Introduction
The Ariidae family has worldwide distribution in tropical and subtropical seas, coastal shelf waters, and the lower basins of coastal rivers and estuaries. Members in this family range from omnivorous to highly specialized feeders (Kobelkowsky and Castillo-Rivera 1995, Mendoza-Carranza 2003, Denadai et al. 2012, Sandoval-Londoño et al. 2015). Ariopsis guatemalensis belongs to this family and is one of the 92 reported fish species in the Barra de Navidad Lagoon (Jalisco, Mexico) (González-Sansón et al. 2014). This species is of demersal habits (Froese and Pauly 2014) and is capable of bioaccumulating heavy metals (Roosevelt-Rodríguez et al. 2014, Aguilar-Betancourt et al. 2016). No studies have been made on its trophic biology.
Through the evolution of structures and organs involved in the feeding process, species develop the characteristics needed for exploiting resources readily available in the environment (Granado-Lorencio 2002). The overall objective of this project was to study the feeding habits of A. guatemalensis and the relationship between these habits and the main macroscopic and microscopic characteristics of the digestive tract.
Materials and methods
This study was carried out in the Barra de Navidad Lagoon, which is located on the Pacific coast of western Mexico (19º11ʹ25ʺ N, 104º39ʹ53ʺ W). The site is a ~334-ha-euryhaline-lagoon system that is permanently connected to the sea. The main freshwater supply comes from the Arroyo Seco and the Marabasco Rivers (more details on the study area can be found in Aguilar-Betancourt et al. 2016).
Fish were captured between 2011 and 2016 using different types of artisanal fishing gear: (a) a cast net 3 m long with a mesh size of 2.5 cm; (b) gill nets 60 m long with mesh sizes of 7.0, 7.6, 8.9, and 10.2 cm; and (c) an experimental seine net 10 m long with a mesh size of 1 cm in the codend. The wide array of fishing techniques was used to reduce any bias introduced by the selectivity of each fishing gear when obtaining the size composition of captured individuals. Each specimen was measured for total length (TL, ±1 mm precision), and the stomach, esophagus, and intestine were extracted for histological analysis. These organs were measured and fixed in 10% neutral formaldehyde. Stomach contents were preserved in 70% ethyl alcohol for subsequent analysis in the laboratory. Prey items were classified, counted, and weighed (±0.001 g). Sizes were determined by measuring (±0.1 cm) the total length of shrimp and similar species (from the tip of the rostrum to the rear end of the telson), the total length of fish, and the carapace width of crabs. Organisms were identified to the lowest taxonomic level possible using the literature and specialized keys available for each group.
Prey items were quantified using the numeric method (%N = Ni/Nt × 100, where Ni is the number of organisms in category i and Nt is the total number of organisms in all categories); the gravimetric method (%W = Wi/Wt × 100, where Wi is the weight of organisms in category i and Wt is the total weight of all categories); and the frequency of occurrence (%FO = Fi/Ne × 100, where Fi is the number of stomachs where category i was found and Ne is the total number of stomachs analyzed). As a complementary approach, we used the prey-specific index of relative importance (PSIRI, %PSIRI = %Fi × %PNi + %PWi/2) to determine the importance of each prey (Brown et al. 2012); this index uses %N and %W values fitted to the specific abundance of prey items.
To examine if sample size would provide an estimate that suitably represented stomach contents, cumulative trophic diversity curves were generated following the method proposed by Figueiredo et al. (2005). Trophic diversity was measured using the Shannon index (H′) (Magurran 2004), which was estimated using 100 randomizations without replacement in EstimateS 9 (Colwell 2017). The curve was considered asymptotic if at least 2 values preceding the trophic diversity value of the total sample (H′max) were within the H′max ±0.05 H′max interval (Alonso et al. 2002).
