Introduction
Tigriopus Norman, 1869 is a copepod genus typically linked to marine rock pools in the supratidal and the uppermost intertidal zones (McAllen 1999), although it is also known to occur subtidally off the Antarctic Peninsula (Waller et al. 2006, Park et al. 2014), Mexico (Ganz and Burton 1995, Edmands 2001), Southern Asia (Jung et al. 2006, Ki et al. 2009), and Sweden (Lang 1948). The supratidal and intertidal coastal rock pools are harsh habitats characterized by the large instability of physical (e.g., temperature or water amount) and chemical conditions (e.g., oxygen content, pH, salinity) (Ganning and Wulff 1970, Underwood and Skilleter 1996). To cope with such continuously changing habitats, behavioral and\or physiological mechanisms aimed at avoiding or overcoming adverse conditions were developed by the rock-pool-inhabiting fauna, which thus became a focal point for studies of these extreme environments (Dethier 1980, Raisuddin et al. 2007).
Like some species of the beetle family Hydraenidae (Antonini et al. 2010) and mosquitoes of the family Culicidae (Mastrantonio et al. 2015) occurring in the same habitat type, Tigriopus developed adaptations to survive these stresses. Adult beetles and mosquitoes are able to fly away from drying pools, switching to nearby filled pools, and in other taxa, late instar larvae can enter into resting stages, or resistant eggs might be produced (Williams 2007). Conversely, Tigriopus spp. survive desiccation as adults in a dormant state (Battaglia 1982) and are not able to produce resistant cysts or diapausing eggs. Moreover, adult Tigriopus californicus may persist in dried pools for some days or weeks, even without entering the “dormancy phase”, retaining themselves in small habitable habitat patches awaiting better conditions to recolonize the same rock pool (Powlik 1998). The means of dispersal of Tigriopus spp. are still unknown, although the passive dispersal of the species mediated by birds, floating algae, and water currents has been proposed (see discussion in Handschumacher et al. 2010)
The genus Tigriopus includes 14 species (Walter and Boxshall 2018), and the following are known to occur in the Atlantic-Mediterranean area: Tigriopus brevicornis (Müller 1776) and Tigriopus brachydactylus (Candeias 1959) from Africa, Tigriopus fulvus (Fischer 1860) from the Mediterranean Sea and the Atlantic island of Madeira, and Tigriopus minutus Bozic 1960 from Senegal; this last species has also been dubitatively reported to occur in the Mediterranean Sea by Lazaretto and Libertini (1986), although no other records of T. minutus are available for this area. The species occurring in the Atlantic-Mediterranean area can be morphologically distinguished based on a few characters whose intra- and interspecific variability has not been exhaustively examined to date, and T. brevicornis was not recognized as taxonomically distinct from T. fulvus until the late 1970s (Carli and Fiori 1977). Moreover, in the early XX century, 2 “varieties” of T. fulvus were described, i.e., T. fulvus var. adriatica by Van Douwe (1913) from specimens collected in Rovinj (Croatia) and T. fulvus var. algirica by Monard (1935) from specimens collected in Tipasa (currently Tipaza, Algeria); oddly, these taxa were characterized based on a morphological comparison of these populations with the Atlantic species T. brevicornis instead of with T. fulvus s.s. (Carli and Fiori 1977). According to article 45.6.4 of the International Code of Zoological Nomenclature (https://www.iczn.org/), if a taxon of infrasubspecific rank was established before 1961, it has to be considered of subspecific rank (ICZN 1999); accordingly, T. fulvus is currently to be considered a polytypic species including the 3 subspecies Tigriopus fulvus fulvus (Fischer 1860), Tigriopus fulvus algiricusMonard 1935, and Tigriopus fulvus adriaticusVan Douwe 1913. As stressed by Lazzaretto and Libertini (1986), the actual genetic diversity pattern of Mediterranean Tigriopus populations is still unknown and yet to be properly explored.
