Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Ciencias marinas
Print version ISSN 0185-3880
Cienc. mar vol.42 n.3 Ensenada Sep. 2016
https://doi.org/10.7773/cm.v42i3.2650
Articles
Calcification of the main reef-building coral species on the Pacific coast of southern Mexico
1 Laboratorio de Ecosistemas Costeros, Departamento de Hidrobiología, Universidad Autónoma Metropolitana (UAM), San Rafael Atlixco 186, Col. Vicentina, Delegación Iztapalapa, 09340 Ciudad de México, México.
2 Centro de Investigación Científica y de Educación Superior de Ensenada (CICESE), Carretera Ensenada-Tijuana no. 3918, Zona Playitas, 22860 Ensenada, Baja California, México.
3 Instituto de Investigaciones Oceanológicas, Universidad Autónoma de Baja California, Carretera Transpeninsular Ensenada-Tijuana no. 3917, Fraccionamiento Playitas, 22860 Ensenada, Baja California, México.
4 Universidad del Mar, Ciudad Universitaria, 70902 Distrito de San Pedro Pochutla, Puerto Ángel, Oaxaca, México.
Global warming and ocean acidification affect coral calcification. Nevertheless, there is not enough information regarding the growth parameters of the main reef-building coral species in marginal growth areas such as the Pacific coast of southern Mexico. In order to fill this gap, coral growth parameters of 8 hermatypic coral species (massive species: Porites panamensis, Porites lobata, Pavona gigantea, and Pavona varians; branching species: Pocillopora meandrina, Pocillopora damicornis, Pocillopora verrucosa, and Pocillopora capitata) were estimated in 2 areas of the southern Mexican Pacific. Branching coral species had a higher calcification rate (2.99-5.23 g CaCO3 cm-2 yr-1) than massive species (0.34-1.13 g CaCO3 cm-2 yr-1). A significant relation between sea surface temperature (SST) and skeletal density was observed in all massive coral species. Also, 2 massive species (P. gigantea and P. varians) showed a significant relation between SST and calcification rate. Upwelling in the Gulf of Tehuantepec transports deep water with low pH and low aragonite saturation, and may be affecting the calcification rate of stony corals in the studied area.
Key words: coral growth; density; Pocillopora; Pavona; Porites
El calentamiento global y la acidificación del océano influyen en la calcificación de los corales. No obstante, existe poca información respecto a los parámetros de crecimiento de las principales especies de corales constructores de arrecifes en áreas de crecimiento marginales como la costa del Pacífico del sur de México. Se obtuvieron los parámetros de crecimiento de 8 especies de corales hermatípicos (especies con crecimiento masivo: Porites panamensis, Porites lobata, Pavona gigantea y Pavona varians; especies con crecimiento ramificado: Pocillopora meandrina, Pocillopora damicornis, Pocillopora verrucosa y Pocillopora capitata) en 2 zonas de la costa del Pacífico del sur de México. Los corales ramificados tuvieron tasas de calcificación más altas (2.99-5.23 g CaCO3 cm-2 año-1) que los corales masivos (0.34-1.13 g CaCO3 cm-2 año-1). Se observó una relación significativa entre la temperatura superficial del mar (TSM) y la densidad del esqueleto para todas las especies de coral masivo. Además, 2 especies de coral masivo (P. gigantea y P. varians) mostraron una relación significativa entre la tasa de calcificación y la TSM. Las surgencias en la región del golfo de Tehuantepec acarrean aguas con bajo pH y baja saturación de aragonita, y podrían estar afectando negativamente la tasa de calcificación de los corales en el área de estudio.
Palabras clave: crecimiento coralino; densidad; Pocillopora; Pavona; Porites
Introduction
Studies aiming to understand the calcification process in hermatypic corals and the factors that limit their growth have been conducted since the 1960s. Wells (1963) determined that coral growth parameters are directly related to seasonal variations in environmental conditions. Knutson et al. (1972) subsequently confirmed this theory and recognized annual pairs of high- and low-density skeletal growth bands that represent the seasons when the calcification rates are higher and lower. This coral banding pattern provides a means of analyzing, retrospectively, the growth parameters of coralline skeleton based on three variables containing complementary information: (1) skeletal extension rate, (2) skeletal density (which depends directly on the density of aragonite [2.94gcm-3], the pores in the skeleton being responsible for affecting this value [Hughes 1987]), and (3) calcification rate (Dodge and Brass 1984, Carricart-Ganivet et al. 2000). Moreover, skeletal bands are associated with the prevailing environmental conditions where corals develop, and are thus an important tool for reconstructing the environmental conditions at coral reef locations (Knutson et al. 1972, Buddemeier 1974, Chalker et al. 1985).
The environmental variables that exert the greatest effect on coral growth are light (Buddemeier 1974, Wellington and Glynn 1983, Falkowski 2000, Yentsch et al. 2002), sedimentation (Dodge et al. 1974, Loya 1976, Barnes and Lough 1999, Fabricius 2005), nutrient concentrations (Atkinson and Bilger 1992, Lough et al. 1999, Fabricius 2005), aragonite saturation state (Ωaragonite) (Gattuso et al. 1998, Kleypas et al. 1999, Langdon et al. 2000, Marubini et al. 2001), pH (Atkinson et al. 1994, Marubini and Atkinson 1999, Kleypas and Langdon 2006), and sea surface temperature (SST) (Dodge et al. 1974, Veron 1995, Lough and Barnes 2000, Cruz-Piñón et al. 2003, Carricart-Ganivet 2004, Carricart-Ganivet et al. 2012, Cabral-Tena et al. 2013, Norzagaray-López et al. 2013).
Coral growth and its variation are considered to be controlled by 2 factors: (1) the amount of energy available for the deposition of the calcareous material (known as calcification rate) and (2) the way the coral uses this material to construct its skeleton. Differences in the latter result in 2 growth strategies: investing calcification resources into skeletal density or into skeletal extension (Carricart-Ganivet 2007).
In the eastern Pacific and Mexican Caribbean, massive corals belonging to the genera Porites and Orbicella exhibit an annual growth pattern: in late summer, when SST is higher, they deposit a denser skeleton and a high-density band is formed; conversely, when SST decreases, the skeletal density diminishes and a low-density band is formed (Highsmith 1979, Barnes and Lough 1993, Lough and Barnes 2000, Carricart-Ganivet 2004). Hence, a pair of growth bands of different density (high, low) represents one year of growth (Barnes and Lough 1993). The annual calcification rate (g CaCO3 cm-2 yr-1), which according to Dodge and Brass (1984) is the product of skeletal density (g CaCO3 cm-3) and extension rate (cm yr-1), can thus be determined by analyzing the pattern of alternating bands in coral skeletons.
