Introduction
Marine organisms, including algae and lichen, have attracted the attention of many researchers as sources of bioactive compounds because of their properties, the diversity of their molecules, and their novel chemical structures which are complex and difficult to synthesize chemically. Seaweeds provide an excellent source of bioactive compounds such as carotenoids, polysaccharides, proteins, lipids, fatty acids, pigments, vitamins, polyphenols, and microelements, among others (Borowitzka 2013). Biochemical compounds from algae are currently used as ingredients with bioactive properties in cosmeceutical products. These cosmetic products differ from classic cosmetics in that they have therapeutic or pharmacological properties. Lichens are also well known due to the diversity of the secondary metabolites that they produce known as lichenic substances. Recently, there is increased interest in natural active compounds as an alternative to synthetic substances. Although these compounds often show lower activity, they are nontoxic and do not generate residues.
Antioxidants present in extracts from algae and lichen are of special interest for use in cosmetics and nutraceuticals (oral and topical formulations) since their potential toxicity and health risks are not as great as that of synthetic antioxi-dants (Thomas and Kim 2013). Pharmacological activities (antitumor and anti-inflammatory) have been closely related to oxidative imbalance. Antioxidant properties of algal natural compounds can increase the shelf life of foods and cosmetics through delayed oxidation (Chintali Ashwini et al. 2013). In addition to antioxidants, algae also provide other ingredients used in the formulation of cosmetic products, such as other bioactive ingredients, minerals, polysaccha-rides, etc. This supports the use of algae and lichen to advance human health, as functional foods and/or dietary supplements, due to the positive impact that their use would have in the prevention and/or treatment of pathologies linked to oxidative stress (Yuan and Walsh 2006).
This study aims to evaluate the antioxidant activity of algal extracts and one marine lichen extract by using different solvents, related to different mechanisms of action, which can be explained by the presence of various metabolites (Batista-González et al. 2009). We quantified the content of algal and lichenic compounds with potential bioactive properties, such as carbohydrates, lipids, and UV-screen substances with antioxidant capacity like polyphenols and mycosporine-like amino acids (MAAs) (Stengel et al. 2011). These compounds show important applications in a range of products in the food, pharmaceutical, and cosmetic industries.
The following species were selected for this study because they can be intensively cultured in integrated multi-trophic aquaculture systems (Gómez-Pinchetti et al. 2011) and because of the potential biotechnological use of the algal biomass: Gelidium corneum (Hudson) JV Lamouroux and Gelidium pusillum (Stackhouse) Le Jolis (Rabiei et al. 2016); Porphyra umbilicalis Kützing (Pereira and Yarish 2010); Halopithys incurva (Hudson) Batters (Güenaga 2011); Gracilariopsis longissima (SG Gmelin) M Steentoft, LM Irvine, and WF Farnham (Hernández et al. 2006); Hydropuntia cornea (J Agardh) MJ Wynne (Figueroa et al. 2012); and Ulva rotundata Bliding (Martínez-Aragón et al. 2002). The culture of Lichina pygmaea (OF Müller) C Agardh is currently not feasible, so it is only possible to cultivate photobionts of the cyanobacterial genus Calothrix for the production of metabolites of interest. The symbiosis between photobiont and mycobiont is probably more favorable for the production of bioactive compounds due to the synergies that may arise between both organisms.
Materials and methods
Seven clean and fresh macroalgae and one marine lichen were collected in coastal areas of different geographic locations at the same or approximately the same moment in time. The biomass was placed in a portable ice chest (approximately -4 °C) for 3-4 h. All species were collected at Tarifa, Cádiz, Spain (36°00'10"N, 5°36'33"W), except Gracilariopsis longissima (San Pedro River, Cádiz, Spain; 36°32'52"N, 6°12'33"W), Hydropuntia cornea (Gran Canaria, Canary Islands, Spain, 27°59'28"N, 15°22'8"W), and U.rotundata (Málaga, Málaga, Spain; 36°42'41"N, 4°19'33"W). Only H. cornea was obtained from cultures (algal densities 6-8 g fresh weight per liter) in tanks located in a greenhouse.
Algae were dried between sheets of paper to remove excess moisture and frozen at -20 °C until the analysis. Extraction was done with various alcoholic and hydroalco-holic solvents: distilled H2O (100%), EtOH (100%), EtOH (50%), and MeOH (20%). For each extract, 10 g fresh weight of algal thallus was used. Thalli were ground in a mortar (placed on a bed of ice) with 150 mL of the above-cited solvents. Sterile beach sand was used to achieve greater abrasion. The extracts were incubated in a thermal bath (SS40-2, Grant Instruments, Cambridge, UK) at 45 ± 2 °C with constant stirring for 6 h. After this time, the first extracts were filtered through a 100-μm mesh and then centrifuged (Beckman GS-15 R centrifuge) for 10 min at 4 °C. Finally, the supernatant was concentrated on a Buchi R-210 rotary evaporator.
