Introduction
Neohelice granulata is a crab with a characteristic burrowing behavior. It digs and keeps open semipermanent burrows, allowing it to adopt a semiterrestrial life from the northerneastern coast of Patagonia, Argentina (42º25′S, 64º36′W), to Río de Janeiro, Brazil (22º57′S, 42º50′W) (Spivak 2010). This species uses these burrows to protect itself from wave action, extreme temperatures, and desiccation. The burrows also provide shelter from aerial and terrestrial predators during periods of low tide and from aquatic predators at high tide, thus avoiding the stress of finding refuge or escaping predators. Moreover, burrows are the places where 2 important events in a crab’s life occur, molting and reproduction, and also where young recruits are safeguarded until they reach larger sizes (Milner et al. 2010, Sal-Moyano et al. 2012).
When building and maintaining their burrows, crabs bring sediments to the surface, forming mounds near burrow entrances (Murray et al. 2002). This bioturbation affects sediment structure, because the cohesive nature of the organic matrix is disrupted by this process. Apart from directly affecting sediment porosity and permeability, this process has ecological significance in the aeration of soils containing anoxic sediments and in the distribution of halophytes. Consequently, high densities of active burrowers can increase erosion rates and sediment mobility (Botto and Iribarne 2000).
Variations in the architecture of N. granulata burrows have been observed in studies conducted at other locations in Argentina, such as Mar Chiquita Lagoon, Buenos Aires Province (36º09′26″S, 60º34′11″W; Iribarne et al. 1997, Botto et al. 2006), and San Antonio Bay, Río Negro Province (40º44′51.43″S, 64º52′5.10″W; Sal-Moyano et al. 2012, Luppi et al. 2013, Bas et al. 2014). These studies revealed differences in terms of size and shape of burrows (diameter, depth, volume, and angle) when different intertidal sites, relative to the tide line, were compared.
In the Bahía Blanca Estuary (30º45′-39º25′S, 61º45′-62º25′W), which is near the southern range of N. granulata, Escapa et al. (2007, 2008) analyzed the potential role of the burrowing crab in the erosion of salt marshes and described some architectural variables; their studies are the only antecedents near our study area. However the Bahía Blanca Estuary is a broad ecosystem, and local and specific studies are necessary to include the different habitats in this singular environment. In this context, the main goal of the present study was to assess differences in burrowing behavior and the structural morphology of N. granulata burrows at different intertidal sites in a mesotidal estuary. We hypothesized that burrowing activity and burrow morphology would vary between contrasting habitats, which were selected on the basis of their differences in tide level, sediment characteristics, and substrate type.
Materials and methods
Study area
The Bahía Blanca Estuary, in the southwest region of the Buenos Aires Province, covers an area of approximately 3,000 km2. It is a mesotidal coastal plain estuary with a semidiurnal tidal regime, and it is characterized by the presence of islands that are interconnected by an extensive system of tidal channels that is affected by up to 4-m tides. Freshwater input to the estuary is weak and comes mainly from 2 tributaries on the northern shore of the interior part of the system (Melo 2004). Salinity in the middle portion of the estuary is about 33.98 (annual mean), whereas the inner zone becomes hypersaline during dry summers because of the high evaporation rate (Piccolo et al. 2008). The sampling area was located in Cuatreros Port (38º44′50″S, 62º23′5″W). It is the site that is most representative of the inner zone of the estuary in terms of hydrodynamic conditions and the site with the most marked temperature and salinity fluctuations. The inner zone acts as a true estuary (Negrín 2011) (Fig. 1a).
Field surveys
We conducted seasonal samplings from autumn 2013 to autumn 2015. Measurements of burrow architecture were performed during the 2013/2014 and 2014/2015 breeding seasons. We selected 2 habitats with visually different vegetation composition and sedimentary and hydrodynamic conditions: a Sarcocornia perennis saltmarsh in the middle intertidal zone and a mudflat in the lower intertidal zone (Fig. 1b).