Given the relationship between coral growth and environmental conditions (Knutson et al. 1972), it is essential to determine the basic parameters of calcification (i.e., density, extension) and the environmental factors that regulate them at regional scale, especially in the case of coral species that most strongly contribute to the structural development of the reef system. The growth of scleractinian corals is responsible for the persistence of coral reefs over time (Manzello 2010). Thus, the state of these species and the health status of the reef in general can be assessed by quantifying the calcification rate of the main reef-forming species and identifying the environmental variables that regulate this process. Moreover, the information provided by the skeletal density bands allows environmental reconstructions of the periods when the coral developed, which can be used to infer the response of coral reefs to future climate scenarios (Buddemeier et al. 2004, IPCC 2007). This is particularly relevant in the case of those species or regions where reefs develop under marginal environmental conditions. Since the end of the 19th century, the Eastern Tropical Pacific is considered to be a suboptimal area for reef development because it has a narrow continental shelf, high productivity, relatively cold waters, and seasonal upwelling (Glynn and Ault 2000), as well as water with a low pH and low Ωaragonite (Manzello et al. 2008, Manzello 2010). These last 2 elements turn the eastern Pacific into a natural laboratory for studying the effects of an ocean with high levels of CO2 on the processes regulating calcification in coral species and reef development. Despite its relevance, only a few studies have been conducted on coral calcification in the eastern Pacific (Glynn and Wellington 1983; Wellington and Glynn 1983; Halfar et al. 2005; Calderón-Aguilera et al. 2007; Reyes-Bonilla and López-Pérez 2009; Cabral-Tena et al. 2013; Norzagaray-López et al. 2013, 2014), and of these studies, even fewer have reported growth values obtained in situ (Glynn and Wellington 1983; Wellington and Glynn 1983; Cabral-Tena et al. 2013; Norzagaray-López et al. 2013, 2014). To date, in situ estimates have been reported for the Galapagos Islands (Glynn and Wellington 1983, Wellington and Glynn 1983) and the Gulf of California (Cabral-Tena et al. 2013; Norzagaray-López et al. 2013, 2014; Tortolero-Langarica 2016). There are no estimates for corals that develop in Pacific waters off southern Mexico.
The reefs on the Pacific coast of tropical southern Mexico are relatively small and discontinuous, and have a low diversity of coral species (Reyes-Bonilla 2003). There is a predominance of Pocillopora spp. and to a lesser extent of Pavona spp. and Porites spp. (Glynn and Leyte-Morales 1997, López-Pérez et al. 2012). The Pacific reefs of Mexico are divided into 5 regions. Of these, the southern Pacific region comprises the reef formations found off Ixtapa-Zihuatanejo in the state of Guerrero (López-Pérez et al. 2012) and off Puerto Ángel (Reyes-Bonilla and Leyte-Morales 1998) and Bahías de Huatulco in the state of Oaxaca (Glynn and Leyte-Morales 1997, López-Pérez and Hernández-Ballesteros 2004). These 2 areas have different climate and oceanographic conditions. Specifically, the Huatulco area is influenced by seasonal upwelling that occurs in the Gulf of Tehuantepec, which elevates subsurface water with low pH, low Ωaragonite and variations in SST to the surface (Chapa-Balcorta et al. 2015). In contrast, the Ixtapa-Zihuatanejo area has more stable SST conditions (Morales et al. 2008) and higher Ωaragonite (Reyes-Bonilla et al. 2014). These differences could be reflected in the growth parameters of reef-forming corals from each area, but they have not been determined for either of these 2 areas or for any of the species. Understanding the growth parameters and the growth-regulating factors is necessary considering that a recent evaluation of the carbon system in the region (Chapa-Balcorta et al. 2015) revealed that reefs may experience stress because of more extreme ocean acidification conditions than previously suggested (Manzello et al. 2008, Manzello 2010).
The present study aimed to determine the extension rate, density, and calcification rate of the main reef-building species (Pocillopora meandrina, Pocillopora damicornis, Pocillopora verrucosa, Pocillopora capitata, Porites panamensis, Porites lobata, Pavona gigantea, and Pavona varians) in Pacific waters off southern Mexico, to assess the effect of SST on the growth of these species, and to compare the calcification parameters obtained with those obtained by other authors at other latitudes. This is relevant at a time when possible climate change (global warming) scenarios and their influence on coastal and reef systems are being discussed.
Materials and methods
Study area
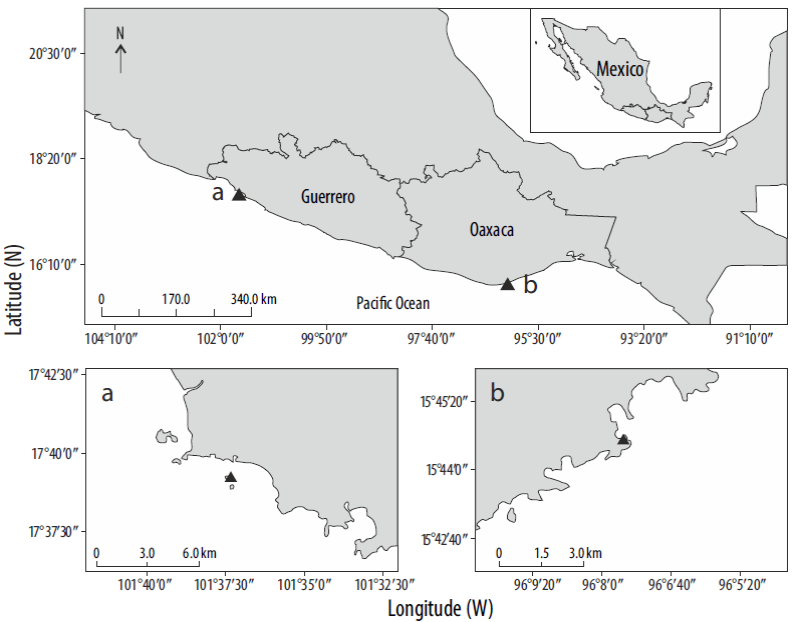
Coral colonies were collected at 2 sites on the Pacific coast of southern Mexico with different oceanographic conditions (Fig. 1). La Entrega (Oaxaca; 15°44'34"N, 96°07'35"W), located on the west side of the Gulf of Tehuantepec, is affected by strong intermittent northerly winds that generate strong upwelling (Chapa-Balcorta et al. 2015). During the upwelling periods (October to April), SST can drop as much as 8 °C (annual mean 28.7 °C), and lower pH and Ωaragonite are recorded (Chapa-Balcorta et al. 2015). At the second site, Zacatoso (Guerrero; 17°39'14"N, 101°37'19"W), the environmental parameters are typical of the Pacific coast of southern Mexico: mean annual SST of 29.2 °C (Morales et al. 2008), with annual thermal variations of less than 2 °C; mean surface salinity of 34; a stable, shallow (20-40 m) thermocline (Fiedler and Talley 2006); and higher Ωaragonite (Reyes-Bonilla et al. 2014). Unlike La Entrega, Zacatoso is not affected by upwelling events that affect SST, pH, and Ωaragonite.
Field work
During September and November 2010, at La Entrega and Zacatoso, 40 fragments of hermatypic corals, previously obtained from healthy colonies, were placed within a PVC structure anchored at 5 m depth and fixed with epoxy resin. The colonies were stained with alizarin red as a reference point to later measure skeletal extension (Barnes 1972). The fixed and stained corals were extracted in October and November 2013. At the end of the period, at La Entrega we collected 4 colonies of Pocillopora meandrina, 9 of Pocillopora damicornis, 2 of Pocillopora capitata, 8 of Porites panamensis, and 4 of Pavona varians. At Zacatoso, we collected 3 colonies of Pocillopora verrucosa, 5 of Porites lobata, and 5 of Pavona gigantea. The branching corals ranged from 9 to 15 cm in height and from 8 to 10 cm in width. The massive corals ranged from 4 to 9 cm in height and from 8 to 12 cm in width.
Growth parameters
The skeletal density (g CaCO3 cm-3) and extension rate (cm yr-1) of each colony was determined. In the case of the Pocillopora colonies (branching corals), skeletal extension was obtained by measuring the distance from the alizarin red line to the apex of the ramification with a digital vernier (±0.1 mm accuracy). Skeletal density was estimated by the displacement method (Brown and Scoffin 1986). The calcification rate (g CaCO3 cm-2 yr-1) was calculated as the product of skeletal density and extension rate (Lough and Barnes 2000).