The extraction yields (η) for each solvent were calculated after extraction. The yields related to the extracted biomass (dry weight) were calculated according to the following formula: (η%) = (solubilized(obtained)/biomass(provided)) x 100. Solubilized(obtained) (in grams dry weight) is the amount of material extracted from the biomass with the different solvents and biomass(provided) is the amount of biomass (in grams) used to make the extraction. The fresh weight:dry weight ratio (FW/DW) was calculated for each species-after keeping the samples for 24 h at 60 °C.
The organic matter content (%) was estimated by measuring the loss on ignition at 550 °C during 3.5 h. Biochemical tests were performed in triplicate.
Methods for testing antioxidant activity
The DPPH assay was performed according to the method developed by Kim et al. (2002). The reaction was complete after 30 min in the dark at room temperature (~20 °C) because light can degrade the generated radical. The absorbance was read at 517 nm in a spectrophotometer (UVMini-1240 model, Shimadzu, Columbia, USA).
The ABTS assay was performed as described by Re et al. (1999). The mixture was allowed to stand for 8 min at room temperature and the absorbance was immediately recorded at 413 nm. The antioxidant activity of the extracts measured by the DPPH and ABTS assays was calculated by following the same mathematical procedure.
The β-carotene bleaching method (BBM) was performed according to Hidalgo et al. (1994). The decrease in absorbance at 470 nm for 90 min was monitored when the extract was added to the solution. The antioxidant activity (AA%) was calculated according to the following equation: [AA% = (slope(sample) - slope(control)/slope(control)) x 100]. Slope refers to the slopes of the obtained discoloration curve (absorbance/time). For this, the part of the curve describing linear behavior was adjusted by linear regression. A Trolox solution (0-15 μM) was used as a standard for the 3 different methods tested. The results were expressed as micromoles of Trolox equivalents per gram of dry weight (μmol TE g-1 DW). The values shown in this study are for each species and for each method used.
Total internal carbon and nitrogen contents were determined in an elemental analyzer (LECO-932 CNHS, Michigan, USA) and C:N ratios were calculated.
Quantification of total carbohydrates was conducted by the anthrone method (Brooks et al. 1986). Results were expressed as starch equivalent (% starch) by multiplying by a factor of 0.9.
Total lipid contents in the extracts were quantified by the sulfo-phospho-vanillin (SPV) method according to Mishra et al. (2014). To obtain the standard curve, triolein was used as standard at the final concentrations of 0.1-0.6 mg L-1.
Phenolic compounds were determined after the incubation period according to Folin and Ciocalteau (1927). Phloroglucinol was used for the standard calibration curve.
The MAAs were analyzed with a Waters HPLC system (Waters 600) as described by Korbee-Peinado et al. (2004), using published extinction coefficients.
Statistical analysis
Analysis of variance (ANOVA) was used to test the differences in the studied variables. Two-way ANOVA was used to detect significant differences among different antioxi-dant assays and solvents among species, and one-way ANOVA was used for the other variables. Homogeneity of variance was tested using the Cochran test and by visual inspection of the residuals for both analysis. Student-Newman-Keuls (SNK) tests were performed after significant ANOVA (post hoc analysis). Correlations among data obtained were calculated using Pearson's correlation coefficient (r). All analyses were done with SPSS v10.0 for Windows (SPSS, Chicago, IL, USA).
Results
The FW/DW ratio was 4.75 for Gelidium pusillum, 3.05 for Gelidium corneum, 5.60 for Porphyra umbilicalis, 3.68 for Halopithys incurva, 7.57 for Gracilariopsis longissima, 9.17 for Hydropuntia cornea, 2.54 for Ulva rotundata, and 3.67 for Lichina pygmaea. The water content in the studied species varied between 60% and 90% (Table 1) and the organic matter content ranged from 9.4% in Halopithys incurva to the lowest values of less than 6% in Gracilariopsis longissima and Hydropuntia cornea (Table 1). Ash content was very high in U. rotundata and L. pygmaea (about 32%), whereas the minimal value of 5.3% was found in H. cornea (Table 1).
Table 1 Carbon and nitrogen content, humidity, organic matter, and ash in the different species analyzed. Each value is presented as mean ± standard error (n = 3): C, N, and C:N ratio based on percent dry weight (v/v); humidity, organic matter, and ash based on percent fresh weight. Different letters in the same column indicate significant differences among species (P < 0.05).

The yield extraction was significantly different (P < 0.01) depending on the solvent used (Fig. 1). The order of the yield extraction, from high to low, was as follows: 20% MeOH = H2O; H2O = 50% EtOH; 50% EtOH = 100% EtOH (Fig. 1).

