Introduction
Among macroalgae, the Rhodophyta constitute the group with the highest diversity in the sea. Calcareous red algae represent a high proportion of species within the Rhodophyta (Lüning 1990). According to Kelaher (2002), calcifying algae contribute significantly to the strength of the intertidal community structure because they provide refuge for many organisms, protecting them against wave action. Coralline algae are an ubiquitous algal group and they are dominant in coral reef communities (Littler 1973, Glynn 1996). However, the abundance of coralline algae in cryptic and shaded environments has been extensively underestimated (Litter 1973). Calcareous algae can grow in deep waters (200 m depth) at the limit of the photic zone (0.0015 µ mol m-2 s-1) and in surface waters exposed to high levels of photosynthetically active radiation (PAR) and ultraviolet (UV) radiation (Littler et al. 1991, Payri et al. 2001). The primary production of crustose coralline macroalgae is considered high due to their great abundance, although the contribution of organic carbon production rate is low (Larkum and Wood 1993). According to Chisholm et al. (1990), the organic productivity has been possibly underestimated since most studies on photosynthetic activity have been conducted using artificial light sources.
With the objective of increasing the knowledge of the performance of calcareous red macroalgae, photosynthetic activities were estimated by using in vivo chlorophyll a fluorescence of photosystem II on samples obtained from 2 different geographical communities of calcareous red macroalgae: (1) Neogoniolithon brassica-florida (Harvey) Setchell & LR Mason 1943, forming the vermetid reefs with Dendropoma petraeum (Prosobranchia, Mollusca) off Almería, Spanish Mediterranean coast, and (2) rhodoliths formed by Lithophyllum margaritae (Hariot) Heydrich 1901, collected from 2 sites in the southern part of the Baja California Peninsula, Mexico. Our aim is to understand the responses of calcareous red macroalgae to changes in the environmental variables by carrying out experiments on the effects of solar radiation and/or temperature variations on the photosynthetic productivity, and, in addition, to evaluate the effect of animals associated or attached to the algae.
Vermetid reefs are formed by sessile marine gastropods and calcareous red macroalgae. The gregarious vermetid gastropod D. petraeum forms dense aggregations cemented by the encrusting red alga N. brassica-florida along the lower mid-littoral area in the warmest areas of the Mediterranean Sea, on the southeastern coast of Spain. They form compact and resistant crusts in moderately exposed zones of oligotrophic waters with low rates of sedimentation (Calvo et al. 1998). Despite their importance as biogenic constructors, modulators of morphological coastal processes, indicators of sea level changes, and ecological habitat promoters of biodiversity (Calvo et al. 1998), very few studies on these marine communities have been done (Keen and Morton 1960). In the case of vermetids, most studies have focused on the gastropods (Milazzo et al. 2014) or on the entire community and have not examined the algae or relationship between both organisms. In this context, it is important to know the factors affecting the associated algae to understand how the changes in the environment could affect the expected equilibrium in the reef. Here, we present the effect of increasing temperature on the photosynthetic activity of N. brassica-florida associated with D. petraeum collected from the Cabo de Gata-Níjar Natural Park (Almería, Spain) (Fig. 1a).
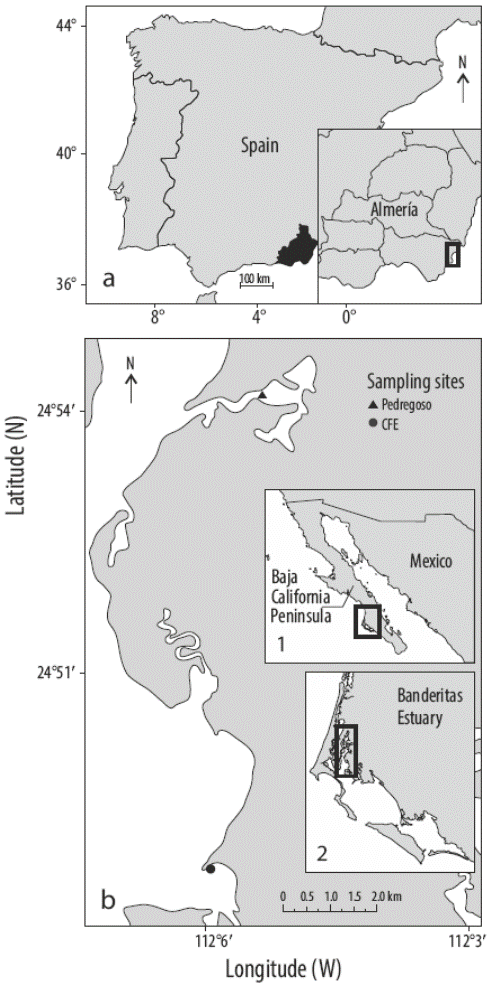
Figure 1 (a) Collection site of vermetids (Dendropoma petraeum) with the red macroalga Neogoniolithon brassica-florida in Cabo de Gata-Níjar Natural Park (southern Spain; 36o48´N, 2o03´W). (b) Collection sites (Pedregoso and CFE) of rhodoliths (Lithophyllum margaritae) in Banderitas Estuary within Magdalena Bay (Baja California Peninsula, Mexico): inset 1 shows the location of Magdalena Bay (black square) in northwestern Mexico and inset 2 shows the location of Banderitas Estuary (black square) within Magdalena Bay.