To analyze if diet composition changed as fish size increased, the total sample was divided into 3 size classes in a way that each class had a similar number of specimens. Size classes were labeled C1, which included specimens having TL <30 cm (n = 30); C2, which included specimens having TL between 30 and 35 cm (n = 32); and C3, which included specimens having TL >35 cm (n = 21). Stomach contents were analyzed using the methods mentioned above. Pairwise comparisons of diet composition (%PSIRI) among size classes were performed using the Bray-Curtis similarity index and the corresponding confidence intervals (95%), which were bootstrapped using SpadeR (Chao et al. 2016) in R (R Core Team 2019). Following the criteria by Cumming and Finch (2005) and Gotelli and Colwell (2011), if the confidence intervals for the Bray-Curtis similarity index overlapped between 2 size-class pairs, then differences in diet composition between those classes were not considered significant.
For histological analysis of stomachs, esophagi, and intestines, a tissue sample was taken from each organ, but samples were also taken from the anterior, middle, and posterior intestinal segments. Processing was done following the routine histological technique: samples were dehydrated in alcohol, embedded in paraffin, and sliced with a microtome (Erma, Japan). Tissues were stained with haematoxylin and eosin using the technique by Mayer and Harris, and slices were preserved in Canada balsam (Lucano-Ramírez et al. 2001). Microscopic descriptions were done following Gómez-Ramírez et al. (2010).
Results
A total of 102 A. guatemalensis stomachs were analyzed, 83 of which had contents. Size data for the analyzed fish were considered homogenous because, although the size interval ranged between 12.3 and 49.0 TL, 65% of TL sizes ranged between 25 and 35 cm. The analysis by size classes produced the following Bray-Curtis similarity index values and confidence intervals (in parentheses): C1-C2, 0.714 (0.613-0.814); C1-C3, 0.544 (0.442-0.646); C2-C3, 0.636 (0.546-0.727). Confidence intervals overlapped in all cases, so changes in diet composition with specimen size were not considered significant. Therefore, all subsequent analyses were done considering all fish specimens included in this study.
The number of stomachs analyzed was considered suitable for the description of the species’ general diet because the cumulative trophic diversity curve showed a clear tendency to reach the asymptote (Fig. 1). Fishes were the main prey (%PSIRI = 53.65, %W = 68.51, %N = 38.79, %FO = 74.39). Though most fishes were in advanced stages of digestion, fishes from the Gobiidae family were identifiable, with notable presence of Gobionellus microdon (Gilbert, 1892) (%PSIRI = 1.84, %W = 2.46, %N = 1.21, %FO = 2.44) and, to a lesser degree, Ctenogobius manglicola (Jordan & Starks, 1895) (%PSIRI = 0.31, %W = 0.02, %N = 0.61, %FO = 1.22) and Ctenogobius sagittula (Günther, 1862) (%PSIRI = 0.31, %W = 0.01, %N = 0.61, %FO = 1.22). Crustaceans were the second most important prey item (%PSIRI = 36.15, %W = 21.39, %N = 50.91, %FO = 64.63). The mud shrimp Upogebia dawsoni (Williams 1986) and the caridean shrimp Alpheus pacificus (Dana 1852) were the most important crustaceans. One peculiar finding was the presence of fish eggs, a prey item with high index values (%PSIRI = 7.22, %W = 7.16, %N = 7.27, %FO = 1.22; Table 1), in one of the A. guatemalensis stomachs.

Figure 1 Cumulative trophic diversity curve for Ariopsis guatemalensis. The horizontal dashed lines indicate H′max ±0.05 H′max.