Figure 1 Extraction yields (% yield [v/v]) for the solvents used. Data are expressed as the mean of all analyzed species (mean ± standard error; n = 27). Different letters indicate significant differences among solvents (P < 0.05).
The antioxidant capacities of the 8 species were tested with 3 antioxidant methods. In the case of the DPPH and ABTS methods, the solvent that showed the highest value in the assays was 50% EtOH (for 66.7% and 44.4% of the species studied, respectively). However, in the case of BBM, the solvent with the best result was 100% EtOH (for 44.7% of the species). For all the macroalgal species, significant interactions were found between the factors "solvent" and "method" (P < 0.01). In the case of Gelidium pusillum, the highest activity in the DPPH assay (9.65 μmol TE g-1 DW) (P < 0.01) was found in 50% EtOH (Fig. 2a). The highest antioxidant capacity was observed in both ethanolic solvents (50% EtOH and 100% EtOH). This same pattern was found for Gelidium corneum, although there was no significant difference between the 50% EtOH and 100% EtOH solvents in the DPPH assay, which showed greater activity (Fig. 2b). In the case of P. umbilicalis (Fig. 2c), the aqueous solvent showed similar results for DPPH and ABTS. Antioxidant activity for DPPH and BBM was also the same for the H2O, 100% EtOH, and 20% MeOH extracts (P > 0.05). The solvent that showed the highest extracting capacity of antioxidant compounds for this species was 50% EtOH (10.7 μmol TE g-1 DW) in the DPPH assay. In the case of Halopithys incurva (Fig. 2d), the highest antioxidant capacity (10.7 μmol TE g-1 DW) was reached in 20% MeOH for DPPH. There were no significant differences between 50% EtOH and 20% MeOH, as well as between H2O and 100% EtOH in BBM. In the case of Gracilariopsis longissima, the DPPH and ABTS assays showed no significant differences in terms of antioxidant capacity for the 20% MeOH and H2O solvents (Fig. 2e).
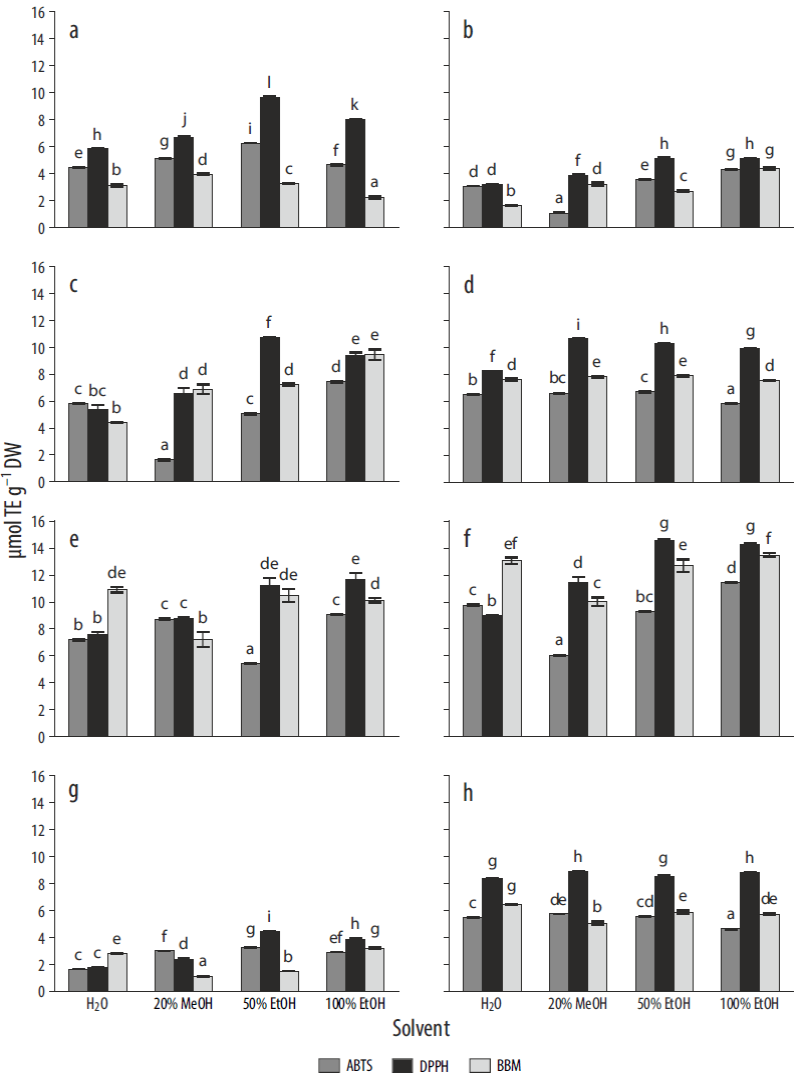
Figure 2 Antioxidant capacity, expressed as micromoles of Trolox equivalents per gram dry weight (μmol TE g-1 DW), in species obtained through ABTS, DPPH, and BBM assays using different solvents. (a) Gelidium pusillum, (b) Gelidium corneum, (c) Porphyra umbilicalis, (d) Halopythis incurva, (e) Gracilariopsis longissima, (f) Hydropuntia cornea, (g) Ulva rotundata, and (h) Lichina pygmaea. Different letters indicate significant differences for solvents and methods (P < 0.05).