In light of the paucity of data currently available, and of the taxonomic uncertainties affecting the genus Tigriopus in the Mediterranean area, the goal of this work is to explore the genetic variability of T. fulvus and its alleged subspecies along the coasts of the Mediterranean Sea, and to contribute to the clarification of their taxonomy. Moreover, we aimed to evaluate if the noteworthy interpopulation genetic diversity observed in several Mediterranean rock-pool-inhabiting taxa (e.g., Antonini et al. 2010, Audiso et al. 2010, Mastrantonio et al. 2015) also characterizes the harpacticoid copepods of the genus Tigriopus.
Materials and methods
In order to investigate the genetic diversity occurring among T. fulvus populations, copepods were collected from intertidal and supratidal rock pools in 21 different locations across the Mediterranean Sea and the eastern Atlantic Ocean (Table 1, Fig. 1). In addition, specimens of the North Atlantic species, T. brevicornis, were collected in Galicia (Spain) to be used as outgroup. Latitude and longitude for each locality was determined with a geographical positioning system (GPS). The map of the sampling sites was done using QGIS software v. 2.18.2 (http://www.qgis.org).
Table 1 Origin and GenBank accession numbers (A.N.) for the analyzed Tigriopus specimens. Geographic coordinates are expressed as decimal degrees (Map Datum: WGS84). *: specimens from the type locality of Tigriopus fulvus algiricus; §: specimen from the type locality of Tigriopus fulvus adriaticus; †: specimens from Madeira, the type locality of Tigriopus fulvus s.s.
| Taxa | Code | Sample | Country | Location | Latitude (N) | Longitude (E) | A.N. | Source |
| T. fulvus* | TIP | TF44 | Algeria | Tipaza | 36.6229 | 2.4081 | MK211338 | Present work |
| T. fulvus* | TIP | TF45 | Algeria | Tipaza | 36.6229 | 2.4081 | MK211337 | Present work |
| T. fulvus | ROV | TF54 | Croatia | Rovigno | 45.1172 | 13.6071 | MK211332 | Present work |
| T. fulvus | TER | TF1 | Italy | Terrasini | 38.1542 | 13.0756 | MK211350 | Present work |
| T. fulvus | TER | TF6 | Italy | Terrasini | 38.1542 | 13.0756 | MK211351 | Present work |
| T. fulvus | TER | TF7 | Italy | Terrasini | 38.1542 | 13.0756 | MK211352 | Present work |
| T. fulvus | BAR | TF2 | Italy | Barcarello | 38.2129 | 13.2916 | MK211354 | Present work |
| T. fulvus | BAR | TF8 | Italy | Barcarello | 38.2129 | 13.2916 | MK211355 | Present work |
| T. fulvus | BAR | TF9 | Italy | Barcarello | 38.2129 | 13.2916 | MK211353 | Present work |
| T. fulvus | MAG | TF3 | Italy | Magnisi | 37.1562 | 15.2369 | MK211345 | Present work |
| T. fulvus | MAG | TF10 | Italy | Magnisi | 37.1562 | 15.2369 | MK211344 | Present work |
| T. fulvus | MAG | TF11 | Italy | Magnisi | 37.1562 | 15.2369 | MK211346 | Present work |
| T. fulvus | PLE | TF12 | Italy | Plemmirio | 37.0021 | 15.3315 | MK211356 | Present work |
| T. fulvus | PLE | TF13 | Italy | Plemmirio | 37.0021 | 15.3315 | MK211357 | Present work |
| T. fulvus | MIL | TF14 | Italy | Milazzo | 38.2700 | 15.2245 | MK211348 | Present work |