In the case of the Porites and Pavona colonies (massive corals), each colony was cut into 7-mm-thick slices along the growth axis using a high-velocity saw. Each slice was washed and left to dry in the sun for 24 h. The slices were X-radiographed with a digital mammography machine (Senographe 600 T Senix HF, Waukesha, WI) at 34-40 kVp for 60 s, using a focal distance of 45 cm. During each exposure an aragonite standard was used consisting of 8 blocks of known thickness and density (2.83 g CaCO3 cm-3) built from a shell of Tridacna maxima (Carricart-Ganivet and Barnes 2007). The digitized X-ray images were used to measure the growth parameters based on optical densitometry (Carricart-Ganivet and Barnes 2007). The coral growth bands from each image were analyzed using Image J v.1.44 (https://imagej.nih.gov/ij/). Annual skeletal extension rate (cm yr-1) was estimated as the linear distance between two consecutive low-density bands. Average annual density (g CaCO3 cm-3) was defined as the average density between adjacent density minima and maxima for each pair of bands. Finally, The annual calcification rate (g CaCO3 cm-2 yr-1) was obtained from the product of average annual density and annual growth of each pair of bands (Lough and Barnes 2000).
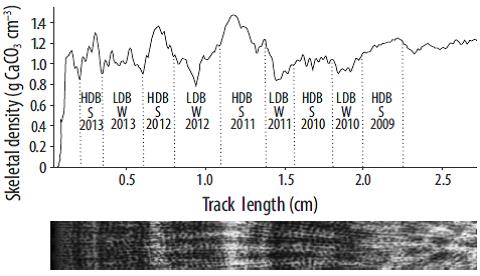
To analyze the seasonal effect of SST on the growth parameters of massive corals (Porites and Pavona), 6-month growth rates were obtained. We performed a sclerochrono-logical analysis using the density profile generated by optical densitometry (Carricart-Ganivet and Barnes 2007) for the annual calcification rate estimation, as well as the dates of collection (October and November 2013) and alizarin staining (September and November 2010). The bands behind the staining line were considered to be prior to 2010. Considering that a pair of different density (high, low) growth bands represents one year of growth (Barnes and Lough 1993), each band was read individually (instead of in pairs, as for the annual estimation) to obtain the 6-month growth values (Fig. 2). The 6-month skeletal extension was calculated as the linear distance occupied by each high- or low-density band. Average 6-month density was estimated as the average density between the minimum and maximum density of each individual band. The 6-month calcification rate was calculated as the product between 6-month average density and linear extension rate.
Sea surface temperature
For each site, SST values (maximum, minimum, and monthly mean ± standard deviation) were obtained from images generated by the MODIS sensor aboard the Aqua satellite at a spatial resolution of 4 km (http://ocean-color.gsfc.nasa.gov/cgi/l3) for the period from January 2007 to December 2013. To compare SST with the coral growth bands, these values were grouped into two 6-month periods: December to May (winter) and June to November (summer). Considering that the resolution (4 x 4 km) of the MODIS-derived SST data may not represent the temperature at the coral growth areas, we compared in situ data obtained at La Entrega for the period 2010-2011 with the satellite data for the same period. A thermograph, installed at 5 m depth, recorded temperature at 1-h intervals. Simple linear regression analysis revealed the same pattern and a highly significant positive relation (r 2 = 0.96, P = 0.0001) for the in situ and satellite data. This suggested that the satellite-derived SST data could be used in the analyses including coral growth parameters.
Data analysis
The relation between the annual growth values (skeletal density, extension rate, and calcification rate) of the massive coral species was analyzed using the Pearson correlation coefficient. Additionally, linear regression models were used to analyze the relation between the SST values and the 6-month growth values. Before these analyses, the assumptions of normality and homoscedasticity of the data were examined. All analyses were performed using α = 0.05 (Zar 2010).
Results
The mean (±SD) annual growth values (period 2010-2013) are shown in Figure 3. Of the branching corals, Pocillopora damicornis (La Entrega, Oaxaca) had the highest density value (1.78 ± 0.31 g CaCO3 cm-3) and an extension rate of 2.94 ± 0.32 cm yr-1, resulting in a calcification rate of 5.23 ± 1.03 g CaCO3 cm-2 yr-1, the highest calcification rate value recorded for the genus. Pocillopora verrucosa (Zacatoso, Guerrero) had the lowest density value (1.47 ± 0.01 g CaCO3 cm-3) and the highest extension rate (3.42 ± 0.32 cm yr-1), resulting in a calcification rate of 5.04 ± 0.44 g CaCO3 cm-2 yr-1. Pocillopora meandrina (La Entrega, Oaxaca) had a mean density of 1.47 ± 0.09 g CaCO3 cm-3, extension rate of 2.02 ± 0.39 cm yr-1, and calcification rate of 2.99 ± 0.74 g CaCO3 cm-2 yr-1. Pocillopora capitata (La Entrega, Oaxaca) had a mean density of 1.67 ± 0.07 g CaCO3 cm-3, extension rate of 2.92 ± 0.23 cm yr-1, and calcification rate of 4.87 ± 0.14 g CaCO3 cm-2 yr-1.
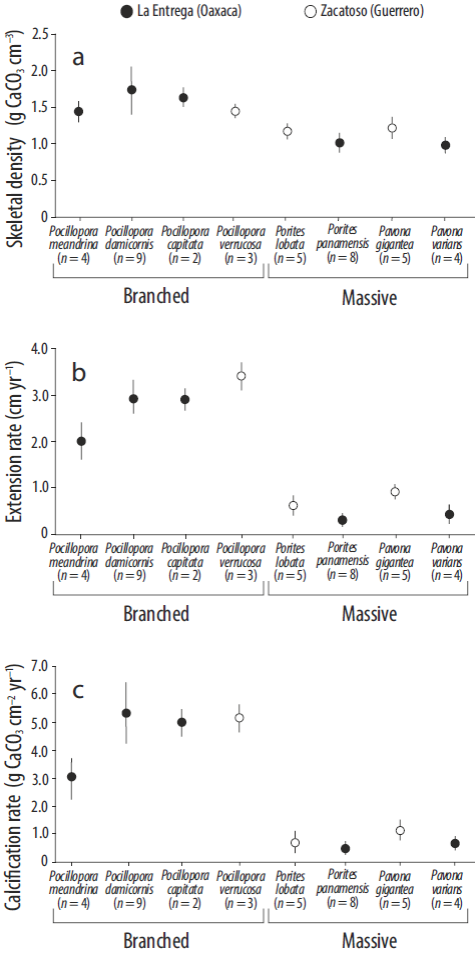
Figure 3 Growth parameters of scleractinian corals on the Pacific coast of southern Mexico for the period 2010-2013: (a) skeletal density (g CaCO3 cm-3), (b) extension rate (cm yr-1), and (c) calcification rate (g CaCO3 cm-2 yr-1). Circles represent the mean, vertical lines the standard deviation, and n the sample size.
Regarding the massive species, at Zacatoso (Guerrero), Pavona gigantea had the highest values of all 3 growth parameters (density: 1.24 ± 0.09 g CaCO3 cm-3; extension: 0.91 ± 0.07 cm yr-1; calcification: 1.13 ± 0.09 g CaCO3 cm-2 yr-1), whereas Porites lobata had a mean density of 1.20 ± 0.07 g CaCO3 cm-3, extension of 0.60 ± 0.16 cm yr-1, and calcification rate of 0.72 ± 0.22 g CaCO3 cm-2 yr-1. At La Entrega (Oaxaca), Porites panamensis had the lowest values of all 3 growth parameters (density: 1.12 ± 0.08 g CaCO3 cm-3; extension: 0.31 ± 0.07 cm yr-1; calcification: 0.34 ± 0.07 g CaCO3 cm-2 yr-1), and Pavona varians had a mean density of 1.04 ± 0.07 g CaCO3 cm-3, extension rate of 0.43 ± 0.07 cm yr-1, and calcification rate of 0.45 ± 0.09 g CaCO3 cm-2 yr-1.