Burrow density was quantified using quadrats (0.5 × 0.5 m), which were randomly placed at each site during low tides, when the area was exposed (10 replicates). The total number of open burrows in each quadrat was counted, and active burrows were identified by the presence of crabs, prints, or removed sediments, which are lighter in color and have different texture (biogenic mounds). Polyester resin and 2 components, accelerator (cobalt octoate solution) and catalyst (methyl ethyl ketone peroxide), were poured into selected active burrows to make burrow casts.
Sediment samples from the adjacent surface were collected to compare sediments from biogenic mounds with sediments from areas without crabs (control) and to determine water and organic matter contents, grain size distribution profiles, and mineralogical composition. Penetrability (N·cm-2) was measured as the pressure needed to compress the spring of a piston that was forced into the sediment to a standard depth (McLachlan and Brown 2006); low pressure values indicated soft sediments and high values indicated firm sediments.
Laboratory measurements and data analysis
Entrance diameter, tunnel diameter, total length, and total depth were measured from each burrow cast with a digital caliper (±0.1 mm). Curvature and number of branches and entrances were also recorded for each burrow cast. Only data from complete casts were used for analyses.
Sediment properties were analyzed. Water content was determined as the difference between wet and dry weight (after drying at 60 ºC for 5 d). Organic matter content was taken to be the difference between dry and ash-free dry weight (after ignition in a muffle furnace at 500 ºC for 7 h).
For grain size analysis, samples were dried to constant weight and organic matter was removed with hydrogen peroxide at 130 volumes, initially cold and then hot (60 ºC) until bubbling of organic material had ceased. This methodology was used to avoid interference with the determination of clay content. A laser diffraction particle size analyzer (Malvern Mastersizer 2000) was used to determine particle size distribution (0.2-2000 µm particles). Data were analyzed using Mastersizer 2000 v5.40 (Malvern Instrument Ltd. 2007) and Gradistat v5.0 (Blott and Pye 2001) software.