Table 1 Percentage values of the numerical (%N), frequency of occurrence (%FO), and gravimetric (%W) indexes and the prey-specific index of relative importance (%PSIRI) for the dietary composition of Ariopsis guatemalensis.
| Group | Category | %FO | %N | %W | %PSIRI |
| Porifera | 4.88 | 1.21 | 2.59 | 1.90 | |
| Alpheidae | Alpheus spp. | 1.22 | 1.21 | 0.31 | 0.76 |
| Alpheus pacificus | 7.32 | 4.85 | 1.94 | 3.39 | |
| Penaeidae | Penaeus californiensis | 2.44 | 1.21 | 0.00 | 0.61 |
| Processidae | Processa spp. | 1.22 | 0.61 | 0.01 | 0.31 |
| Upogebiidae | Upogebia dawsoni | 7.32 | 16.97 | 2.54 | 9.75 |
| Shrimp remains | 3.66 | 2.42 | 0.83 | 1.63 | |
| Grapsidae | Grapsus spp. | 3.66 | 1.82 | 2.22 | 2.02 |
| Portunidae | Callinectes spp. | 1.22 | 0.61 | 0.04 | 0.32 |
| Callinectes arcuatus | 2.44 | 3.64 | 0.45 | 2.04 | |
| Xanthidae | Panopeus spp. | 2.44 | 2.42 | 1.13 | 1.78 |
| Leucosiidae | Randallia bulligera | 1.22 | 0.61 | 0.05 | 0.33 |
| Brachyuran larvae | 2.44 | 1.21 | 0.02 | 0.61 | |
| Paguridae | 2.44 | 1.21 | 0.23 | 0.72 | |
| Crab remains | 2.44 | 0.61 | 0.47 | 0.54 | |
| Crustacean remains | 23.17 | 11.52 | 11.16 | 11.34 | |
| Insecta | 2.44 | 1.82 | 0.35 | 1.08 | |
| Fish eggs | 1.22 | 7.27 | 7.16 | 7.22 | |
| Gobiidae | Ctenogobius sagittula | 1.22 | 0.61 | 0.01 | 0.31 |
| Ctenogobius manglicola | 1.22 | 0.61 | 0.02 | 0.31 | |
| Gobionellus microdon | 2.44 | 1.21 | 2.46 | 1.84 | |
| Goby remains | 1.22 | 1.21 | 0.44 | 0.83 | |
| Engraulidae | Anchoa spp. | 1.22 | 0.61 | 0.05 | 0.33 |
| Fish remains | 67.07 | 34.55 | 65.54 | 50.04 |
The sizes of fishes and invertebrates found in stomach contents turned out to be homogeneous in most cases because, although TL intervals were large, standard errors were relatively low and oscillated between 3.6% and 22.9% of mean values. When the lengths of prey were compared with the lengths of the fish that contained them in their stomachs, the positive correlation was significant only for specimens of the family Callinectidae (Table 2).
Table 2 Mean (± standard error), minimum, and maximum size values for select food categories. The correlation coefficient (r) and its associated probability (P) are given for cases with N ≥ 6. NI, not identified.
| Category | N | Mean | Min | Max | r | P |
| Shrimp NI | 3 | 0.83 ± 0.03 | 0.80 | 0.90 | - | - |
| Upogebia | 27 | 2.20 ± 0.12 | 0.70 | 2.90 | -0.127 | 0.527 |
| Alpheidae | 10 | 2.86 ± 0.35 | 1.30 | 4.90 | 0.379 | 0.279 |
| Callinectidae | 7 | 0.36 ± 0.04 | 0.20 | 0.50 | 0.816 | 0.025 |
| Crabs NI | 10 | 1.09 ± 0.21 | 0.50 | 2.40 | -0.274 | 0.443 |
| Insects NI | 3 | 0.93 ± 0.13 | 0.80 | 1.20 | - | - |
| Fishes | 6 | 5.98 ± 1.37 | 2.20 | 9.70 | 0.463 | 0.355 |
Macroscopic description of Ariopsis guatemalensis
The mouth is in a subterminal position and is relatively large. Teeth are villiform and arranged in plates: 2 premaxillary plates; 2 oval vomerine plates, barely separated from the midline and adjacent to 2 palatine plates; and 2 pairs of pharyngeal tooth plates. It has 3 pairs of barbels: 1 maxillary pair and 2 mandibular pairs. Gill rakers are short and scarce, with large spaces between them. The esophagus is short and bulky. The stomach is large and J-shaped. No pyloric caeca were observed. The intestine is relatively short. The average quotient of total intestine length to total fish length was 1.4 ± 0.05 (Fig. 2).