On average, the massive coral species had 4 pairs of growth bands. This represents a 4-year growth record (2009-2013) per colony and suggests that the studied colonies on average had one year of growth prior to seeding. The correlation analysis of the three annual growth parameters (density, extension, and calcification) revealed a positive and significant relation between extension rate and calcification rate for all the species studied (Porites lobata: r = 0.97, P = 0.0001; Pavona gigantea: r = 0.98, P = 0.0001; Porites panamensis: r = 0.98, P = 0.0001; Pavona varians: r = 0.99, P = 0.0001). The correlation between skeletal density and calcification rate was low (P > 0.05) for all the species.
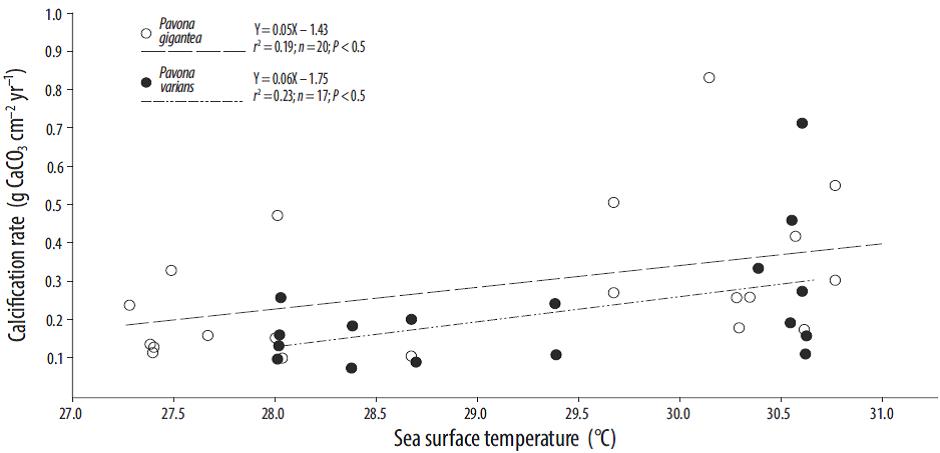
A comparison of the SST and growth parameter values (Table 1) revealed that the density values for the Porites and Pavona colonies were positively related to SST (Fig. 4). At Zacatoso, a significant relation between SST and skeletal density was observed for Porites lobata (r2 = 0.55, P = 0.0001) and Pavona gigantea (r 2 = 0.62, P = 0.0001). At La Entrega, Porites panamensis (r2 = 0.36, P = 0.01) and Pavona varians (r 2 = 0.55, P = 0.001) showed a significant positive relation. A low, though significant, correlation between SST and calcification rate was observed for Pavona gigantea (r 2 = 0.19, P < 0.5) and Pavona varians (r 2 = 0.23, P < 0.5; Fig. 5). The extension rates for Porites lobata, Porites panamensis, Pavona gigantea, and Pavona varians were not significantly correlated with SST.
Table 1 Mean (± SD) 6-month (summer/winter) skeletal density, extension, and calcification values for the massive coral species studied.

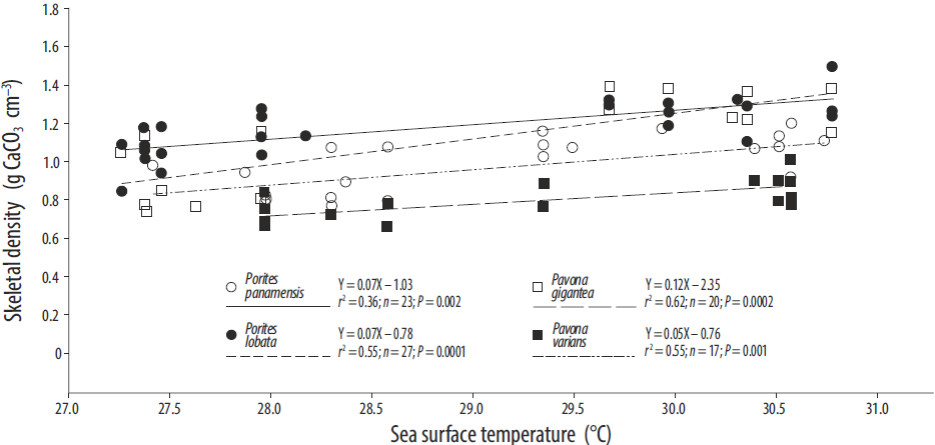
Figure 4 Relation between skeletal density and sea surface temperature in the massive corals collected on the Pacific coast of southern Mexico.
Discussion
Growth parameters: branching species
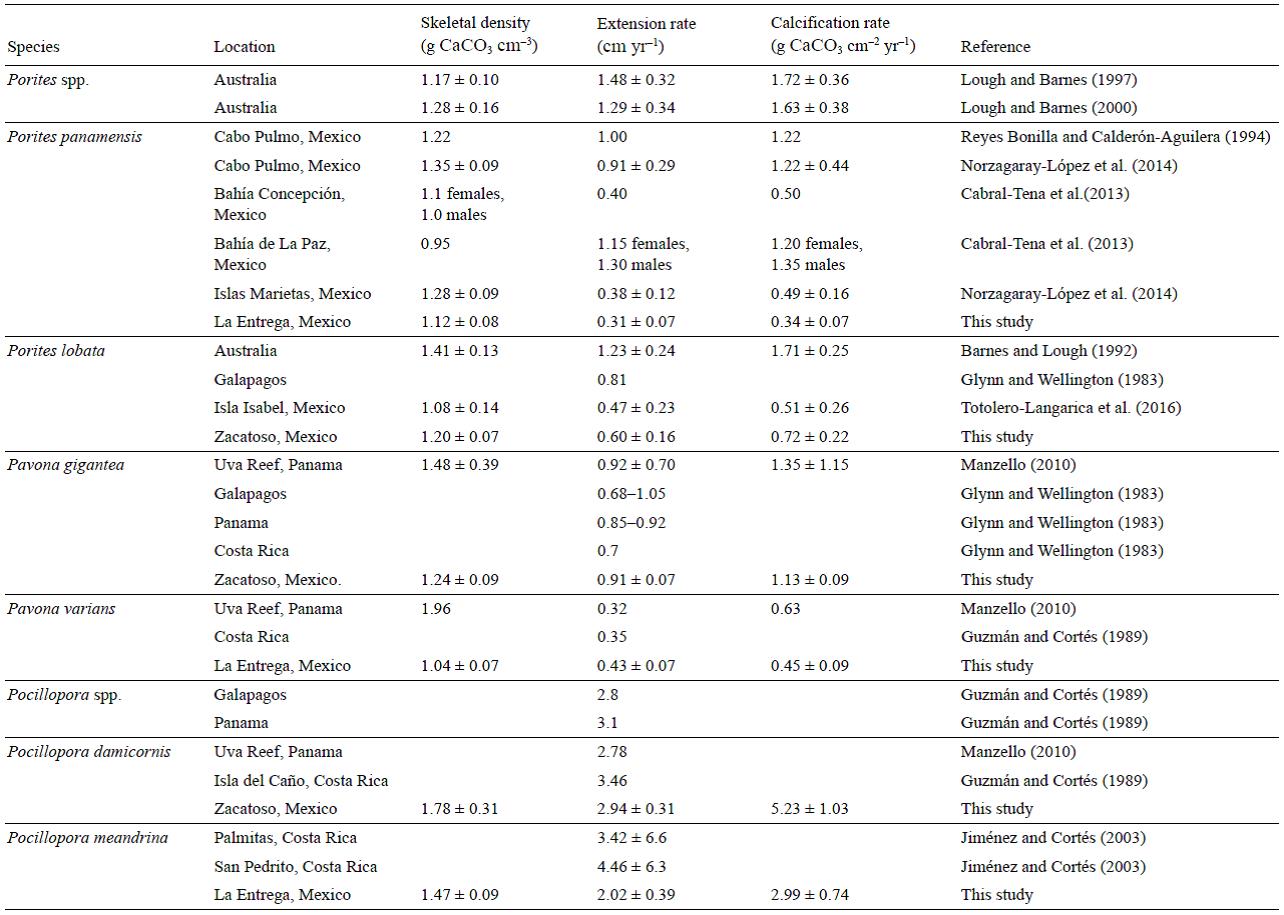
The extension rates reported herein for the 4 Pocillopora species studied (P capitata, P. damicornis, P. meandrina, and P. verrucosa) fall within the ranges reported in other studies conducted on the Pacific coast of Central America (Glynn 1977, Guzmán and Cortés 1989, Jiménez and Cortés 2003, Manzello 2010). Considering that the environmental conditions at our study sites have been described as unfavorable for coral growth (e.g., upwelling, SST fluctuations, low Ωaragonite; Reyes-Bonilla et al. 2002, Cupul-Magaña and Calderón-Aguilera 2008, Chapa-Balcorta et al. 2015), they must not be sufficiently adverse or extreme to negatively influence the calcification process of Pocillopora spp. and, in turn, their contribution of calcium carbonate to reef development. Except for one reef (Morros del Cerro Colorado) off Ixtapa, Guerrero (López-Pérez et al. 2012), where Porites lobata is the species that contributes the most to coral growth and reef structure, the reefs in our study area are relatively small, have low species diversity, and are primarily formed by Pocillopora spp. (Cortés 2003, Reyes-Bonilla 2003).
Among the factors that may explain the predominance of Pocillopora spp. in the region is their symbiotic relationship with stress-tolerant Symbiodinium clade D (LaJeunesse et al. 2010, Walther-Mendoza et al. 2016), which could prove advantageous during El Niño events (Carriquiry et al. 2001, Reyes-Bonilla et al. 2002, Baker et al. 2004, López-Pérez et al. 2016). Also, the ability of Pocillopora spp. to tolerate increased nutrient levels, mainly phosphorus and ammonium (Stambler et al. 1991, Muller-Parker 1994), as occurs during seasonal upwelling events in the region, may also be advantageous. Two other factors may also contribute to the predominance of this genus in the region: (1) the ability of pocilloporid corals to reproduce asexually via fragmentation and (2) the high extension and calcification rates of Pocillopora spp. In both the Indo-Pacific and eastern Pacific (Carriquiry and Reyes-Bonilla 1997, Ayre et al. 1997), corals belonging to the genus Pocillopora have been found to easily colonize other reef areas after their branches are detached by waves and cyclones. If they are not buried (López-Pérez et al. 2007), the broken-off fragments become attached to the substrate and continue to develop, allowing branching corals to have greater success in competition for space than the species with low probability of fragmentation (Glynn and Ault 2000, Reyes-Bonilla 2003, Reyes-Bonilla et al. 2013). Pocillopora spp. have relatively high extension and calcification rates compared to Porites spp. and Pavona spp. in the study area (Fig. 3) and to Pocillopora spp. growing at other sites (Table 2).