To determine the mineralogical composition of sediments, samples were dried to constant weight and then milled using an agate mortar. An X-ray diffraction analysis was performed with a Rigaku D-Max III-C diffractometer with CuKα radiation and a graphite monochromator at 35 Kv and 15 mA for the qualitative determination of minerals. The loose grain method was also applied under a magnifying glass using a B2-UMA trinocular Olympus stereomicroscope and computerized image processing programs.
To analyze the differences in mean burrow densities and sediment properties, two-way analyses of variance (ANOVA) were used. If a significant interaction was obtained, the main effects of fixed factors were not considered since this type of interaction indicates lack of independence (Underwood 1997). The null hypothesis of no differences in burrow architectural variables between microhabitats was assessed using one-way ANOVA (Zar 1999). For all statistical analyses, data were previously transformed to comply with the normality and homoscedasticity assumptions. In the case of significant differences with ANOVA, a posteriori multiple-comparison tests (Tukey test, Zar 1999) were used to identify significant differences.
Results
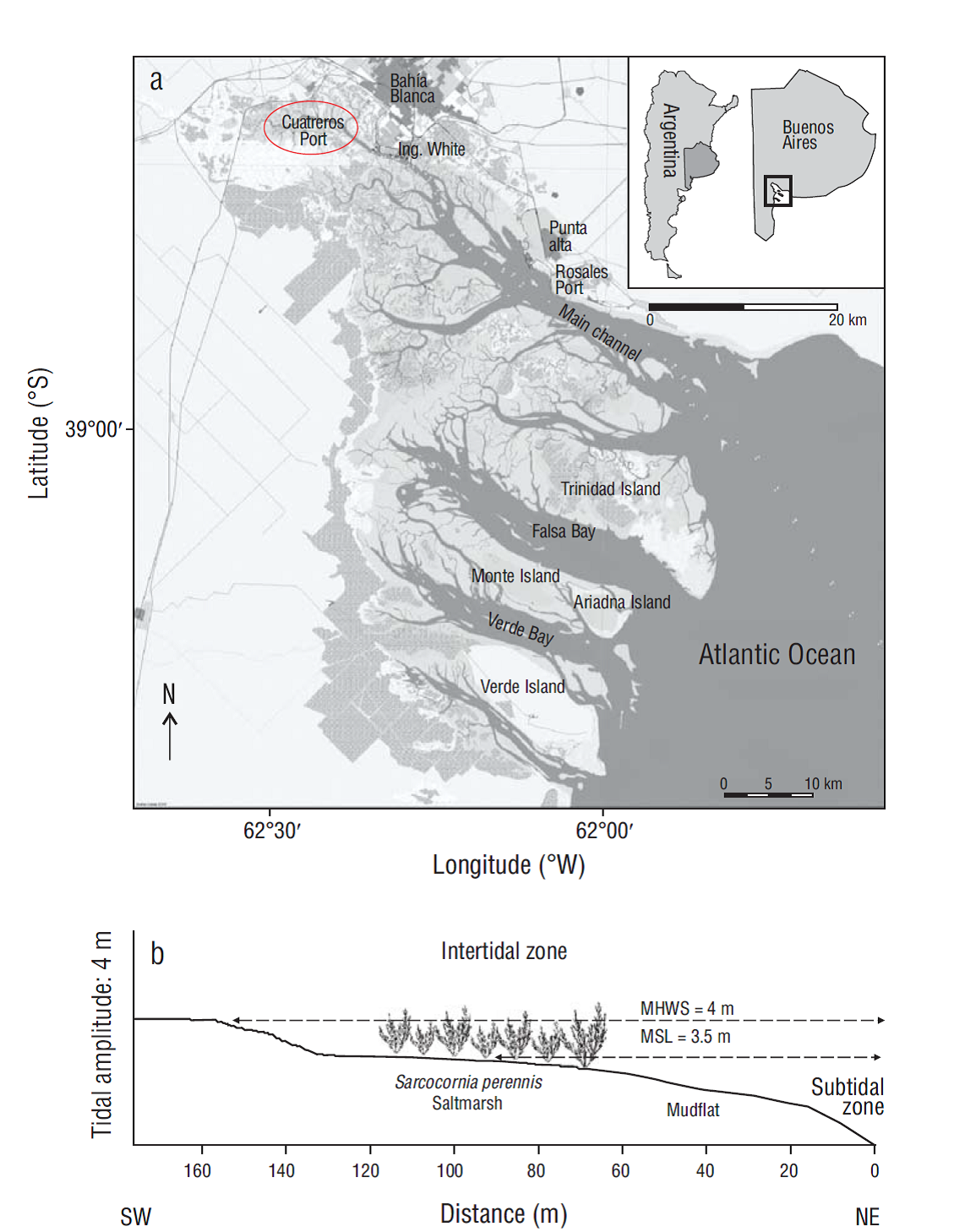
The total number of burrows (per square meter) differed significantly between sites (F = 236.78, P < 0.001) but not between seasons (F = 1.26, P > 0.05). The effects of interaction between sites and seasons were also not significant (two-way ANOVA interaction: F = 0.19, P > 0.05). Maximum was 172 burrows·m-2 in autumn in the mudflat (Fig. 2a). When only the active burrows were considered, differences occurred between sites (F = 55.36, P < 0.001) and between seasons (F = 6.61, P < 0.001). The effects of interaction between sites and seasons were also significant (two-way ANOVA interaction: F = 19.47, P < 0.001). The highest number of active burrows was found in the mudflat too, but unlike the previous case, significantly higher values were observed in spring and summer. The maximum number of active burrows (144 burrows·m-2) was recorded in summer in the mudflat, where all the burrows showed signs of activity (Fig. 2b).