There were no significant differences between BBM and DPPH in regard to 50% EtOH. In general, the highest antioxidant activity was found in the ethanolic solvent. Hydropuntia cornea showed the highest antioxidant capacity among the species studied, with maximum values of approximately 14.5 μmol TE g-1 DW in the ethanolic extracts (Fig. 2f). Ulva rotundata presented lower antioxidant capacity than that in the red algae analyzed, the highest value being obtained with 50% EtOH in the DPPH assay (4.46 μmol TE g-1 DW) (Fig. 2g).
In the case of L. pygmaea (Fig. 2h), using the DPPH method the antioxidant activity was slightly higher for 20% MeOH and 100% EtOH than that in the other 2 solvents. In the ABTS assay, the antioxidant activity in water and 100% EtOH was lower than that in the other extracts (Fig. 2). However, the highest value according to BBM was found in H2O.
The carbon content was significantly different in the analyzed species (P < 0.01), except for the Gelidium species (P > 0.05). The carbon content ranged from 22.2% in U. rotundata to 39% in the marine lichen L. pygmaea. Values up to 30% were also found in Gelidium pusillum, Gelidium corneum, P. umbilicalis, and Gracilariopsis longissima. The nitrogen content turned out to be even more heterogeneous (P < 0.01) than that of carbon. The highest level was reached in L. pygmaea (6.2%), followed by the Gelidium species (higher than 3.5%) (Table 1). Gracilariopsis longissima and Hydropuntia cornea presented similar values. However, the species with different carbon and nitrogen content showed the same C:N ratio (Table 1).
Total carbohydrates, expressed as percentage, showed the highest value in 50% EtOH and 20% MeOH (Table 2). In 20% MeOH, Halopithys incurva extracts showed the highest percentage of total carbohydrates among the algae studied (16.1%), followed by Gracilariopsis longissima (14.8%). Levels of 4-6% were observed in Gelidium corneum, P. umbilicalis, and U. rotundata, whereas levels higher than 6% were detected in Gelidium pusillum and Hydropuntia cornea. In 20% MeOH, the lowest level of total carbohydrate was observed in L. pygmaea (3.28%).
Table 2 Total carbohydrates and total lipids (% dry weight) for the various solvents and species. Each value is presented as mean ± standard error (n = 3). Different letters in the same row indicate significant differences among solvents for each species (P < 0.05); n.p.q. = not possible to quantify.
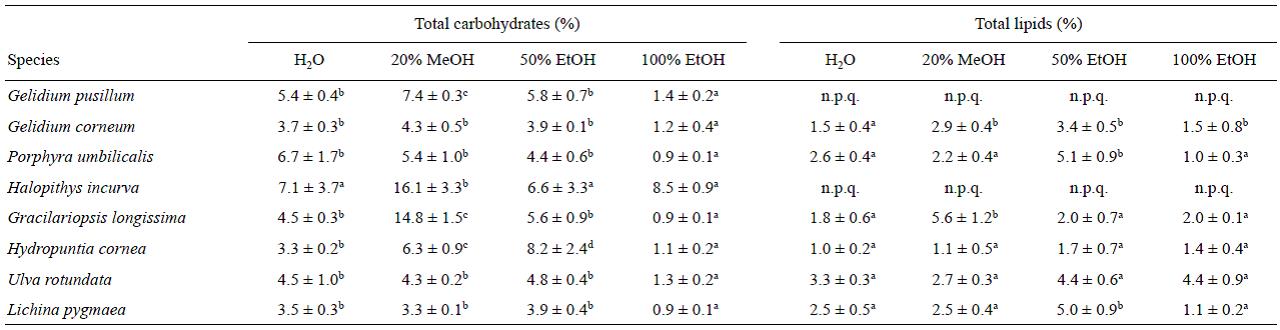
The highest lipid content was obtained with 50% EtOH in 6 species. The lipid content in Halopithys incurva and Gelidium pusillum could not be quantified as there was high interference between the phenolic content and the SPV method (Sun et al. 1998). The highest values (higher than 5%) were found for P. umbilicalis, and L. pygmaeae. The other algae presented values between 4.4% and 1.7% (Table 2). In contrast, the lowest content was obtained in P. umbilicalis with 100% EtOH.
