 cm apart from each other. Animals were captured and branded with the research and collection permits granted by the General Directorate for Wildlife (Dirección General de Vida Silvestre, Mexico). Branding had no negative effects on pup survival, nor did it cause any infection or scarring issue that could compromise legibility (Aurioles and Sinsel 1988, Hernández-Camacho 2001). The hot-iron branding method has been used to identify California sea lions at San Miguel Island, California, since 1987, with no reported negative effects on pup survival or reproductive success (DeLong et al. 2017).
In total 94 females were branded accross the study period. Males were also branded (n = 96, Table 1), but resightings were insufficient for estimating the fine-scale site fidelity index. Of the 96 branded males, only 29 were resighted in 1994, when we had started recording the reproductive status and location of the animals in the colony. During the 9-year study (1994-2003), 16 males from all cohorts were observed in only one breeding season, 10 males from all cohorts were observed in 2 to 3 seasons, and only 3 males from the 1983 and 1984 cohorts were observed in 6 to 9 consecutive seasons.
Table 1 Number of male and female pups that were branded during the mating seasons from 1980 to 1984 at the Los Islotes rookery, Gulf of California. The total number of pups born each year was estimated from counts performed on board and on land during the second week of July, when most births had occurred (García-Aguilar and Aurioles-Gamboa 2003).
| Cohort | Males | Females | Branded relative to pups born (%) |
| 1980 | 17 | 8 | 65.8 |
| 1981 | 18 | 17 | 76.0 |
| 1982 | 19 | 18 | 68.5 |
| 1983 | 24 | 26 | 100.0 |
| 1984 | 18 | 25 | 87.8 |
| Total | 96 | 94 | 79.6 |
After branding, the colony was systematically visited for 9 to 15 days during the breeding seasons from 1994 to 2003. The site (A, B, or C) where each individual was observed and the reproductive status (female with pup or no pup) of adults were recorded in every visit (Hernández-Camacho et al. 2008a, b). Sightings were made from a vessel sailing around the island and from land at the main study sites using binoculars. The number of resighted females decreased over time because individuals died with the passing years (Hernández-Camacho et al. 2008a). In the first year of the study (1994), 38 females were recorded, and in the last (2003) only 17 females were recorded.
Fine-scale site fidelity index
To measure fine-scale site fidelity, we developed the following index using data of females with more than 4 sightings in every breeding season to increase certainty (n = 38 females):
where FI i is the fine-scale site fidelity index for individual i, P i is the probability of sighting individual i at the preferred site (P i = SP/A), SP is the number of times individual i was sighted at the preferred site, A is the total number of times individual i was sighted in a season, and TSP is the total number of sites preferred by individual i.
The return rate was estimated to determine the degree of fidelity to sites A, B, and C in one year with respect to the previous year and to sites A and B in each year with respect to the first year of sightings (1994) since data for site C was insufficient. In addition, we could not determine the differences in the return rates between sites because of the low number of samples obtained for sites A and B. The return rate (RT) was estimated using the method described by Acevedo et al. (2006):
where N rx is the total number of California sea lion females recaptured in year x, and N tx is the total number of branded California sea lion females sighted in year x.
A Pearson correlation was used to explore whether there was a correlation between the fine-scale site fidelity index and the reproductive success of each individual during the study period. For the correlation, the reproductive success value was calculated by dividing the number of pups each female had (one pup per season) by the number of sampling years (n = 9). A chi-squared (χ2) test was used to determine if there were differences between the percentage of branded females that had pups and were always sighted at site A and the percentage of females that had pups and were always sighted at site B. Only the last 2 generations of females were considered because these were the only generations for which there was sufficient data. Analyses were done in R (R Core Team 2018); differences were considered significant when P < 0.05. Given our small sample size, we were unable to associate age and sex with the degree of site fidelity.