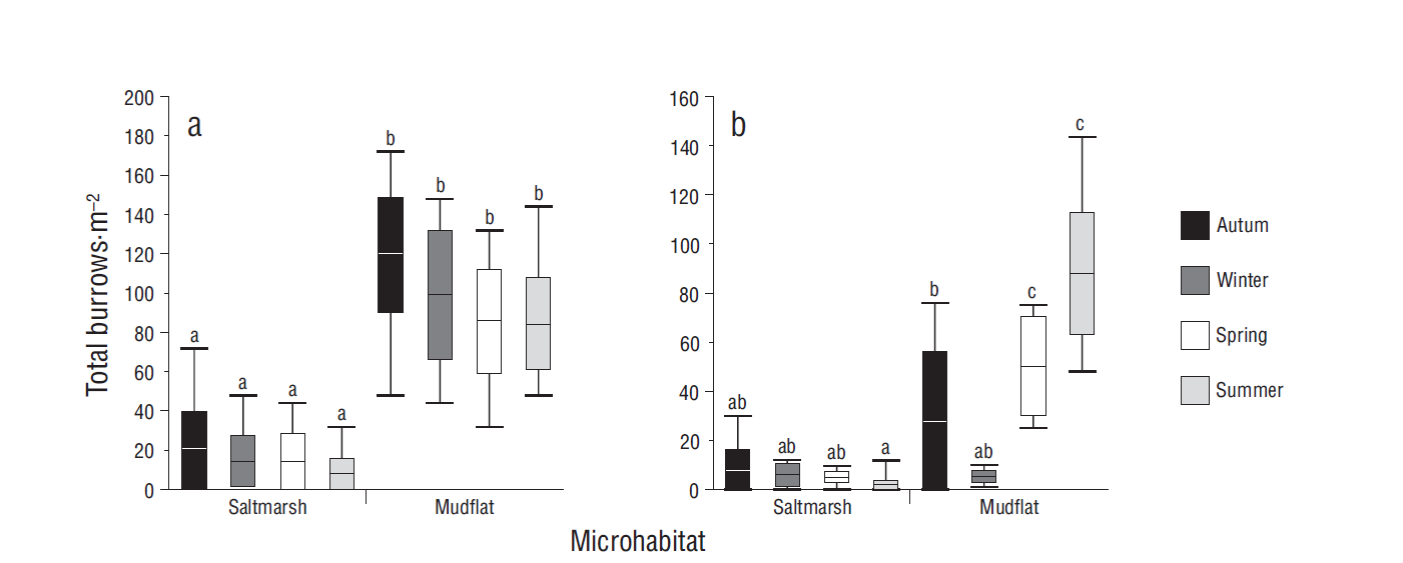
Figure 2 Density of Neohelice granulata burrows. Number of total burrows (a) and number of active burrows (b) in 2 microhabitats throughout the seasons. Lines inside boxes are median values, box limits are standard errors of the mean, and error bars represent non-outlier ranges. Different letters above the boxes denote significant differences between groups of data (Tukey test after ANOVA, P < 0.05).
Water content of sediments varied significantly between microhabitats (F = 18.90, P < 0.001). The sediments from the mudflat had higher water content than those from the saltmarsh. The effect of crab bioturbation on sediment condition was also significant (F = 6.71, P < 0.05); removed sediments had higher water content than control sediments. The effect of this interaction was not significant (F = 0.16, P > 0.05) (Fig. 3a). Organic matter content did not show significant differences between microhabitats (F = 0.04, P > 0.05) or between sediment conditions (F = 0.09, P > 0.05). The effect of the interaction was also not significant (F = 0.10, P > 0.05) (Fig. 3b). When sediment penetrability was analyzed, significant differences were found between microhabitats (F = 7.89, P < 0.05) and sediment conditions (F = 9.44, P < 0.05). Sediments from the mudflat had higher penetrability (lower pressure to penetrate the substrate) than sediments from the saltmarsh. Sediments removed from burrows had higher penetrability than control sediments. The interaction effect of these factors was not significant (F = 0.53, P > 0.05) (Fig. 3c).