Microscopic description of the digestive tract
The 4 major layers of the digestive tract were observed: mucosa, submucosa, muscular layer, and serosa. In the esophagus, the mucosa is composed of the simple columnar epithelium and loose connective tissue; goblet cells and, in smaller quantities, alarm cells were observed in this layer. The submucosa is composed of loose connective tissue, followed by a circular striated muscle layer (the thicker inner layer) and a longitudinal striated muscle layer (the outer layer) (Fig. 3). In the stomach, the lumen was larger than in other organs (esophagus and intestine). The mucosa is composed of the simple columnar epithelium; gastric glands were observed in this layer. The submucosa is composed of loose connective tissue followed by a circular smooth muscle layer and a longitudinal smooth muscle layer (Fig. 4). Tissue conformation in the intestine was similar. The mucosa is composed of the simple columnar epithelium; unlike in the stomach and the esophagus, no gastric glands or goblet cells were observed here. The submucosa showed lymphoid tissue, followed by a smooth muscle layer with 2 orientations, circular (inner layer) and longitudinal (outer layer) (Fig. 5).

Figure 3 Ariopsis guatemalensis esophagus. (a) Lumen (L), mucosa (M), submucosa (S), loose connective tissue (LCT), circular striated muscle (CSM), and longitudinal striated muscle (LSM) (5×). (b) Goblet cells (GC) (20×). (c) Alarm cells (AC) (20×).
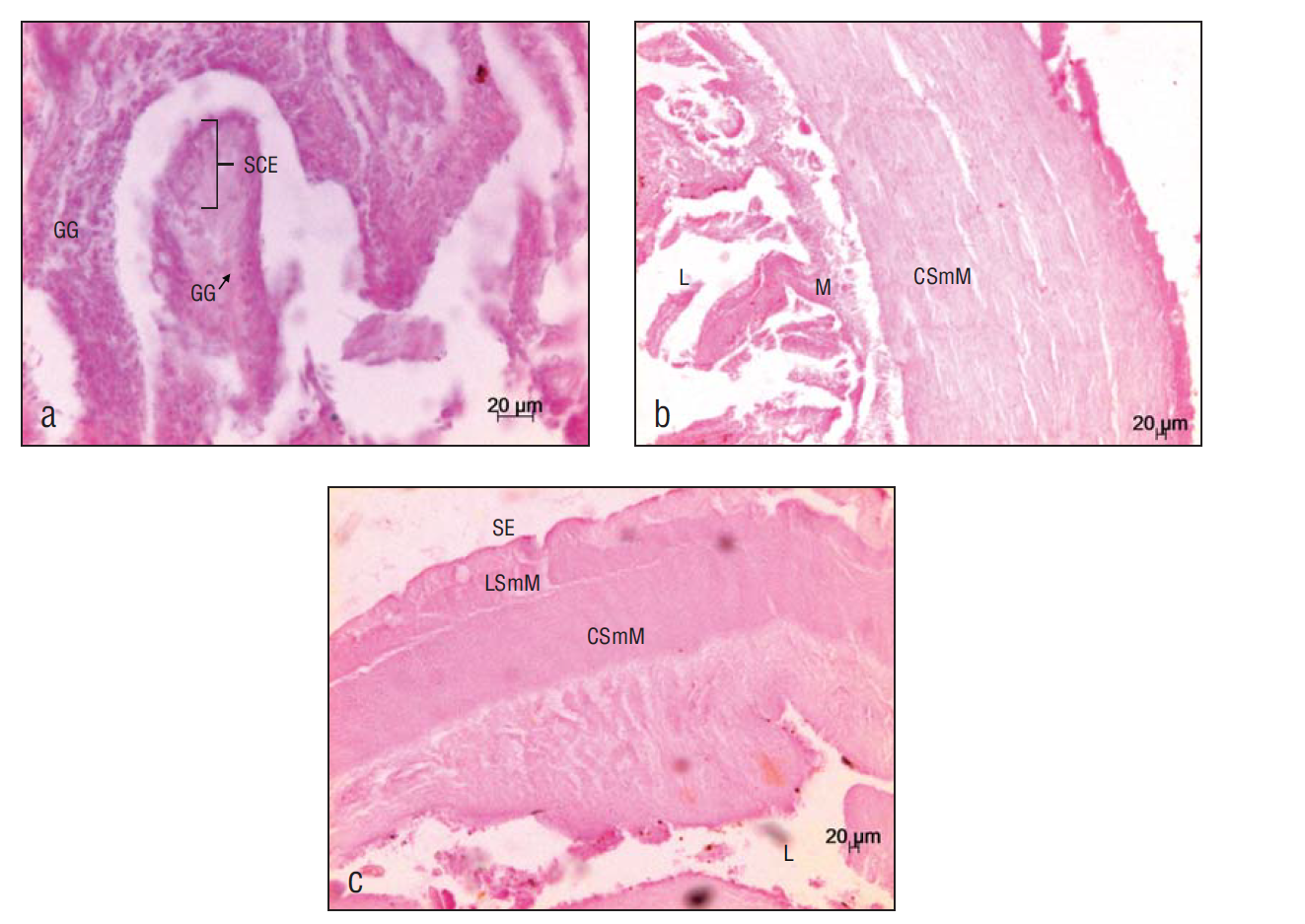
Figure 4 Ariopsis guatemalensis stomach. (a) Simple columnar epithelium (SCE) and gastric glands (GG) (20×). (b) Lumen (L), mucosa (M), and circular smooth muscle (CSmM) (5×). (c) Lumen (L), serosa (SE), and longitudinal smooth muscle (LSmM) (5×).