Growth parameters: massive species
The calcification rates obtained for the massive coral species are, for the most part, lower than those previously recorded by other authors at other latitudes (Table 2). In the case of Porites panamensis, our value was 32% lower than that reported for Concepción Bay in the Gulf of California (0.5 g CaCO3 cm-2 yr-1; Cabral-Tena et al. 2013) and for the Marietas Islands off the Pacific coast of central Mexico (0.49 ± 0.16 g CaCO3 cm-2 yr-1; Norzagaray-López et al. 2013), and up to 74.8% lower than that reported for La Paz Bay (Cabral-Tena et al. 2013) and Cabo Pulmo (Reyes-Bonilla and Calderón-Aguilera 1994) in the Gulf of California. All 3 growth parameter values recorded in this study for Porites lobata were slightly higher than those reported by Tortolero-Langarica et al. (2016) for free-living coral (i.e., rhodoliths) off Isabel Island (Nayarit); thus, the corals growing off Ixtapa (this study) have extension values that are 21.6% higher (0.60 ± 0.16 cm yr-1), they are 10% denser (1.20 ± 0.07 g CaCO3 cm-3), and they calcify, on average, 29.1% more (0.72 ± 0.22 g CaCO3 cm-2 yr-1). Nonetheless, the calcification rate obtained for the species is 57.8% lower than that recorded by Barnes and Lough (1992) in the Great Barrier Reef, Australia (1.71 ± 0.25 g CaCO3 cm-2 yr-1). Likewise, the calcification rate obtained for Pavona gigantea was 17% lower than that reported for La Uva reef in Panama (1.35 g CaCO3 cm-2 yr-1; Manzello 2010). Finally, the calcification rate for Pavona varians was 28.57% lower than that recorded by Manzello (2010) in Panama (0.63 g CaCO3 cm-2 yr-1).
The relatively low calcification values reported herein for the massive species are the result of a low mean extension rate. The highest extension rates of each colony were recorded during the years that the highest calcification values were recorded, suggesting that the Porites panamensis, Porites lobata, Pavona gigantea, and Pavona varians colonies that develop off the Pacific coast of southern Mexico invest more resources into extension than into density, as a result of a positive relation between extension rate and calcification rate (Lough and Barnes 2000, Carricart-Ganivet et al. 2007, Cabral-Tena et al. 2013). These species appear to adopt an extension growth strategy that allows them to compete for space and occupy a greater area in as little time as possible (Lough and Barnes 2000). This is the same strategy adopted by the branching species in the region (see previous section). Nevertheless, the skeletal extension values of any coral belonging to the genus Pocillopora are sensibly higher than those observed for any massive species; hence, the pocil-loporid corals in the region contribute most strongly (>90%) to the construction of the reef structure, whereas the contribution of Pavona spp. (>5%) and Porites spp. (>1%) is marginal (Glynn and Leyte-Morales 1997, Carriquiry et al. 2001, López-Pérez et al. 2012).
Relation between coral growth and SST
Even though the mechanisms and timing of high- and low-density band formation on coral skeletons and the factors that regulate coral calcification are still under discussion, the following variables are known to influence these processes: light (Buddemeier 1974, Wellington and Glynn 1983, Falkowski 2000, Yentsch et al. 2002), sedimentation (Carricart-Ganivet and Merino 2001), nutrient levels (Lough et al. 1999), Ωaragonite (Kleypas et al 1999, Gattuso et al. 1998, Langdon et al. 2000, Marubini et al. 2001), and SST (Dodge et al. 1974; Carricart-Ganivet 2004, 2007; Cabral-Tena et al. 2013; Norzagaray-López et al. 2013). In particular, SST has been observed to substantially affect coral calcification rates (Dodge et al. 1974, Veron 1995, Lough and Barnes 2000, Cruz-Piñón et al. 2003, Carricart-Ganivet 2004, Carricart-Ganivet et al. 2012). Cabral-Tena et al. (2013) and Norzagaray-López et al. (2013) reported that SST affects skeletal density and extension of massive corals on the Pacific coast of Mexico. As extension is the main growth strategy of all the massive corals studied (see previous section), a relation between extension and SST would be expected; however, the results do not indicate that there is a significant relation (P > 0.05) between these two variables, which suggests that skeletal extension may be regulated by another environmental variable not considered in this region of southern Mexico (Guerrero and Oaxaca).