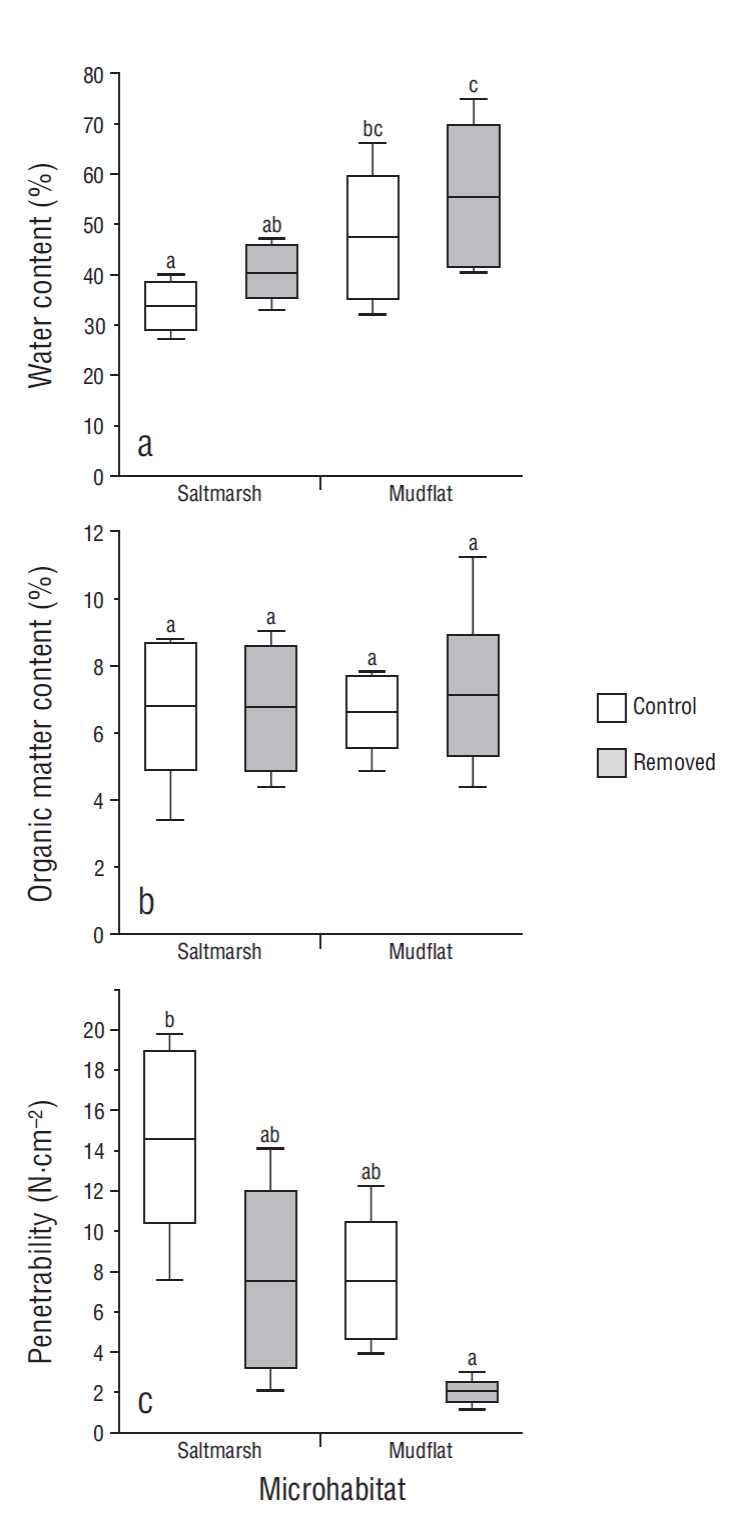
Figure 3 Sediment properties in 2 microhabitats: water content (a), organic matter content (b), and penetrability (c) in control sediments and sediments removed by crabs. Lines inside boxes are median values, box limits are standard errors of the mean, and error bars represent non-outlier ranges. Different letters above the boxes denote significant differences between groups of data (Tukey test after ANOVA, P < 0.05).
The grain size distribution profiles and mineralogical analysis did not show significant differences between sediments from the saltmarsh and sediments from the mudflat. These sediments were composed of thick and very thick limes and very fine sands. Most of the measurements were characterized by one mode between approximately 6 and 60 µm, corresponding to silt particles (Fig. 4). The mineralogical composition of the intertidal sediments was represented mostly by quartz, feldspar, calcite, and halite (salt), and to a lesser extent by clays such as illite and montmorillonite (Fig. 5). No seasonal variations were observed.
Figure 5 X-ray diffraction analysis. (a) Saltmarsh. (b) Mudflat. Qz, quartz; Pl, feldspar; Ar, clay; Ha, halite; Ca, calcite.