| T. fulvus | MIL | TF15 | Italy | Milazzo | 38.2700 | 15.2245 | MK211347 | Present work |
| T. fulvus | MIL | TF16 | Italy | Milazzo | 38.2700 | 15.2245 | MK211349 | Present work |
| T. fulvus | COR | TF17 | Italy | Cornino | 38.0900 | 12.6583 | MK211343 | Present work |
| T. fulvus | PAN1 | TF26 | Italy | Pantelleria | 36.7793 | 11.9541 | MK211358 | Present work |
| T. fulvus | PAN2 | TF27 | Italy | Pantelleria | 36.8158 | 11.9263 | MK211359 | Present work |
| T. fulvus | TRI | TF37 | Italy | Tricase | 39.9330 | 18.3975 | MK211333 | Present work |
| T. fulvus | CEF | TF38 | Italy | Cefalù | 38.0415 | 14.0218 | MK211342 | Present work |
| T. fulvus | CEF | TF39 | Italy | Cefalù | 38.0415 | 14.0218 | MK211341 | Present work |
| T. fulvus | POR | TF42 | Italy | Portoscuso | 39.2065 | 8.3763 | MK211361 | Present work |
| T. fulvus | POR | TF43 | Italy | Portoscuso | 39.2065 | 8.3763 | MK211362 | Present work |
| T. fulvus | CAS | TF46 | Italy | Castiglioncello | 43.4012 | 10.4045 | MK211339 | Present work |
| T. fulvus | CAS | TF48 | Italy | Castiglioncello | 43.4012 | 10.4045 | MK211340 | Present work |
| T. fulvus | GAR | TF83 | Italy | Gargano | 41.9264 | 15.6435 | MK211331 | Present work |
| T. fulvus | SDI | TF85 | Morocco | Sidi Ifni | 29.3467 | -10.1961 | MK211329 | Present work |
| T. fulvus | CAT | TF87 | Morocco | Cape Tamry | 30.5465 | -9.7180 | MK211330 | Present work |
| T. fulvus† | SXL | TF58 | Portugal | Seixal | 32.8270 | -17.1145 | MK211326 | Present work |
| T. fulvus† | SXL | TF59 | Portugal | Seixal | 32.8270 | -17.1145 | MK211325 | Present work |
| T. fulvus† | SXL | TF72 | Portugal | Seixal | 32.8270 | -17.1145 | MK211324 | Present work |
| T. fulvus† | PDC | TF60 | Portugal | Porto da Cruz | 32.7763 | -16.8264 | MK211328 | Present work |
| T. fulvus† | PDC | TF67 | Portugal | Porto da Cruz | 32.7763 | -16.8264 | MK211327 | Present work |
| T. fulvus | JAV | TF49 | Spain | Jàvea | 38.7635 | 0.2050 | MK211336 | Present work |
| T. fulvus | JAV | TF50 | Spain | Jàvea | 38.7635 | 0.2050 | MK211335 | Present work |
| T. fulvus | BEN | TF52 | Spain | Benitachell | 38.7080 | 0.1664 | MK211334 | Present work |
| T. fulvus | MEN | TF78 | Spain | Menorca | 39.9980 | 3.8274 | MK211360 | Present work |
| T. fulvus | - | - | France | Banylus Sur Mer | 42.4833 | 3.1333 | AF315361 | Edmands 2001 |
| T. fulvus | - | - | Spain | Blanes | 41.6666 | 2.8000 | AF315364 | Edmands 2001 |
| T. brevicornis | GLC | TF79 | Spain | Sanxenxo | 42.3898 | -8.7767 | MK211363 | Present work |
| T. brevicornis | GLC | TF81 | Spain | Sanxenxo | 42.3898 | -8.7767 | MK211364 | Present work |

Figure 1 Geographic location of the sampling sites. Circles indicate sites where Tigriopus fulvus was sampled and square indicates sampling site for Tigriopus brevicornis. See Table 1 for the coordinates of the sampling sites and for more information on the collected species. Codes refer to those listed in Table 1. Color codes refer to those reported in Figure 2 .