Figure 5 Ariopsis guatemalensis intestine. (a, b) Anterior intestine (5× and 10×, respectively). (c, d, e) Middle intestine (5×, 10×, and 20×, respectively). (f) Posterior intestine (5×). Lumen (L), mucosa (M), submucosa (S), longitudinal smooth muscle (LSmM), circular smooth muscle (CSmM), muscular layer (Mu), lymphoid tissue (LT), simple columnar epithelium (SCE), erythrocytes (ER).
Discussion
The main prey item consumed by A. guatemalensis was composed of fishes (mainly gobies). Díaz-González and Soto (1988) reported a similar result for Ariopsis caerulescens in the Huizache-Caimanero lagoon system, Sinaloa, Mexico, which predominantly fed on gobies. The results obtained in the present study are similar to those reported for A. caerulescens (Melchor-Aragón 1980) in El Verde Estuary and Caimanero Lagoon in Sinaloa, Mexico, for Bagre marinus (Kobelkowsky and Castillo-Rivera 1995) in lagoons in Veracruz, Gulf of Mexico, and for Ariopsis sp. (aff. assimilis) in a neotropical costal lagoon in Colombia (Sandoval-Londoño et al. 2015), all which fed mainly on fishes, followed by crustaceans. By contrast, Arius liropus (Melchor 1980) in El Verde Estuary and Caimanero Lagoon, Ariopsis canescens in the Huizache-Caimanero lagoon system (Díaz-González and Soto 1988), Cathorops melanopus and Ariopsis felis in the Gulf of Mexico (Kobelkowsky and Castillo-Rivera 1995), Sciades herzbergii in an estuary north of Brazil (Giarrizzo and Saint-Paul 2008, Krumme et al. 2008), and Ariopsis sp. in the Sinú River in Colombia (Olaya-Nieto et al. 2012) fed mainly on crustaceans, and fishes were their secondary prey.