Results
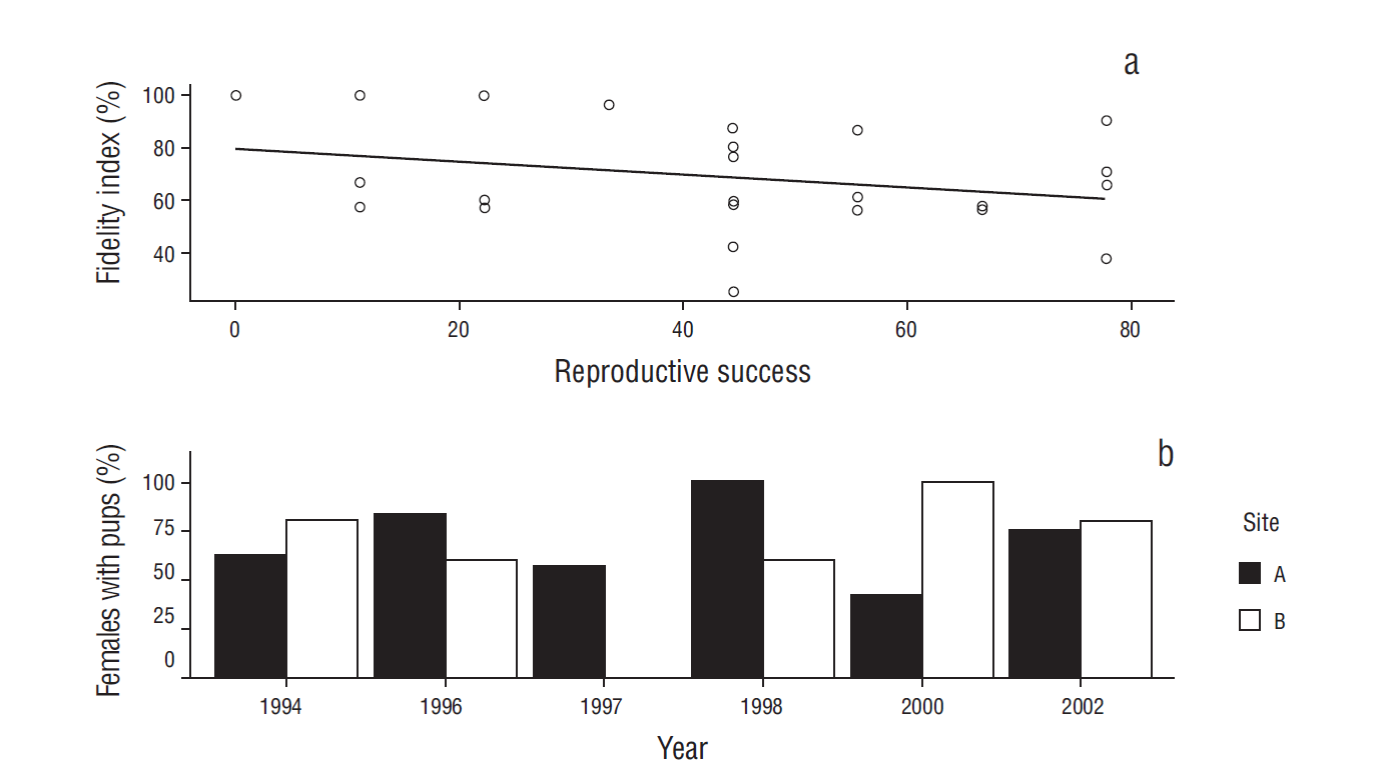
Most California sea lion females showed site fidelity indices >40%. In all, 34% of individuals showed indices >80% (Fig. 2). Yearly return rates with respect to the previous sighting year were highest for sites A and B; however, site A was used the most across all seasons. The decrease in the return rate for site C was attributed to the lower number of females present at this site compared to the other sites, so when an individual from that site was not resighted, the return rate decreased notably. For this analysis, each interval on the x-axis in Figure 3a shows the number of recaptured animals from the previous year; for example, the first interval shows that more than 60% of the animals identified at site A in 1994 were recaptured in 1996. The return rate with respect to the first year of analysis (1994) did not show a clear pattern, though individuals did show preference for site A (Fig. 3b).

Figure 2 Individual fine-scale site fidelity index for female California sea lions (Zalophus californianus) at Los Islotes rookery, Gulf of California, Mexico.