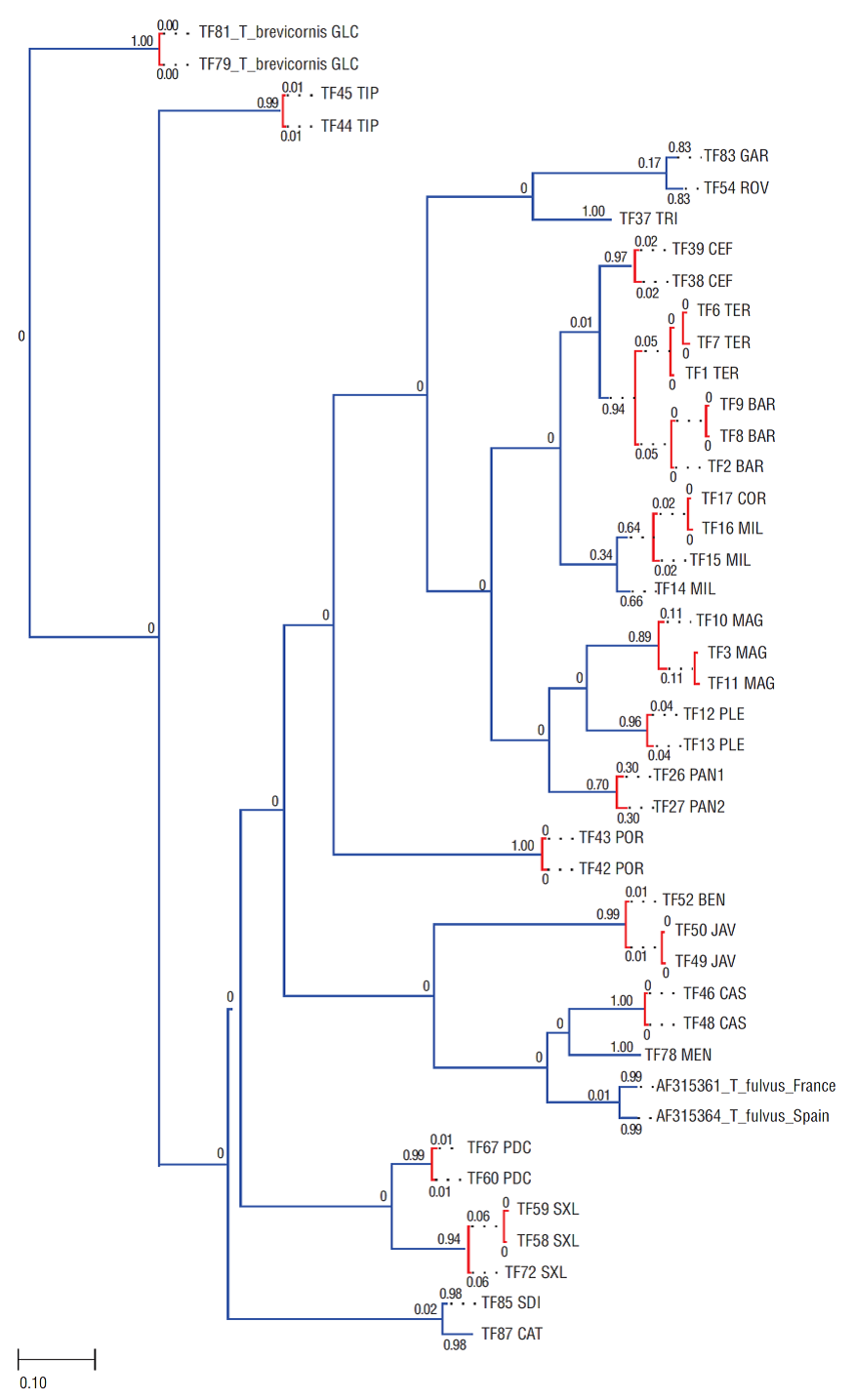
Figure 2 Bayesian phylogram (95% majority rule consensus tree) for Tigriopus spp. based on the 552 bp fragment of the mtDNA COI. Samples of Tigriopus brevicornis were used as outgroup to root the tree. Node statistical support is reported as nodal posterior probabilities (Bayesian Inference of phylogeny, BI)/bootstrap values (Maximum Likelihood, ML). Asterisks indicate a bootstrap support value lower than 50. Rectangles refer to MOTUs as indicated by the K/Θ ratio (white rectangle), ABGD (gray rectangles), and bPTP (black rectangles). Square brackets group the samples according to the current taxonomy of the genus. The analyzed specimens are reported using the codes listed in Table 1.