Biogenic mounds did not have a specific orientation. No general pattern was observed with their position in relation to the mouth of the burrow or to the location relative to the subtidal zone. The diameters of burrow entrances measured in the field showed significant differences between microhabitats (F = 29.71, P < 0.01). Burrow entrances from the mudflat were found to be significantly narrower, compared to the burrows from the saltmarsh (Fig. 6).

Figure 6 Entrance diameters of burrows measured in the field (EDF) in 2 microhabitats. Lines inside boxes are median values, box limits are standard errors of the mean, and error bars represent non-outlier ranges. Different letters above the boxes denote significant differences between groups of data (Tukey test after ANOVA, P < 0.05).
From the casts obtained during field sampling, we observed that burrows had both simple and complex morphologies, ranging from vertical tunnels with a single entrance to complex interconnected tunnels with multiple entrances. Entrance diameters did not show significant differences between the microhabitats (F = 0.53, P > 0.05), nor did the tunnel diameters (F = 0.06, P > 0.05). While for the length variable, significant differences occurred between microhabitats (F = 6.88, P < 0.05). We also found significant differences in burrow depth between microhabitats (F = 5.85, P < 0.05). The casts from the saltmarsh were significantly longer and deeper than those from the mudflat (Fig. 7). The relationship between the depth of the tunnel and the diameter of the entrance (ratio >1) allowed all casts to be classified as tubular. Chambers were found inside burrows from the saltmarsh. These chambers were recorded only within the tunnels, not at the entrances of the burrows.

Figure 7 Parameters measured in burrow casts. (a) Entrance diameter. (b) Tunnel diameter. (c) Total length. (d) Total depth. Lines inside boxes are median values, box limits are standard errors of the mean, and error bars represent non-outlier ranges. Different letters above the boxes denote significant differences between groups of data (Tukey test after ANOVA, P < 0.05).
Discussion
Neohelice graulata density decreases towards the upper intertidal zone, as noted by Bortolus and Iribarne (1999). However the colonization of the high marsh by N. granulata is facilitated by the presence of plants. Sarcocornia perennis is a good colonizer of high and saline intertidal areas. It generates a shading area allowing the sediment to stay wetter, therefore softer and more liable to being excavated. It also dampens various environmental factors, such as desiccation and high surface temperatures (Bortolus et al. 2002).
The maximum density of total N. granulata burrows recorded in this study was 72 burrows·m-2 for the saltmarsh and 172 burrows·m-2 for the mudflat. These densities are comparatively similar, and sometimes higher, to those reported by other authors for the Argentinean coast. An average of 64.8 burrows·m-2 in saltmarshes and a maximum of 75 burrows·m-2 in mudflat areas were found by Escapa et al. (2008) and Montemayor et al. (2011) in the internal section of the Bahía Blanca Estuary. In the Mar Chiquita Lagoon, burrows can reach a maximum density of 60 burrows·m-2 in saltmarshes and 100 burrows·m-2 in mudflats (Iribarne et al. 1997, Bortolus et al. 2002). By contrast, in the wetlands of Santa Lucía, Uruguay, a maximum of 95 burrows·m-2 were recorded in saltmarshes (Merentiel-Ferreyra 2014).
A higher density of burrows in autumn might be related to the molting phenomenon. According to Luppi et al. (2013), N. granulata is almost inactive on the intertidal surface of the Mar Chiquita Lagoon and Bahía Blanca Estuary during this season, regardless of the daily variation in environmental conditions. So the burrows increase in number and their entrances are closed up with mud deposits. Crabs remain inside the burrows during the pre- and post-molt phases, suggesting a rather synchronized molting season. Burrows are the best refuge for the species during this delicate stage.
Total and active burrow densities showed high variation throughout the year in the mudflat, possibly one of the most dynamic areas in the intertidal zone. In this microhabitat we found the highest densities of active burrows in the summer, compared to the rest of the year. In addition, ovigerous females have been found only in this microhabitat; this could be interpreted as a reproductive advantage for the species. Settling in areas near the coastline during the summer months would allow ovigerous females to better oxygenate their eggs and readily release their larvae (Anger et al. 1994).