On the other hand, rhodoliths are non-geniculate freeliving coralline algae that form extensive beds in some seabeds. They constitute important biogenic carbonate ecosystems (Foster 2001) and refuge for many organisms (Amado-Filho et al. 2007, Foster et al. 2007). In addition, they are also feed for invertebrate species and support species of commercial importance in many coastal areas (Kamenos et al. 2003, Steller et al. 2003). However, physiological knowledge of this algal community is scarce. At geological level, coralline algae play an important role in maintaining beach stability because they constitute a major source of carbonate sediment (Russell and Johnson 2000). In the Gulf of California, rhodoliths form extensive beds and can resist extremely variable physical conditions such as high temperature ranges, i.e., 8 to 32 oC (Foster et al. 1997, Riosmena Rodríguez et al. 1999). Lithophyllum margaritae is the most abundant non-geniculate, rhodolith-forming coralline species in this region.
In this study, the hypothesis is that the photosynthetic activity of calcareous algae will increase under the conditions favoring the respiration rate of the animals associated with the macroalgae (i.e., increased temperature in the case of D. petraeum [vermetids] in the first set of experiments and the presence of attached epifauna [dominated by sponges] in rhodoliths in the case of the second set of experiments), since the CO2 available for photosynthesis will increase. Other variables like irradiance and nutrient availability were also considered.
Materials and methods
Sampling
Specimens of N. brassica-florida associated with vermetid reefs (D. petraeum) were collected from Cabo de Gata-Níjar Natural Park (Almería, Spain: 36o48´N, 2o03´W) (Fig. 1a) and transported in temperature-controlled tanks to the mesocosm system at Malaga University's Unit for Microbiology, Ecophysiology and Genetics of Aquatic Organisms (UMEGOA). Twelve vermetid reef units of 10 × 10 cm2 in size were collected.
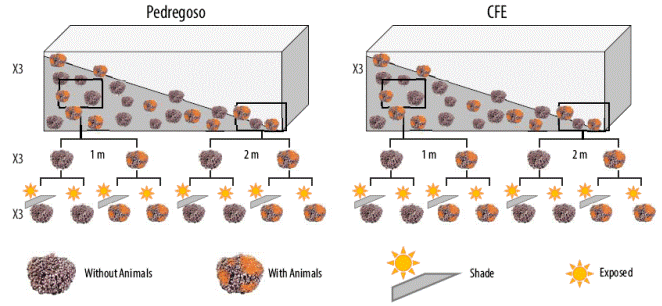
On the other hand, L. margaritae samples were collected from 2 sites at Banderitas Estuary, a coastal lagoon located within Magdalena Bay on the Pacific side of the Baja California Peninsula (Mexico): (1) Pedregoso (24o54´12´´N; 112o05´24´´W) and (2) CFE (24o48´45´´N; 112o05´59´´W)
(Fig. 1b). At each site, rhodoliths were sampled at 2 depths (1 and 2 m), and a total of 72 rhodoliths were collected, 36 covered with epifauna (mainly sponges) and 36 without attached epifauna. After collection, field studies were conducted at this coastal lagoon.
Experimental designs and physiological variables
In the present study, 2 sets of experiments were conducted to estimate both photosynthesis yield and production in calcareous red macroalgae. In the first, N. brassica-florida was incubated in a mesocosm system (Experiment 1) and in the second, rhodoliths were cultivated in tanks (Experiment 2).
For fluorescence measurements, 2 different fluorometers were used: a Water-PAM fluorometer for the measurements in the outdoor mesocoms and in situ in the coastal area, and a PAM-2000 fluorometer for laboratory measurements. Both fluorometers present red light as measured light, but the actinic light is red in Water-PAM and provided by a halogen lamp in PAM-2000. The technical characteristics of these fluorometers can be found in Figueroa et al. (2013).
Experiment 1
In the first set of experiments conducted in Spain, the mesocosm system consisted of 3 open vessels (0.094 m2 surface area, 14 L volume) per treatment, connected in parallel to a separate tank of 102 L capacity. The water flow between each box and its header tank was 0.84 ± 0.05 L min-1, representing a turnover rate of 26 ± 1% h-1 (according to Stengel et al. 2014) with a system to regulate water temperature (Titan, Aqua Medic, Bissendorf, Germany). Samples were incubated in individual tanks during a 6-month period (February to August 2013) under outdoor semi-natural solar conditions (reduced by 37% of EPAR [400-700 nm] and 41% of UVA [320-400 nm] and UVB ]280-320 nm]) and at 2 different temperatures: ambient temperature (T) and ambient temperature plus 2 oC (T+) (Fig. 2a, b). Physicochemical variables (pH and temperature) were controlled daily in each tank with AT Control System (T2001HC, Aqua Medic). Solar incident radiation was measured constantly with a multifilter radiometer NILU-UV6 (Geminali AS, Norway). General in situ incident irradiance was measured with a Zipo Hobbo U12-UV radiometer and for punctual incident irradiance, a radiometer equipped with a LI-COR spherical PAR sensor (Walz GmbH, Effeltrich, Germany) was used (Quintano et al. 2013).