For 3 of the 8 species studied, phenolic compounds (expressed as phloroglucinol) showed higher values for the 20% MeOH solvent (Fig. 3). Significant differences among solvents were found for all the species (P < 0.01), except for L. pygmaea. The highest values of phenolic compounds were obtained for Gelidium pusillum (4.5-6 mg g-1 DW), followed by L. pygmaea (7 mg g-1 DW), Halopithys incurva (4.5 mg g-1 DW), and Hydropuntia cornea (3 mg g-1 DW) in 20% MeOH (Fig. 3). The other species presented values close to or lower than 3 mg g-1 DW (Fig. 2).
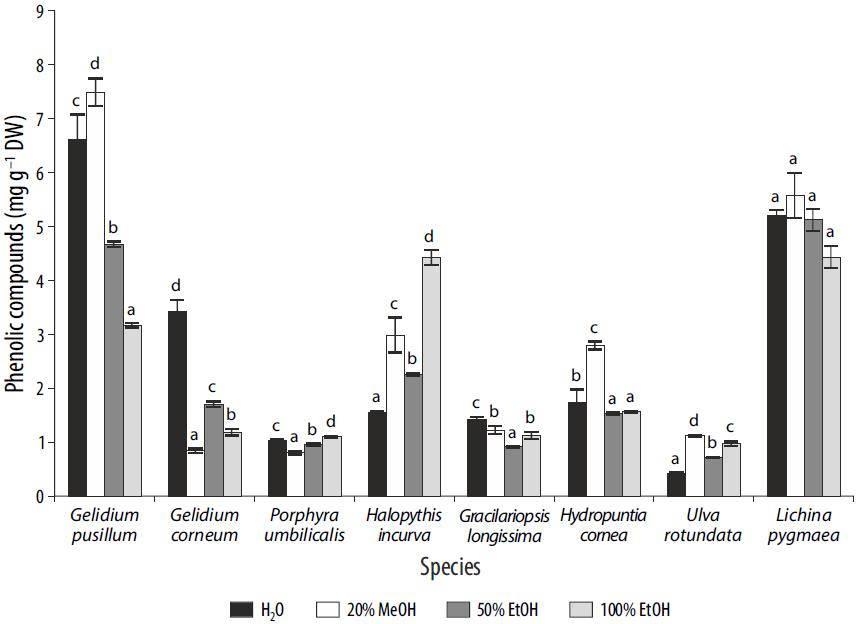
Figure 3 Phenolic compounds (mg g 1 dry weight) in different solvents for each species. Differences among solvents are indicated by different letters for each species (P < 0.05). Data are expressed as mean ± standard error (n = 3).
The amount and composition of MAAs was significantly different among the species studied. Six different MAAs were detected in different proportions: mycosporine-serinol, palythine, palythinol, shinorine, porphyra-334, and asterina-330. The last 5 MAAs were only present in Gelidium pusillum and P. umbilicalis, whereas mycosporine-serinol was present only in L. pygmaea (Table 3). The content of total MAAs in Gelidium pusillum was about 1.7 times higher than that of Gelidium corneum. For these UV absorbing compounds, no significant differences were found in terms of solvent used during the extraction except for 100% EtOH, where the content of MAAs was much lower than elsewhere.
Table 3 Composition of mycosporine-like amino acids (MAAs) and total concentration of MAAs in different species and solvents. Data are expressed as mean ± standard error (n = 3) (% w/w dry weight). Different letters indicate differences among solvents for each species (P < 0.05); n.f. = not found.

The second dominant MAA was palythinol, which was detected in all species except in L. pygmaeae. Palythine was observed in all species except in the marine lichen and Gracilariopsis longissima. In contrast, mycosporine-serinol was only found in L. pygmaeae, whereas porphyra-334 was found in Gelidium pusillum, P. umbilicalis, and Hydropuntia cornea. Finally, asterina-330 was detected in both Gelidium species and in P. umbilicalis.
The antioxidant capacity in Gelidium pusillum was correlated with the lipid content in the ABTS assay (P < 0.05; r = 0.98; n = 12), and with the total carbohydrate content (P < 0.05; r = 0.98; n = 12) and the MAA asterina-330 (P < 0.05; r = 0.67; n = 9) when BBM was used. However, in the case of Gelidium corneum, in the ABTS assay, the anti-oxidant capacity was related to only total MAAs (P < 0.01; r = 0.78; n = 12). For P. umbilicalis, in the ABTS assay, the antioxidant capacity was correlated with the phenolic content (P < 0.01; r = 0.99), the MAA shinorine (P < 0.01; r = 0.83; n = 12), porphyra-334 (P < 0.01; r = 0.92; n = 12), and total MAAs (P < 0.01; r = 0.91; n = 12). In Halopithys incurva (P < 0.01; r = 0.80; n = 12) and U. rotundata (P < 0.01; r = 0.72; n = 12), the phenolic content correlated positively with the antioxidant capacity. In Gracilariopsis longissima, the antioxidant capacity measured with BBM correlated with lipids (P < 0.01; r = 0.80; n = 12). The antioxidant capacity in Hydropuntia cornea correlated with the MAA palythinol (P < 0.05; r = 0.82) in the DPPH assay. Finally, the antioxi-dant capacity in L. pygmaea was correlated with the phenolic content (P < 0.05; r = 0.969; n = 12) and total carbohydrates (P < 0.01; r = 0.89; n = 12) in the ABTS assay. The MAA shinorine was correlated with antioxidant activity (P < 0.01; r = 0.80; n = 12) in the DDPH assay.