The physical properties of the sediments from the mudflat (e.g., the high water content and penetrability) might be correlated with their proximity to the subtidal zone, which is usually saturated with water. Sediments removed by crab activity had higher water content and penetrability than control sediments; this is in agreement with Botto and Iribarne (2000) and Escapa et al. (2007), who indicated that bioturbation increases the values of these parameters. No significant differences were found in the contents of organic matter between sediments, because remixing of sediments by the species kept organic matter homogeneously dispersed in the sedimentary column (Bortolus and Iribarne 1999, Gutiérrez et al. 2006).
The predominant fractions in the grain size distribution profile corresponded to the medium size (medium to fine sands) and the fine size (silts and clays). Homogeneous sediments indicate a low-energy environment. Tidal channels in low-energy environments branch out and leave wide, shallow flood plains, and energy in these areas consequently decreases (Gelós et al. 2004). No significant variations were found in the mineralogical composition of sediments, only in the proportion of minerals present in the sediments from the 2 microhabitats. This study constitutes the first mineralogical results for 2 intertidal zones from the Bahía Blanca Estuary that had not been previously studied.
Burrows in mudflats had a weak structure. They sometimes collapsed when the polyester resin was poured into them. In some cases burrows entrance diameters were overestimated because of this. This is the reason we found differences in the diameters of the burrow entrances from the mudflats when we used both methodologies. However, burrows in the upper intertidal zone were well structured. We believe that the diameters measured in the field are more accurate estimates of the actual dimensions of the entrances than those measured from the casts.
In our study site we found burrows with ramifications and multiple entrances, and this was directly associated with the type of substrate on which the burrows were built. Several experiments with Austrohelice crassa crabs showed that the burrows from sandy sites were unique and simple, while those at sites with a higher proportion of the clay-silt fraction were more complex in structure, with multiple vertical ramifications that were horizontally interconnected through tunnels (Morrisey et al. 1999). These multiple entrances may provide easy access to the shelter, especially in un-vegetated mudflats. In addition, there is evidence suggesting that a larger number of burrow entrances in intertidal mudflats increase the ability to entrap organic particles that may serve as food (Iribarne et al. 1997, Botto et al. 2006). This phenomenon was also observed for species of the genus Uca (Genoni 1991, Qureshi and Saher 2012) and in our study would be supported by the high densities of burrows found in the mudflat.
Burrows from the saltmarsh were longer and deeper than those from the mudflat near the subtidal zone for the internal zone of the Bahía Blanca Estuary. Crabs could change the depth of their burrows in relation to water table depth, to reach groundwater levels. In this way, the burrows would contain water throughout the tide cycle, an adaptation to maintain the humidity in the high areas of the marshes (Iribarne et al. 1997, Escapa et al. 2007).
The burrows with chambers are not mentioned in the bibliography for the Bahía Blanca Estuary. However, copulation is likely to occur not only on the surface but also within the burrows, as was found for the Grapsoidea superfamily by Brockerhoff and McLay (2005). In contrast, burrows from mudflats could have chambers in their tunnels, although they would not be as conspicuous as the ones described by these authors, since the value that was used to detect them (18 mm, Sal-Moyano et al. 2012) could be a very high parameter for this microhabitat.
Our observations and field experiments showed that burrow features and burrowing activity of N. granulata differed between study sites, according to biotic and abiotic factors, indicating that this species has an adaptive burrowing behavior. Moreover, in this study we demonstrated that burrowing activity affects some sediment properties. It is important to highlight that in environments with intermediate levels of energy (such as Bahía Blanca Estuary), burrowing organisms play a very significant role. They destabilize the substrate, making fresh material available to tidal currents and waves, which ultimately increases the load of suspended sediments within the environment. This favors the bioavailability of sediment in the water column, particularly when there are high densities of bioturbators as in mudflats. This may have important implications for the functioning of estuarine ecosystems.











 nueva página del texto (beta)
nueva página del texto (beta)