Harpacticoids were sampled with a 200-µm mesh hand net. Collected specimens were fixed in situ in 96% ethanol, sorted out under a stereomicroscope and identified according to Wells (2007). In an attempt to identify the alleged T. fulvus subspecies, the identification keys provided by Monard (1935) and Van Douwe (1913) were used.
After morphological identification, specimens were dipped in double distilled water for 15 min and processed for DNA extraction using the BIORON GmbH Ron’s Tissue DNA Mini Kit following the manufacturer’s instructions. The selective amplification of a cytochrome c oxidase subunit I (COI) fragment was carried out by the polymerase chain reaction (PCR) using the primers L1384-COI and H2612-COI (Machida et al. 2004).
The PCR mix consisted of 18.05 µL double-distilled water, 2.5 µL Buffer 10X including 15 mM MgCl2 solution, 0.25 µL dNTPs (10 mM of each), 0.9 µL of each primer (10 µM), 0.4 µL BIORON DFS-Taq DNA Polymerase 5U/µL, and 2 µL of DNA template, for a total volume of 25 µL. The thermal cycle consisted of 35 cycles of denaturing (95 ºC for 50 s), annealing (48 ºC for 50 s), and extension (72 ºC for 50 s), followed by 7 min at 72 ºC for the final extension step. After PCR, 5 µL of each PCR product were separated by electrophoresis on a 2% agarose gel at 90 V for 20 min and visualized with a UV Transilluminator. When PCR products showed a clear and single band of the expected length, they were purified using the Exo-SAP-IT kit (Affymetrix USB) and sequenced by Macrogen Inc. (Seoul, South Korea) with an ABI 3130xL (Applied Biosystems) sequencer. The same primers used for the PCR were subsequently used for direct sequencing of the PCR product, and the quality of the obtained chromatograms was checked through the measurement of their Phred scores (Richterich 1998). Only sequences with continuous reads of high-quality bases (QV > 20) were used. Chromatograms were analyzed and manually proofread with the software Chromas v.2.6.2 (Technelysium, Pty. Ltd. 1998; Queensland, Australia) and aligned with ClustalX v.2.1 (Larkin et al. 2007).
The 39 novel mitochondrial sequences for T. fulvus and the 2 for T. brevicornis were deposited in GenBank (see Table 1 for their accession numbers). In addition, the only 2 T. fulvus sequences available on GenBank were downloaded and included in the analyses (see Table 1 for their accession numbers).
MEGA v.7.0 (Kumar et al. 2016) was used to translate the mtDNA sequences to amino acids in order to check for the possible presence of frameshifts or stop codons, which would indicate the presence of sequencing errors or pseudogenes, a widespread phenomenon among crustaceans (e.g., Song et al. 2008, Schizas 2012), and to calculate the pairwise uncorrected ‘p’ distance based on the entire mtDNA dataset.
The molecular identification of the studied specimens and the reconstruction of the phylogenetic relationships among the taxa was performed with Bayesian inference (BI) and maximum likelihood (ML) methods as implemented in MrBayes v.3.2.6 (Ronquist et al. 2012) and PhyML v.3 (Guindon and Gascuel 2003), respectively. As a measure of branch support, bootstrap values (Felsenstein 1985) were calculated with 1,000 replicates in the ML tree, and posterior probability values were reported on the BI tree. The choice of the best evolutionary model was made using PartitionFinder v.1.0.1 (Lanfear et al. 2012) according to the Akaike information criterion (AIC, Akaike 1974). The BI and ML analyses were performed using a general time-reversible model of sequence evolution with a proportion of invariant sites (GTR+I; number substitution types = 6). In the BI analyses, two independent Markov chain Monte Carlo analyses were run with 1 million generations (temp.: 0.2; default priors). Trees and parameter values were sampled every 100 generations, resulting in 10,000 saved trees per analysis; in the analysis, convergence was reached (effective sample size above 254.10); 2,500 trees were conservatively discarded as “burnin”.