Correlation between antioxidant activity was found among the 3 methods: ABTS-DPPH (P < 0.01; r = 0.859; n = 36), ABTS-BBM (P < 0.01; r = 0.825; n = 36), and DPPH-BBM (P < 0.01; r = 0.841; n = 36).
The quantity of soluble carbohydrates was correlated with antioxidant activity measured with ABTS (P < 0.01; r = 0.28; n = 96) and DPPH (P < 0.01; r = 0.30; n = 96). The lipid content was related to antioxidant activity in Gracilariopsis longissima with ABTS (P < 0.01; r = 0.25; n = 96) and with BBM (P < 0.01; r = 0.99; n = 96).
The phenolic compound content was correlated with the ABTS (P < 0.05; r = 0.21; n = 96) and DPPH (P < 0.01; r = 0.28; n = 96) methods. Finally, total MAA levels were correlated with the 2 antioxidant methods used: ABTS (P < 0.05; r = 0.408; n = 36) and DPPH (P < 0.05; r = 0.431; n = 36).
Discussion
In general, it is noteworthy that in the aqueous medium (H2O), the antioxidant capacity observed by the DPPH and ABTS methods was similar in 55% of the species analyzed; an explanation for this result is that both methods have the same action mechanism. Between these 2 antioxidant assays, the solvent with the highest antioxidant capacity extraction was 50% EtOH for 44% and 56% of the species studied with the ABTS and DPPH methods, respectively. These results are in agreement with those obtained by Plaza et al. (2010). However, for the BBM assay, the best solvent was 100% EtOH, probably related to the fact that this method is suitable for the determination of antioxidant compounds in a lipophilic medium.
Another important difference between the assays is that the ABTS radical can be dissolved both in organic and aqueous medium; therefore, the antioxidant activity can be measured considering the hydrophilic or lipophilic nature of the compounds in the sample (Wojdylo et al. 2007). On the contrary, the DPPH radical can only be measured in an organic medium, limiting the interpretation of the antioxidant capacity of hydrophilic compounds present in the extracts (Surveswaran et al. 2007). On the other hand, a synergism among different bioactive compounds may also be occurring in our extracts.
In order to select a macroalgal species it is necessary to develop appropriate, quick, cost-efficient, and environmentally friendly methods of extraction that aim to isolate biologically active compounds of interest without loss of the activity. In numerous studies, the most common solvents for the extraction of antioxidants include water, ethanol, metha-nol, and aqueous mixtures thereof (Alam et al. 2013). These have good polarity and are therefore preferably used for extraction of polar compounds such as phenolic compounds or MAAs, among others. Ultimately, the polarity of the extracting solvent as well as the technique of extraction have a critical effect on the extraction yield (López et al. 2011). Methanol, however, should be discarded for cosmetic applications due to its toxicity.
Among the radical scavenging assays used in this study, the ABTS and DPPH methods are the fastest, simplest (involving fewer steps), and have a lower cost compared to BBM and other methods. In addition, BBM requires many reagents, a long time, and an advanced level of technology, and the result only provides a vision of lipophilic antioxi-dants. The ABTS assay is the only method tested in this study that is applicable to both hydrophilic and lipophilic antioxi-dants (Alam et al. 2013), so its use is justified in studies where it is intended to have an integrated vision of the extract. We found a positive correlation between the DPPH and ABTS methods, between ABTS and BBM, and between BBM and DPPH. A positive correlation between the DPPH and ABTS methods was also found by Floegel et al. (2011), probably because these assays are based on electron transfer, while BBM is based on a mechanism of hydrogen atom transfer (Huang et al. 2005).