A significant relation was observed between skeletal density and SST in all the massive species studied, indicating that maximum annual skeletal density occurs when the temperature is higher. This finding concurs with Barnes and Lough (1992) who reported that the high-density bands of Porites corals in Australia formed in summer. Regarding the relation between calcification rate and SST, in Pavona gigantea and Pavona varians (Fig. 5) maximum calcification rate occurred above 29 °C. Other studies, however, have reported that it occurs at 26-28 °C (Edmunds 2005, Cooper et al. 2008). This difference could be attributed to the adaptation of reef-building corals to the local environmental conditions they experience off Mexico's Pacific coast. There is evidence of the acclimatization of coral populations to lower or higher local and regional temperatures (Kleypas et al. 1999) than those reported here, and that maximum calcification levels can occur at different temperature ranges probably in concordance with different symbionts (Baker 2004, Jones et al. 2008, LaJeunesse et al. 2010, Walther-Mendoza et al. 2016).
Finally, even though the sampling size for some species (e.g., P. varians) was small and the results should be interpreted with caution, this study reports, for the first time, the density, extension rate, and calcification rate of the main reef-building corals on the Pacific coast of southern Mexico. The results are relevant and when they are analyzed in a regional context (i.e., eastern Pacific) they provide insight into the large-scale processes that regulate the coral calcification parameters, in particular those related to Ωaragonite and pH. The results of this study, together with studies on the carbonate system in this region (Chapa-Balcorta et al. 2015), are essential in order to conduct real-time studies in the near future of the effects of pH and Ωaragonite on the development of reef-forming corals.
Acknowledgments
This study was financed by grants from the National Council for Science and Technology (CONACYT, Mexico, Basic Science project 236654) and PRODEP UAM-PTC (509) to ALP. We thank Omar Valencia-Méndez (UAM-I) for his support and 2 anonymous reviewers for their valuable comments that helped to improve this paper.
REFERENCES
Atkinson MJ, Bilger RW. 1992. Effects of water velocity on phosphate uptake in coral reef-flat communities. Limnol. Oceanogr. 37: 273-279. [ Links ]
Atkinson MJ, Kotler E, Newton P. 1994. Effects of water velocity on respiration, calcification, and ammonium uptake of a Porites compressa community. Pac. Sci. 48: 296-303. [ Links ]
Ayre DJ, Hughes TP, Standish RJ. 1997. Genetic differentiation, reproductive mode, and gene flow in the brooding coral Pocillopora damicornis along the Great Barrier Reef, Australia. Mar. Ecol. Prog. Ser. 159: 175-187. [ Links ]
Baker AC. 2004. Symbiont diversity on coral reefs and its relationship to bleaching resistance and resilience. In: Coral Health and Disease. Springer, Berlin, Heidelberg, pp. 177-194. [ Links ]
Baker AC, Starger CJ, McClanahan TR, Glynn PW. 2004. Coral reefs: Corals' adaptive response to climate change. Nature 430: 741. http://dx.doi.org/10.1038/430741a [ Links ]
Barnes DJ. 1972. The structure and formation of growth-ridges in Scleractinian coral skeletons. Proc. R. Soc. Lond. (Biol. Sci.) 182: 331-350. [ Links ]
Barnes DJ, Lough JM. 1992. Systematic variations in the depth of skeleton occupied by coral tissue in massive colonies of Porites from the Great Barrier Reef. J. Exp. Mar. Biol. Ecol. 159: 113-128. [ Links ]
Barnes DJ, Lough JM. 1993. On the nature and causes of density banding in massive coral skeletons. J. Exp. Mar. Biol. Ecol. 167: 91-108. [ Links ]
Barnes DJ, Lough JM. 1999. Porites growth characteristics in a changed environment: Misima Island, Papua New Guinea. Coral Reefs 18: 213-218. [ Links ]
Brown BE, Scoffin TP. 1986. Measuring growth rates of reef corals as an indicator of the effects of pollution and environmental disturbance. In: Brown BE (ed.), Human Induced Damage to Coral Reefs. UNESCO Rep. Mar. Sci. 40: 12-24. [ Links ]
Buddemeier RW. 1974. Environmental controls over annual and lunar monthly cycles in hermatypic coral calcification. Proc. 2nd Int. Coral Reef Symp. 2: 259-26. [ Links ]
Buddemeier RW, Kleypas JA, Aronson RB. 2004. Coral reefs and global climate change: Potential contributions of climate change to stresses on coral reef ecosystems. Pew Center on Global Climate Change, Arlington, 44 pp. [ Links ]
Cabral-Tena R, Reyes-Bonilla H, Lluch-Cota S, Paz-García D, Calderón-Aguilera LE, Norzagaray-López O, Balart E. 2013. Different calcification rates in males and females of the coral Porites panamensis in the Gulf of California. Mar. Ecol. Prog. Ser. 476: 1-8. http://dx.doi.org/10.3354/meps10269 [ Links ]
Calderón-Aguilera LE, Reyes-Bonilla H, Carriquiry JD. 2007. El papel de los arrecifes coralinos en el flujo de carbono en el océano: Estudios en el Pacífico mexicano. In: Hernández-Torre B, Gaxiola-Castro G (eds.), Carbono en Ecosistemas Acuáticos de México. SEMARNAT/INE, CICESE, México, pp. 215-226. [ Links ]
Carricart-Ganivet JP. 2004. Sea surface temperature and the growth of the West Atlantic reef building coral Montastraea annularis. J. Exp. Mar. Biol. Ecol. 302: 249-260. [ Links ]
Carricart-Ganivet JP. 2007. Annual density banding in massive coral skeletons: Result of growth strategies to inhabit reefs with high microborers' activity? Mar. Biol. 153: 1-5. [ Links ]
Carricart-Ganivet JP, Barnes DJ. 2007. Densitometry from digitized images of X-radiographs: Methodology for measurement of coral skeletal density. J. Exp. Mar. Biol. Ecol. 344: 67-72. [ Links ]
Carricart-Ganivet JP , Beltrán-Torres AU, Merino M, Ruiz-Zarate MA. 2000. Skeletal extension, density and calcification rate of the reef building coral Montastraea annularis (Ellis and Solander) in the Mexican Caribbean. Bull. Mar. Sci. 66: 215-224. [ Links ]
Carricart-Ganivet JP, Cabanillas-Terán N, Cruz-Ortega I, Blanchon P. 2012. Sensitivity of calcification to thermal stress varies among genera of massive reef-building corals. PLOS One 7(3): e32859. http://dx.doi.org/10.1371/journal.pone.0032859 [ Links ]
Carricart-Ganivet JP, Lough JM, Barnes DJ. 2007. Growth and luminescence characteristics in skeletons of massive Porites from a depth gradient in the central Great Barrier Reef. J. Exp. Mar. Biol. Ecol. 351: 27-36. [ Links ]