A haplotype network including all the available T. fulvus COI sequences was constructed through the software PopART (v.1.7; http://popart.otago.ac.nz), using the minimum spanning method (Kruskal 1956) (Fig. S1).
In this study, the “evolutionary genetic species concept” proposed by Birky et al. (2010) was followed. According to this concept, species are inclusive populations that are evolving independently of each other, either because they are reproductively isolated, or because they are separated by environmental or physical barriers, or both. Those lineages that evolve separately from others were thus considered different taxa of putative species rank. In order to single out evolutionary lineages of species rank, DNA taxonomy approaches based on different assumptions were implemented, i.e., a quantitative approach based on coalescent model (ABGD, Puillandre et al. 2012), a phylogenetic criterion based on branching rates (bPTP, Zhang et al. 2013), and a genetic population criterion based on genetic isolation (K/Θ ratio; Birky and Barraclough 2009, Birky et al. 2010, Birky 2013). Both ABGD and bPTP methods were implemented through their online interfaces (http://www.abi.snv.jussieu.fr/public/abgd/abgdweb.html and http://species.h-its.org/ptp/). Following Korn and Hundsdoerfer (2016), the K/Θ ratio was computed based on the uncorrected p-distance matrices within and among the detected clades. This method tests if the reciprocal monophyly of sister lineages is statistically significant, which would suggest that they are independently evolving entities, hence bonae species sensuBirky et al. (2010).
Results
Overall, 41 Tigriopus specimens belonging to T. fulvus s.l. and T. brevicornis were analyzed, and included in the analyses (Table 1). According to the morphological study of the collected samples, the Algerian and Adriatic samples coming from the type localities of the alleged T. fulvus subspecies showed no consistent morphological differences when compared to the samples coming from Madeira Island, where the type locality of T. fulvus s.s. occurs, thus casting some doubts on the actual subspecific status of these populations. No specimens morphologically ascribable to the poorly characterized T. minutus were collected in this survey.
After having trimmed out the sequences, a properly aligned fragment 552 bp long of the COI mtDNA gene was obtained. All the sequences were deposited in GenBank (accession numbers MK211324-MK211364).
The BI and ML trees based on the mitochondrial COI fragment and rooted on T. brevicornis showed a congruent topology, with a sister-taxa relationship between the Algerian samples from the T. fulvus algiricus type locality and the rest of the T. fulvus s.l. specimens, whereas the alleged T. f. adriaticus sample was nested well within the ingroup (Fig. 2). Well-supported Moroccan, Madeiran, and Mediterranean T. fulvus clades stemmed from a basal polytomy. The Mediterranean clade showed noteworthy internal molecular structuring, with intra-clade pairwise uncorrected p distances ranging from 0% to 19%. Interestingly, the occurrence of private monophyletic haplogroups was observed in different Mediterranean subbasins (see Figs. 1, 2), and no haplotypes were shared among different rock pools even within subbasins.
The initial and recursive partitions of the ABGD analysis found 16 groups of putative species rank within the ingroup, with prior maximal divergence of intraspecific diversity values (p) ranging from 0.0129 to 0.0359 (Fig. S2). Among these 16 groups, two correspond to the Madeiran clade of T. fulvus s.s., as shown in the mtDNA based tree (Fig. 2). bPTP analysis estimated the presence of 21 putative species in the ingroup, i.e., finding 11 of the groups highlighted by ABGD analysis, and further splitting the other ones. Madeiran T. fulvus samples were ascribed to two different groups also by bPTP, as shown in Figure 2.
The K/Θ ratio showed the presence of a single species in the ingroup, with inter-clade distances (K) much lower than 4Θ, i.e., 4 times the average sequence divergence among individuals of each clade (Table 2). The K/Θ ratio thus does not support the existence of independently evolving lineages of species rank within the studied dataset.