In our study, in general, the antioxidant activity in the macroalgae and marine lichen analyzed is high (5-15 μmol TE g-1 DW) compared with other results published for several microalgae (7-11 μmol TE g-1 DW) and other macroalgae (1.6-2.2 μmol TE g-1 DW) (Matanjun et al. 2008). This could be explained by the fact that most species analyzed in other studies are subjected to stressors in their habitat or in the culture system. The increase in stress provokes an activation of both enzymatic and nonenzymatic antioxidant substances (Stengel et al. 2011). Regarding the effect of type of solvent on the antioxidant activity, Yuan et al. (2005) estimated antioxidant activity related to poly-phenols in Palmaria palmata and obtained the highest levels of these substances using methanol as extractor. Hydrophilic constituents are extracted with polar solvents such as water, methanol, or ethanol. O'Sullivan et al. (2011) used methanol and water to extract bioactive compounds (antioxidants) from different brown algae, Fucus being the genus with the highest antioxidant activity. The results of this study are consistent with the results found by Sarikurkcu et al. (2009) in extracts of terrestrial plants, in which a strong correlation between the polarity of the extracts and their antioxidant potential was observed. The most polar extracts showed a greater potential for capturing free radicals. To have a high biotechnological production of bioactive compounds, potentially cultivable species should ideally present low levels of water and high levels of organic matter. The analyzed red algae have much higher internal water levels and lower organic matter levels than L. pygmaea and U. rotundata (Chlorophyta). The latter 2 species presented low levels of internal water resulting in low FW/DW values. Holdt and Kraan (2011) reported a similar percentage of water in Ulva species (78-80%) compared to red macroalgae (72-85%). This depends on the characteristics of the species itself, as well as on the zone occupied in the ecosystem. The ash and organic matter content in the different species harvested from the natural environment also depends on the phylum, the geographical origin, and seasonal, environmental and physiological variables. In any case, minerals in the ash in algae are also interesting from a nutrition point of view.
Extraction of biologically active compounds from algal biomass is not selective. The extract is a mixture of different compounds. The factors that influence the composition and the activity of algal extracts depend on the species, environmental conditions, season of the year, age, geographical location, and processing technologies (Kadam et al. 2013). The environmental stress to which algae are exposed (e.g., irradiance, temperature, desiccation, osmotic stress) leads to the formation of free radical and oxidizing agents that provoke photodynamic damage (Gupta and Abu-Ghannam 2011).
Marine macroalgae are a source of bioactive compounds with different biological activities that are not found in terrestrial plants. Seaweeds contain polysaccharides (polymers of monosaccharides linked together by glycosidic bonds) that have numerous biotechnological applications in products such as stabilizers, thickeners, emulsifiers, food, beverages, etc. The total carbohydrate concentrations in the seaweed species of interest range from 4% to 76% DW. Carbohydrates can be chemically separated into soluble and insoluble fractions. The amount of soluble carbohydrates varies between taxa but, according to the results of this study, it is highest in red algae. The 2 species that showed the highest values of carbohydrates are Gracilariopsis longissima and Halopithys incurva (16.1% and 14.8%, respectively) in the methanol:water extract (20% MeOH). These species have been shown to have a high growth rate in integrated multitrophic aquaculture systems. At a stocking density of 6 g FW L-1, a biomass production of 22-29 g DW m-2 d-1 was found (Viera et al. 2011). A growth rate of 10-16% d-1 was reported for G. longissima and H. incurva (Robledo et al. 2014). In extensive aquaculture ponds, the growth rate of G. longissima was 9% d-1 (Hernández et al. 2006). Halopithys incurva has been cultivated in fishpond effluents at 8 g FW L-1 with a production of about 5 g DW m-2 d-1 and nitrogen uptake efficiency of 90-99%; however, at 6 g FW L-1, the average biomass productivity was 16-19 g DW m-2 d-1 (Viera et al. 2011).
Polyphenols were detected in all the analyzed species, but were higher in the red algae Gelidium pusillum and Halopithys incurva and in the marine lichen L. pygmaeae than in the other species. The lowest level of polyphenols was found in the green alga U. rotundata. In 44% of the species studied, the methanol:water extract (20% MeOH) was the most efficient for polyphenol extraction. This agrees with the results obtained by López et al. (2011) who describe a more efficient extraction of polyphenols in methanol or ethanol than in water; however, the water extract for the brown alga Stypocaulon scoparium showed both the highest activity and phenolic contents compared to the 50% metha-nol, 100% methanol or 100% ethanol extracts used in that study. López et al. (2011) observed that the levels of phenolic compounds could be 3 times different depending on the extraction method used. In this study, the content of phenolic compounds in some of the red algae and in the lichen studied are as high as in brown macroalgae, the microalgal group with the highest reported level of polyphenols (Connan et al. 2006, Stengel et al. 2011). For example, the content of phenols in the different solvents was 3.2-6.1 mg g-1 DW for Gelidium pusillum, 1.5-4.4 mg g-1 DW for Halopithys incurva, 1.5-2.8 mg g-1 DW for Hydropuntia cornea, and 4.4-5.2 mg g-1 DW for L. pygmaea. Güenaga (2011) previously reported high levels of phenols in Halopithys incurva. In addition, sulphated phenols and bromophenols have been detected in several species of red macroalgae (Zhao et al. 2004). Among the macroalgae, Rhodophyta possess the highest abundance of halogenated phenols (mainly bromi-nated and chlorinated) with antioxidant, antimicrobial, anti-cancer, antidiabetic, and antithrombotic effects. The results obtained for phenolic compounds in this study agree with the values found by other authors. Souza et al. (2011) reported values of 1.1 and 0.89 mg GAE (gallic acid equivalents) g-1 DW for Gracilaria birdiae and Hydropuntia cornea, respectively. For Porphyra sp., Kuda et al. (2005) obtained 0.88 mg CE (catechin equivalents) g-1 DW in ethanolic solvent. For Gracilariopsis tenuifrons, Zubia et al. (2014) reported values of 1-1.4 mg PGE (phloroglucinol equivalents) g-1 DW for phenols extracted with methanolic solvents.