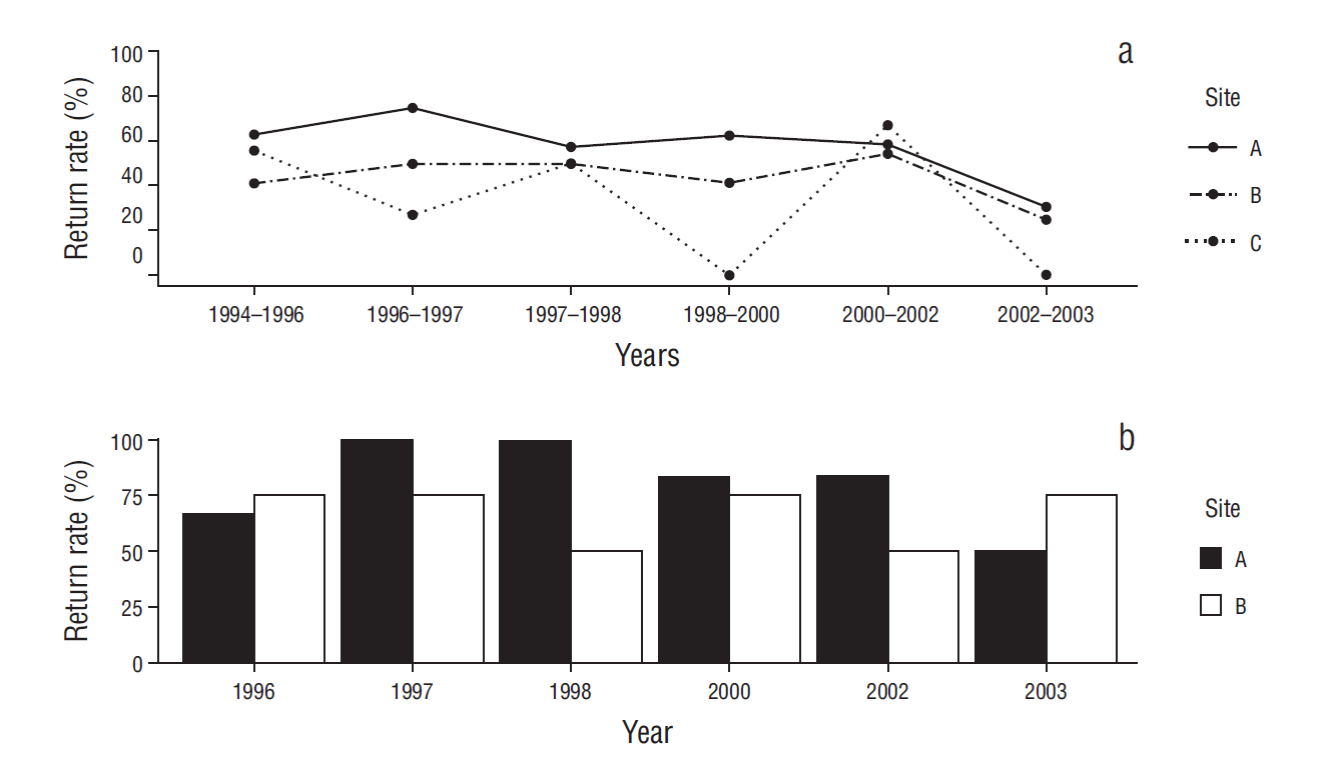
Figure 3 Return rate for female California sea lions (Zalophus californianus) at sites A, B, and C for each year with respect to the previous year (a) and for each year with respect to 1994 (b). Each interval on the x-axis shows the number of recaptured animals from the previous year.
No significant correlation was found between reproductive success and the site fidelity index (P > 0.05, ρ xy = -0.2852) (Fig. 4a). The trend, however, was negative, meaning that when reproductive success increased, the fine-scale site fidelity index decreased. No significant differences were observed in the percentage of females with pups between sites A and B (χ2 = 8.66) (Fig. 4b); that is, no site was preferred for nursing.
Discussion
Fine-scale site fidelity is an important trait that determines the home range of species and the social structure within colonies (Amos and Harwood 1998, Chesser 1998, Pomeroy et al. 2000). California sea lion females at Los Islotes showed a high degree of fine-scale site fidelity, as has been reported for Antarctic fur seals, Galápagos sea lions, and Weddell seals (Cameron et al. 2007, Wolf and Trillmich 2007, Hoffman and Forcada 2012).
In some species, fine-scale site fidelity changes depending on the age category, reproductive success, and population density in the colonies (Cameron et al. 2007, Wolf and Trillmich 2007). In general, adults show higher site fidelity than juveniles because juveniles explore alternative sites in their natal colony or in other colonies when they are excluded from the breeding sites (Baker et al. 1995). We were unable analyze site fidelity by age categories for the California sea lion because we had more data of females in younger age classes, as some females from the first cohorts had already died by the time we started the site-fidelity study in 1994.
The degree of fine-scale site fidelity is positively correlated with female reproductive success or the number of pups in a territory in species like the Antarctic fur seal (Lunn and Boyd 1991), the Galápagos sea lion (Wolf and Trillmich 2007), and the Weddell seal (Cameron et al. 2007). However, in the present study we did not find a positive correlation between reproductive success and fine-scale site fidelity, nor did we identify a preferred site for nursing; we believe the opposing trends in the percentage of females with pups between years and sites are due to chance. If one of the sites were the more suitable for nursing, then the percentage of females with pups at that site would have had to have been higher throughout the study period.