Table 2 Application of the K/ϴ ratio to Tigriopus fulvus s.l. mitochondrial lineages. n: number of individuals; p-dist.: uncorrected p distance; π: nucleotide diversity; ϴ: intra-clade variation; K: inter-clade distance; Tfulvcm: T. fulvus from the central Mediterranean area; Tfadr: T. f. adriaticus; Tfalg: T. f. algiricus; Tfulvss: T. fulvus s.s. from Madeira; Tfulvsl: T. fulvus s.l.
| Group | n | p-dist. | π | 4\3π | ϴ | K | K\ϴ ratio | Sample |
| Tfulvcm | 19 | 0.112 | 0.118 | 0.157 | 0.140 | 0.188 | 0.868 | TF1, TF2, TF3, TF6, TF7, TF8, TF9, TF10, TF11, TF12, TF13, TF14, TF15, TF16, TF17, TF26, TF27, TF38, TF39 |
| Tfadr | 3 | 0.112 | 0.168 | 0.224 | 0.216 | 0.188 | 0.868 | TF37, TF54, TF83 |
| Tfulvsp1 | 22 | 0.062 | 0.064 | 0.086 | 0.071 | 0.212 | 2.981 | Tfulvcm + Tfadr |
| Tfulvsp2 | 2 | 0.002 | 0.005 | 0.006 | 0.005 | 0.212 | 2.981 | TF42, TF43 |
| Tfulvsp3 | 24 | 0.143 | 0.149 | 0.198 | 0.186 | 0.228 | 1.053 | Tfulvsp1 + Tfulvsp2 |
| Tfulvsp4 | 6 | 0.140 | 0.168 | 0.224 | 0.216 | 0.228 | 1.053 | TF46, TF48, TF49, TF50, TF52, TF78 |
| Tfulvsp5 | 30 | 0.033 | 0.034 | 0.045 | 0.035 | 0.225 | 0.357 | Tfulvsp3 + Tfulvsp4 |
| Tfulvsp6 | 2 | 0.171 | 0.342 | 0.456 | 0.628 | 0.220 | 2.545 | TF85, TF87 |
| Tfulvss | 5 | 0.062 | 0.077 | 0.103 | 0.086 | 0.221 | 0.351 | TF58, TF59, TF60, TF67, TF72 |
| Tfulvsl | 37 | 0.187 | 0.192 | 0.256 | 0.258 | 0.207 | 0.801 | Tfulvsp5 + Tfulvsp6 + Tfulvss |
| Tfalg | 2 | 0.001 | 0.002 | 0.003 | 0.002 | 0.207 | 0.801 | TF44, TF45 |
Discussion
Taxonomical notes
The ABGD and bPTP DNA taxonomy approaches used in the frame of this work suggested the presence of an unexpectedly high number of species within the ingroup. Conversely, according to the same dataset, the K/Θ ratio identified the presence of a single species in the whole study area, namely T. fulvus. However, sample size is known to differently affect the accuracy of the molecular taxonomy approaches implemented in our analyses as ABGD and bPTP are more sensitive to the size of the sample (Pulliandre et al. 2012, Zhang et al. 2013), whereas the K/Θ ratio should be not significantly influenced by the sample size (cf. Birky 2013). Moreover, it should be kept in mind that DNA taxonomy approaches just delimit primary species hypotheses, i.e., indications that, taken alone, are not sufficient to clarify the taxonomical rank to be attributed to the studied clades. It is thus necessary to adopt an integrative approach and search for a consensus between their outputs before delineating species (Fontaneto et al. 2015). For these reasons, lacking a consensus among the outputs of the different DNA taxonomy approaches implemented and pending a wider sampling coverage of Tigriopus molecular diversity in the whole Atlantic-Mediterranean area, we opted to rely on the results of the K/Θ ratio approach, which is considered the most conservative method among the implemented molecular taxonomy approaches (Birky and Barraclough 2009, Bode et al. 2010). Moreover, the morphological study of the samples highlighted that the morphological features used to distinguish the subspecies fall, in fact, within the internal variability of the species (L Vecchioni in prep.); this, coupled with the branching pattern of the phylogenetic tree (Fig. 2), suggests that the currently described T. fulvus subspecies are to be considered as junior synonyms of T. fulvus s.s., although a wider sampling of the overall morphological and molecular diversity of Atlantic-Mediterranean Tigriopus populations is desirable. Currently available morphological and molecular data therefore suggest that only T. fulvus occurs in the study area, but more surveys aimed at obtaining a clearer picture of its overall diversity are needed.