Carricart-Ganivet JP, Merino M . 2001. Growth responses of the reef-building coral Montastraea annularis along a gradient of continental influence in the southern Gulf of Mexico. Bull. Mar. Sci. 68: 133-146. [ Links ]
Carriquiry JD, Cupul-Magaña AL, Rodríguez-Zaragoza F, Medina-Rosas P. 2001. Coral bleaching and mortality in the Mexican Pacific during the 1997-98 El Niño and prediction from a remote sensing approach. Bull. Mar. Sci. 69: 237-249. [ Links ]
Carriquiry JD, Reyes-Bonilla H. 1997. Community structure and geographic distribution of the coral reefs of Nayarit, Mexican Pacific = Estructura de la comunidad y distribución geográfica de los arrecifes coralinos de Nayarit, Pacífico de México. Cienc. Mar. 23(2): 227-248. [ Links ]
Chalker B, Barnes D, Isdale P. 1985. Calibration of X-ray densitometry for the measurement of coral skeletal density. Coral Reefs 4: 95-100. [ Links ]
Chapa-Balcorta C, Hernández-Ayón JM, Durazo R, Beier E, Alin SR, López-Pérez A. 2015. Influence of post-Tehuano oceanographic processes in the dynamics of the CO2 system in the Gulf of Tehuantepec, Mexico. J. Geophys. Res. (Oceans) 120(12): 7752-7770. http://dx.doi.org/10.1002/2015JC011249 [ Links ]
Cooper TF, De'Ath G, Fabricius KE, Lough JM. 2008. Declining coral calcification in massive Porites in two nearshore regions of the northern Great Barrier Reef. Global Change Biol. 14: 529-538. [ Links ]
Cortés J. 2003. Latin American Coral Reefs. Elsevier, Amsterdam, 497 pp. [ Links ]
Cruz-Piñón G, Carricart-Ganivet JP, Espinoza-Avalos J. 2003. Monthly skeletal extension rates of the hermatypic corals Montastraea annularis and Montastrae faveolata: Biological and environmental controls. Mar. Biol. 143: 491-500. [ Links ]
Cupul-Magaña A, Calderón-Aguilera L. 2008. Cold water bleaching at Islas Marietas National Park, Nayarit, Mexico. 15th National Oceanography Conference, 13-18 October 2008, Veracruz, Mexico. [ Links ]
Dodge RE, Aller RC, Thomson J. 1974. Coral growth related to resuspension of bottom sediments. Nature 247: 574-577. [ Links ]
Dodge RE, Brass GW. 1984. Skeletal extension, density and calcification of the reef coral, Montastrea annularis: St. Croix, US Virgin Islands. Bull. Mar. Sci. 34: 288-307. [ Links ]
Edmunds PJ. 2005. Effect of elevated temperature on aerobic respiration of coral recruits. Mar. Biol. 146: 655-663. [ Links ]
Fabricius KE. 2005. Effects of terrestrial runoff on the ecology of corals and coral reefs: Review and synthesis. Mar. Pollut. Bull. 50: 125-146. [ Links ]
Falkowski P. 2000. The global carbon cycle: A test of our knowledge of earth as a system. Science 5490: 291-296. [ Links ]
Fiedler PC, Talley LD. 2006. Hydrography of the eastern tropical Pacific: A review. Prog. Oceanogr. 69: 143-180. [ Links ]
Gattuso JP, Frankignoulle M, Bourge I, Romaine S, Buddemeier RW . 1998. Effect of calcium carbonate saturation of seawater on coral calcification. Global Planet. Change 18: 37-46. [ Links ]
Glynn PW. 1977. Coral growth in upwelling and non-upwelling areas off the Pacific coast of Panama. J. Mar. Res. 35: 567-585. [ Links ]
Glynn PW, Ault JS. 2000. A biogeographic analysis and review of the far eastern Pacific coral reef region. Coral Reefs 19: 1-23. [ Links ]
Glynn PW, Leyte-Morales GE. 1997. Coral reefs of Huatulco, west Mexico: Reef development in upwelling Gulf of Tehuantepec. Rev. Biol. Trop. 45: 1033-1047. [ Links ]
Glynn PW, Wellington GM. 1983. Corals and Coral Reefs of the Galapagos Islands. University of California Press, Berkeley, 330 pp. [ Links ]
Guzmán HM, Cortés J. 1989. Growth rates of eight species of Scleractinian corals in the eastern Pacific (Costa Rica). Bull. Mar. Sci. 44: 1186-1194. [ Links ]
Halfar J, Godinez-Orta L, Riegl B, Valdez-Holguín JE, Borges JM. 2005. Living on the edge: High-latitude Porites carbonate production under temperate eutrophic conditions. Coral Reefs 24: 582-592. [ Links ]
Highsmith RC. 1979. Coral growth rates and environmental control of density banding. J. Exp. Mar. Biol. Ecol. 37: 105-125. [ Links ]
Hughes T. 1987. Skeletal density and growth form of coral. Mar. Ecol. Prog. Ser. 35: 259-66. [ Links ]
[IPCC] Intergovernmental Panel on Climate Change. 2007. Climate Change 2007: The Physical Science Basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. Solomon S, Qin D, Manning M, Chen Z, Marquis M, Averyt KB, Tignor M, Miller HL (eds.). Cambridge Univ. Press, 396 pp. [ Links ]
Jiménez C, Cortés J. 2003. Growth of seven species of scleractinian corals in an upwelling environment of the eastern Pacific (Golfo de Papagayo, Costa Rica). Bull. Mar. Sci. 72: 187-198. [ Links ]
Jones AM, Berkelmans R, Van Oppen, MJ, Mieog JC, Sinclair W. 2008. A community change in the algal endosymbionts of a scleractinian coral following a natural bleaching event: Field evidence of acclimatization. Proc. R. Soc. Lond. (Biol. Sci.) 275: 1359-1365. [ Links ]
Kleypas JA, Langdon C. 2006. Coral reefs and changing seawater carbonate chemistry. Coral Reefs and Climate Change: Science and Management, Coastal and Estuarine Studies 61, American Geophysical Union, pp. 73-110. [ Links ]
Kleypas JA, McManus JW, Meñez LA. 1999. Environmental limits to coral reef development: Where do we draw the line? Am. Zool. 39: 146-159. [ Links ]
Knutson DW, Buddemeier RW, Smith SV. 1972. Coral chronometers: Seasonal growth bands in reef corals. Science 177: 270-272. http://dx.doi.org/10.1126/science.177.4045.270 [ Links ]
LaJeunesse TC, Smith R, Walther M, Pinzón J, Pettay DT, McGinley M, Aschaffenburg M, Medina-Rosas P , Cupul-Magaña AL , López-Pérez A , Reyes-Bonilla H, Warner ME. 2010. Host-symbiont recombination versus natural selection in the response of coral-dinoflagellate symbioses to environmental disturbance. Proc. R. Soc. Lond. (Biol. Sci.) 277(1696): 2925-2934. http://dx.doi.org/10.1098/rspb.2010.0385 [ Links ]
Langdon C, Takahashi T, Sweeney C, Chipman D, Goddard J, Marubini F, Aceves H, Barnett H, Atkinson MJ. 2000. Effect of calcium carbonate saturation state on the calcification rate of an experimental coral reef. Global Biogeochem. Cycles 14: 639-654. http://dx.doi.org/10.1029/1999GB001195 [ Links ]