The absence of change in dietary composition among size classes and the lack of correlation between prey size and fish size can be explained by the fact that this species has a large mouth, which allows individuals, even the smallest ones in the analyzed sample, to capture large-sized prey. The only case for which a significant correlation was found (Callinectidae) should be taken with much caution because the sample size for this case was small (n = 7).
The position of the mouth and dental plates enable this species to manipulate live prey and break up food before passing it on to the esophagus. This has been reported by Kobelkowsky and Castillo-Rivera (1995) for C. melanopus, A. felis, and B. marinus, which feed on fishes and crustaceans, just as A. guatemalensis did in the present study. Gill rakers in A. guatemalensis, as in Ariopsis seemanni (Gómez-Ramírez et al. 2010), do not carry out any digestive function because of the low number of gill rakers and the spacing between them (low filtration capacity).
Ariopsis guatemalensis specimens had relatively short intestines compared to total body length. This concurs with the bentho-icthyophagous habits shown by this species (Sis et al. 1979, Kramer and Bryant 1995, Wagner et al. 2009, Gómez-Ramírez et al. 2010, Karachle and Stergiou 2010).
The histological analysis of the digestive tract of A. guatemalensis indicated the presence of 4 layers: mucosa, submucosa, muscularis, and serosa, which coincides with reports for other teleosts, such as Pimelodus pictus (Olaya et al. 2007), A. seemanni (Gómez-Ramírez et al. 2010), Glyptosternum maculatum (Xiong et al. 2011), Rhamdia quelen (Hernández et al. 2009), and Pelteobagrus fulvidraco (Cao and Wang 2009).
The mucosa in the esophagus of A. guatemalensis showed abundant goblet cells, which lubricate and protect the mucosa when food is passed on to the stomach. These cells are a characteristic feature of fish that ingest hard-bodied prey (Rodríguez-Rodríguez et al. 2004, Olaya et al. 2007, Gómez-Ramírez et al. 2010). Another feature found in the esophagus of A. guatemalensis was the presence of alarm cells, which according to Olaya et al. (2007), act as immune alarm signals. The layer covering the esophagus showed 2 orientations (circular and longitudinal), indicating that the species has the ability to control regurgitation of unwanted prey or to prevent reflux of gastric contents from the stomach and that there is greater distension of the esophagus during ingestion and movement of food in a single direction (dos Santos et al. 2015).
Enzyme secretion occurs in the stomach, and gastric glands help degrade food before it is passed on to the intestine (Olaya et al. 2007). According to Gómez-Ramírez et al. (2010), the presence of 2 orientations (inner circular and outer longitudinal) in the muscular layers of the stomach allows food to be broken down and mixed with gastric secretions, which results in smaller particles or a semiliquid paste that increases food absorption in the intestine.
The short intestine with mucosal folds in A. guatemalensis is characteristic of fish having carnivorous habits. According to some authors (Sis et al. 1979, Olaya et al. 2007, Gómez-Ramírez et al. 2010), food absorption is proportional to surface area and it increases when there are more mucosal and submucosal folds or apical microvilli. These general considerations adequately describe the characteristics of the intestine in A. guatemalensis.
In conclusion, A. guatemalensis is a benthic carnivorous fish that prefers feeding on fishes and it has adapted to feed on hard-bodied prey (crustaceans). The results obtained here indicate that the characteristics of the digestive tract are strongly related to the feeding habits of A. guatemalensis. The size and position of the mouth, the presence of dental plates on the vomer, the palatine, the premaxilla, and the pharynx, and the presence of goblet cells in the digestive tract are traits in carnivorous fishes that allow them to ingest hard-bodied prey, manipulate live prey, and break up food before it is passed on to the esophagus. The relatively short intestine and mucosal folds in A. guatemalensis are also characteristic of carnivorous fishes.











 text new page (beta)
text new page (beta)