The absorption spectra of the extracts presented characteristic peaks in the UVB (310 nm) and UVA (330-334 nm) region of the spectra (data not shown). This interval corresponds to absorption by photoprotective molecules (MAAs) present in some marine organisms, such as red algae and lichens. In U. rotundata, no absorption in this spectral range was found and no MAAs were detected by HPLC. The maximal absorption at 310 nm in the L. pygmaeae extracts corresponds to the oxo-MAA mycosporine-serinol, whereas the absorption peaks with maximum around 320-334 nm are related to imino-MAAs of red algae (palythine, palythinol, shinorine, porphyra-334, and asterina-330).
In our study, Gelidium pusillum and G. corneum presented palythinol and shinorine as the main MAAs. In general for all the studied species, the content of MAAs was much lower than elsewhere using 100% EtOH as solvent.
This may be explained by the lower polarity of pure ethanol to interact with the hydrophobic part of amino acids and peptides. MAAs can be different among the species due to environmental conditions (UV radiation, nitrogen availability, etc.) in which the specimens grow. The content of total MAAs in G. pusillum was about 1.7 times higher than that of G. corneum. The latter species is found in the lower part of the intertidal zone and in subtidal areas, whereas the former is found in the upper part, where the daily integrated irradiance is higher (Figueroa and Gómez 2001). MAAs are regulated by both irradiance and light quality, and also by nitrogen availability (Barufi et al. 2011). Torres et al. (2015) found 4 MAAs (asterina-330, palythinol, palythene, and usijerene) in Gracilariopsis tenuifrons after extraction with ethanol, whereas in this study only palythinol was found in G. longissima, but also shinorine using 20% MeOH or 50% EtOH. The low extraction yield found using ethanol as solvent could explain this result; shinorine was probably present but only in traces and therefore not detectable.
The C:N ratio is used as an indicator of nutrient status. Under limited nutrient resources, especially nitrogen, growth is restricted in photosynthetic organisms, generating an increase in the C:N ratio and this means an increase in the production of secondary metabolites (Bryant et al. 2012). Thus, C:N is a good indicator of increased production of secondary metabolites related to a decrease in nitrogen-enriched compounds. In relation to the content of antioxidant compounds, Ibrahim and Jaafar (2011) found, in terrestrial plants, that low nitrogen content and therefore high levels of C:N are related to the accumulation of secondary metabolites, that is, an increase in the antioxidant potential of the methanol extracts. These results are consistent with those observed in this study, where species with a high C:N ratio presented higher values of antioxidant activity (ABTS and BBM assays).
In conclusion, the 2 species of this study with the highest antioxidant capacity and molecules of biotechnological interest were the red algae Gracilariopsis longissima and Hydropuntia cornea. Halopithys incurva is also a species of interest because of its high levels of phenolic compounds and because it has previously been successfully cultivated (Güenaga 2011). On the other hand, the red alga Gelidium pusillum has high levels of MAAs and polyphenols but there are no available culture techniques. Moreover, numerous studies have pointed out that the first 2 species possess a high capacity to grow by effluent biofiltration (Figueroa et al. 2012), so outdoor culture would be economically viable and environmentally sustainable.
The most suitable solvent for a biotechnological purpose is ethanol (50% EtOH), an organic solvent with low toxicity and price. It could be used for the extraction of antioxidant compounds without affecting future application of the extracts. The ABTS method is proposed to determine the antioxidant capacity of algal extracts, as it is an easy, quick, and cheap test, and provides a comprehensive view of the entire extract in both lipophilic and hydrophilic media.
In order to gain a better understanding of the mechanisms underlying the antioxidant effects in algae it is necessary to study the structure-activity relationship and the synergy that could occur among molecules, to perform purification and structural analyses of potential antioxidant molecules, and to develop new extraction and purification techniques.











 texto en
texto en 