López-Pérez A, Guendulain-García S, Granja-Fernández R, Hernández-Urraca V, Galván-Rowland L, Zepeta-Vilchis R, López-López D. 2016. Reef community changes associated with the 2009-2010 El Niño in the southern Mexican Pacific 1. Pac. Sci. 70: 175-190. [ Links ]
López-Pérez RA, Calderón-Aguilera LE, Reyes-Bonilla H, Carriquiry JD, Medina-Rosas P, Cupul-Magaña AL, Luna-Salguero BM. 2012. Coral communities and reefs from Guerrero, southern Mexican Pacific. Mar. Ecol. 33: 407-116. [ Links ]
López-Pérez RA, Hernández-Ballesteros LM. 2004. Coral community structure and dynamics in the Huatulco area, western Mexico. Bull. Mar. Sci. 75: 453-472. [ Links ]
López-Pérez RA, Mora-Pérez MG, Leyte-Morales GE . 2007. Coral (Anthozoa: Scleractinia) recruitment at Bahías de Huatulco, western México: Implications for coral community structure and dynamics. Pac. Sci. 61(3): 355-369. [ Links ]
Lough JM, Barnes DJ. 1997. Several centuries of variation in skeletal extension, density and calcification in massive Porites colonies from the Great Barrier Reef: A proxy for seawater temperature and a background of variability against which to identify unnatural change. J. Exp. Mar. Biol. Ecol. 211: 29-67. [ Links ]
Lough JM, Barnes DJ. 2000. Environmental controls on growth of the massive coral Porites. J. Exp. Mar. Biol. Ecol. 245: 225-243. [ Links ]
Lough JM, Barnes DJ, Devereux MJ, Tobin BJ, Tobin S. 1999. Variability in growth characteristics of massive Porites on the Great Barrier Reef. Tech. Rep. no. 28, CRC Reef Research Center, Townsville, 95 pp. [ Links ]
Loya Y. 1976. Effects of water turbidity and sedimentation on the community structure of Puerto Rican corals. Bull. Mar. Sci. 26: 450-466. [ Links ]
Manzello DP. 2010. Coral growth with thermal stress and ocean acidification: Lessons from the eastern tropical Pacific. Coral Reefs 29: 749-758. [ Links ]
Manzello DP, Kleypas JA, Budd DA, Eakin CM, Glynn PW , Langdon C. 2008. Poorly cemented coral reefs of the eastern tropical Pacific: Possible insights into reef development in a high-CO2 world. Proc. Natl. Acad. Sci. USA 105: 10450-10455. [ Links ]
Marubini F, Atkinson MJ. 1999. Effects of lowered pH and elevated nitrate on coral calcification. Mar. Ecol. Prog. Ser. 188: 117-121. [ Links ]
Marubini F , Barnett H , Langdon C , Atkinson MJ . 2001. Dependence of calcification on light and carbonate ion concentration for the hermatypic coral Porites compressa. Mar. Ecol. Prog. Ser. 220: 153-162. [ Links ]
Morales R, Vélez H, Mejía A, Ramírez I, Izurieta J, Saldaña P. 2008. Hidrodinámica de la Bahía de Zihuatanejo, México. XXIII Congreso Latinoamericano de Hidráulica, 2-6 September 2008, Cartagena de Indias, Colombia. [ Links ]
Muller-Parker G, McCloskey LR, Hoegh-Guldberg O, McAuley PJ. 1994. Effect of ammonium enrichment on animal and algal biomass of the coral Pocillopora damicornis. Pac. Sci. 48: 273-283. [ Links ]
Norzagaray-López CO, Ávila-López MC, Chapa-Balcorta C, Calderón-Aguilera LE, Hernández-Ayón M. 2013. Reducida producción de CaCO3 por Porites panamensis en tres comunidades arrecifales del Pacífico mexicano. In: Pellat FP, González JW, Bazan M, Saynes V (eds.), Estado Actual del Conocimiento del Ciclo del Carbono y sus Interacciones en México: Síntesis a 2013. Programa Mexicano del Carbono, México, pp. 437-441. [ Links ]
Norzagaray-López CO , Calderón-Aguilera LE , Hernández-Ayón JM , Reyes-Bonilla H , Carricart-Ganivet JP , Cabral-Tena RA, Balart EF . 2014. Low calcification rates and calcium carbonate production in Porites panamensis at its northernmost geographic distribution. Mar. Ecol. 36: 1244-1255. [ Links ]
Reyes-Bonilla H. 2003. Coral Reefs of the Pacific Coast of Mexico. Latin American Coral Reefs, 331 pp. [ Links ]
Reyes-Bonilla H, Calderón-Aguilera LE . 1994. Parámetros poblacionales de Porites panamensis (Anthozoa: Scleractinia), en el arrecife de Cabo Pulmo, México. Rev. Biol. Trop. 42: 121-128. [ Links ]
Reyes-Bonilla H, Calderón-Aguilera LE, Mozqueda-Torres MC, Carriquiry JD . 2014. Presupuesto de carbono en arrecifes coralinos de México. Interciencia 39: 645. [ Links ]
Reyes-Bonilla H, Carriquiry J, Leyte-Morales G, Cupul-Magaña A. 2002. Effects of the El Nino-Southern Oscillation and the anti-El Nino event (1997-1999) on coral reefs of the western coast of Mexico. Coral Reefs 21: 368-372. [ Links ]
Reyes-Bonilla H, Escobosa-González LE, Cupul-Magaña AL, Medina-Rosas P, Calderón-Aguilera LE. 2013. Estructura comunitaria de corales zooxantelados (Anthozoa: Scleractinia) en el arrecife coralino de Carrizales, Pacífico mexicano. Rev. Biol. Trop. 61: 583-594. [ Links ]
Reyes-Bonilla H , Leyte-Morales GE . 1998. Corals and coral reefs of the Puerto Angel region, west coast of Mexico. Rev. Biol. Trop. 46: 679-681. [ Links ]
Reyes-Bonilla H, López-Pérez RA. 2009. Corals and coral-reef communities in the Gulf of California. In: Johnson ME, Ledesma-Vásquez J (eds.), Atlas of Coastal Ecosystems in the Gulf of California. Past and Present. University of Arizona Press, pp. 45-57. [ Links ]
Stambler N, Popper N, Dubinsky Z, Stimson J. 1991. Effects of nutrient enrichment and water motion on the coral Pocillopora damicornis. Pac. Sci. 45(3): 299-307. [ Links ]
Tortolero-Langarica JA, Rodríguez-Troncoso AP, Carricart-Ganivet JP , Cupul-Magaña AL. 2016. Skeletal extension, density and calcification rates of massive free-living coral Porites lobata Dana, 1846. J. Exp. Mar. Biol. Ecol. 478: 68-76. [ Links ]
Veron JEN. 1995. Corals in Space and Time: The Biogeography and Evolution of the Scleractinia. Cornell Univ. Press, Sydney, 321 pp. [ Links ]
Walther-Mendoza M, Reyes-Bonilla H, LaJeunesse TC, López-Pérez A. 2016. Distribución y diversidad de dinoflagelados simbióticos en corales pétreos de la costa de Oaxaca, Pacífico de México. Rev. Mex. Biodivers. 87: 417-426. [ Links ]
Wells JW. 1963. Coral growth and geochronometry. Nature 197: 948-950. http://dx.doi.org/10.1038/197948a0 [ Links ]
Wellington GM, Glynn PW. 1983. Environmental influences on skeletal banding in eastern Pacific (Panama) corals. Coral Reefs 1: 215-222. [ Links ]
Yentsch CS, Yentsch CM, Cullen JJ, Lapointe B, Phinney DA. 2002. Sunlight and water transparency: Cornerstones in coral research. J. Exp. Mar. Biol. Ecol. 268: 171-183. [ Links ]
Zar JH. 2010. Biostatistical Analysis. Prentice-Hall, New Jersey, 944 pp. [ Links ]
Received: March 2016; Accepted: August 2016











 text in
text in