Regarding the density of animals in colonies, Matthiopoulos et al. (2005) indicated that populations that slowly decrease in size or have low densities tend to show higher site fidelity and philopatry than populations with fast growth rates or high densities. In the present study, we did not have data on animal density for every site and every year, except for 2000, 2002, and 2003 (Young and Gerber 2008), and we were thus unable to analyze the correlation between population density and the fine-scale site fidelity index for the whole study period. Nonetheless, analysis of those 3 years showed that site B had the highest density (0.20 ind·m-2), followed by site C (0.09 ind·m-2), and lastly site A (0.05 ind·m-2). Site A, with the lowest density, showed the highest fine-scale site fidelity index and the highest return rate, as in the finding by Matthiopoulos et al. (2005).
At Los Islotes, the fine-scale site fidelity index for the California sea lion was high despite the rapid increase in the size of the colony (Szteren et al. 2006). This could be due to the fact that the population size during the study period was small (between 300 and 400 individuals), and there was plenty of space available for all individuals. At present, population size (~600 individuals) and density (see Study area above) have been increasing, so it is possible that the fine-scale site fidelity index is lower. This condition could reduce endogamy and altruism in the colony (Greenwood 1980, Matthiopoulos et al. 2005).
High levels of philopatry and fine-scale site fidelity promote endogamous breeding, resulting in low genetic variability, which in turn, affects the reproductive success of individuals and, eventually, the growth of a population (Chilvers and Wilkinson 2008, Serna-Lagunes and Díaz-Rivera 2011). The California sea lion colony at Los Islotes does not show low genetic variability (González-Suárez et al. 2009), indicating endogamous mating is not taking place there. We believe there are 3 mechanisms preventing endogamy and generational overlap in organisms showing high philopatry and fine-scale site fidelity. The first has to do with the breeding time gap between birth and the first reproduction event, which exceeds the average reproductive life span of the parents (4-5 years) and reduces the probability of parents breeding with their descendants (Clutton-Brock 1989, Hernández-Camacho 2001). California sea lion females reproduce for the first time at 4 or 5 years of age and continue reproducing until they are 25 years old (Hernández-Camacho et al. 2008b, Melin et al. 2012); males reproduce at 8 or 9 years of age and their territorial life time lasts for 4.2 ± 1.7 yr (Peterson and Bartholomew 1967). Thus, when a female starts to reproduce, her father is no longer reproductively active; however, when a male starts his reproductive life, its mother is still reproductively active, but their reproductive years overlap in only 4 of the 20 years of the reproductive life time of that female. The second mechanism deals with the higher male mortality rates (Hernández-Camacho et al. 2008a), which prevent adult males from breeding with their mothers or sisters. Only 23% of all males reach adulthood (9 years), whereas 47% of females reach adulthood (Hernández-Camacho 2001). Therefore, the probability of them mating is low. Finally, the third mechanism has to do with the fact that few adult males have territories inside the colony, which reduces the probability of inbreeding. However, genetic evidence indicates that this mechanism could not be operating here. Using microsatellite markers for pups and territorial males in 2 colonies from the Gulf of California, Flatz et al. (2012) found that territorial males fathered a low percentage of pups born at these sites and suggested that females were impregnated outside the breeding territories.
If territorial males are not mating with females, then they must be obtaining another benefit from staying in the territories and defending them. One hypothesis is that territorial defense favors male fitness by increasing the survival of pups from related females, as predicted by the kin selection theory (Hamilton 1964). For example, territorial Galápagos sea lion males confront sharks that approach their territories; it is believed that this risky behavior is to defend pups (Trillmich 1996), although other authors believe pups indirectly benefit from this behavior (Miller 1974). In the case of the California sea lion, territorial males could play a role in preventing other males from altering the tranquility of the colony where females are giving birth and nursing their pups.
Though in this study we did not analyze fine-scale site fidelity for males because of the small sample size, of the 96 males we branded only 29 were resighted when we started recording the reproductive status and geographic location of branded individuals in the colony. During the 9-year study period (1994-2003), only 3 males from the 1983 and 1984 cohorts were sighted in 6 to 9 consecutive seasons. The fine-scale site fidelity indices for these 3 individuals were 93%, 23%, and 52%. The possibility exists that the males that were not observed in one breeding season could have been present in another colony. Nonetheless, we believe this number was low because during cruises carried out in the Gulf of California from the first branding year to 2003, only one 15-year old female was recorded at San Esteban Island (508 km north of Los Islotes) in 1997, one young female at Granito Island (638 km north of Los Islotes) in 1984, and one young male at San Pedro Mártir (465 km north of Los Islotes) in 1985. These 3 individuals were resighted at Los Islotes in later years (Hernández-Camacho 2001).
Genetic studies are needed to determine the degree of kinship between individuals from different age classes in this colony. Furthermore, evaluating territorial behavior during the breeding season will allow us to further understand the polygonous mating system in this pinniped species.











 nueva página del texto (beta)
nueva página del texto (beta)