Pattern of molecular diversity
The phylogenetic analysis of the studied Atlantic-Mediterranean T. fulvus populations revealed noteworthy geographic structuring of the genetic diversity among the samples (Figs. 1, 2), with inter-population mitochondrial divergence values reaching up to 19%. This is in accordance with the known occurrence of high genetic diversity in the North Atlantic T. brevicornis (0-21%, Handschumacher et al. 2010), the Pacific Japanese T. japonicus Mori 1938 (0-23%, Ki et al. 2009), and the Pacific North American T. californicus (Baker 1912) (0-23%, Edmands 2001). According to Handschumacher et al. (2010), the extensive inter-population divergence observed in T. brevicornis could be ascribed to the “paradox of Rockall”. This paradox states that species with limited dispersal and scarce inter-population gene flow are very effective in colonizing remote areas after fortuitous long-range passive dispersal events (Johannesson 1988), thus resulting in the establishment of isolated but widespread populations.
Southern populations of T. californicus and T. brevicornis have been found to be genetically distinct from each other, while the northernmost populations appear to show substantially lower inter-population divergences (Edmands 2001, Handschumacher et al. 2010). This pattern is in accordance with the “Southern richness vs. Northern purity” paradigm (Hewitt 2004, Marrone et al. 2010), where the “northern purity” is attributed to the post-glacial recolonization of the northern area by a subset of the diversity survived in peripheral or southern refugia during glacial events, and the “southern richness” to the continuous persistence of the populations in these areas.
A similar pattern was observed in other species inhabiting rock pools (e.g., in the dipteran genus Aedes, Mastrantonio et al. 2015), whereas the high genetic structure in rock-pool dwelling coleopterans of the genus Calobius was considered in good accordance with the theory of the “refugia within refugia” (Gómez and Lunt 2006, Antonini et al. 2010). However, the role that recent bottlenecks and/or founder effects might have on the genetic structuring of rock-pool dwelling organisms should also be considered (cf. Audisio et al. 2010).
The high level of geographically based genetic structure observed in the Atlantic-Mediterranean Tigriopus populations seems in good accordance with the non-cosmopolitanism paradigm of aquatic taxa and the monopolization hypothesis (De Meester et al. 2002, Incagnone et al. 2015). This hypothesis predicts that the monopolization of water bodies by the first colonizers leads to a long-lasting persistent founder effect; this generates a structured pattern of genetic diversity that mirrors the history of colonization rather than deterministic environmental factors (e.g., Ventura et al. 2014, but see also Kappas et al. 2017). However, the present case study slightly differs from this pattern since Tigriopus populations are known to be frequently wiped out by physical events (e.g., exceptional droughts, storms, or rainfalls), leading to recurrent local extinctions and recolonizations. Accordingly, those Tigriopus haplotypes that colonize a Tigriopus-free rock pool rapidly monopolize it preventing the establishment of other haplotypes, but this monopolization only lasts a relatively short amount of time, i.e., until a new event wipes this population out. Thus, unlike in the monopolization hypothesis, a long-lasting founder effect is not achieved, but rather just a temporary occurrence of monopolizing haplotypes can be observed. Such a pattern could be defined as a “clockwork monopolization”, related to the great instability of the rock pool habitats and to the inability of their inhabitants to produce long-lasting resting stages.











 nueva página del texto (beta)
nueva página del texto (beta)