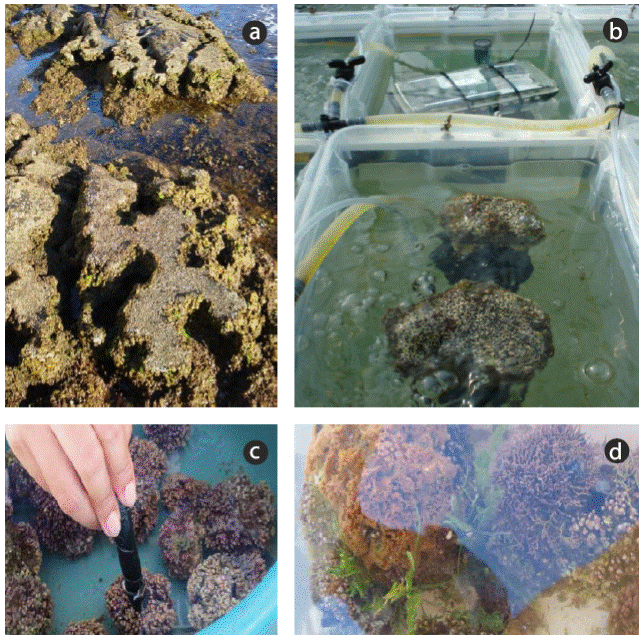
Figure 2 Photographs of vermetids at the harvesting site (Cabo de Gata-Níjar Natural Park, southern Spain) (a) and in the experimental containers (b), and of rhodoliths in the experimental containers without epifauna (c) and with attached animals, predominantly sponges (d).
Photosynthetic activity was estimated using in vivo chlorophyll a fluorescence, under 3 different conditions: in outdoor mesocosms, in situ, and in the laboratory.
Under outdoor conditions, daily cycles of photosynthetic responses were evaluated under natural solar radiation. The effective quantum yield (ΔF/Fm´) was measured with a Water-PAM fluorometer approximately every 2 h on 6 days with different intensity of irradiance (19 and 25 April, 3 and 29 May, 18 July, and 3 August 2013). This parameter was calculated as follows:
where Ft is the intrinsic fluorescence of alga incubated in light and Fm´ is the maximal fluorescence reached after a saturation pulse of algae incubated in light.
The electron transport rates (ETR, μ mol electrons m-2 s-1) were calculated according to Schreiber et al. (1995) as follows:
where ΔF/Fm´ is the effective quantum yield, E is the incident PAR irradiance (μ mol photons m-2 s-1), A is the thallus absorptance calculated as the fraction of incident irradiance that is absorbed by the algae (see Figueroa et al. 2003), and PSII is the fraction of chlorophyll related to photosystem II (400-700 nm), which is 0.15 in red macroalgae (Figueroa et al. 2014a). The ETR data calculated from daily cycles were designated as ETRdc and the the maximum ETR value obtained from all daily cycle data was designated as ETRmax(dc).
In the coastal area where N. brassica-florida was growing (Cabo de Gata-Níjar), in situ measurements of ΔF/Fm´ and ETR (designated as ETRin situ) were taken using a Water-Pam fluorometer.
In the laboratory, using a PAM-2000 fluorometer, rapid light curves (RLCs), i.e., ΔF/Fm´ versus irradiance, were obtained after preincubation of the sample for 15 min in the dark. Each replica was exposed to a saturation pulse with several progressive levels of light intensity for ten 20-s intervals (55, 199, 258, 330, 488, 666, 891, 1260, 1830, and 2700 μmol photons m-2 s-1). From the RLCs, maximum electron transport rate (ETRmax) was obtained from the tangential function reported by Jassby and Platt (1976).
ETRdc, ETRin situ, and ETRmax data were converted into oxygen production values with a conversion factor of 0.1 (10 photons are necessary to produce 1 mol O2), according to Williams and Robertson (1991) and as described by Kromkamp et al. (2008) for microalgae.
Experiment 2
In the experiment conducted in Mexico, the rhodoliths collected at the Pedregoso and CFE sites (Banderitas Estuary) were transported to an area set up to provide shade. All thalli were placed in 4-L vessels filled with seawater (Fig. 3). After acclimation for 6 h, rhodoliths were exposed to 2 treatments, full sunlight and shade (Figs. 2c, d; 3), and RLCs were assessed according to Schreiber et al. (1995) using a Water-PAM fluorometer. Solar irradiance was monitored with 2 Zipo Hobbo U12-UV radiometers. Samples were placed in 0.5-L chambers and incubated for 15 min in darkness to determine maximum quantum yield (Fv/Fm). After that, RLCs were generated with 10 incremental irradiances (7, 16, 23, 34, 51, 116, 166, 231, 315, and 564 (mol photons m-2 s-1) with 20-s intervals in each irradiance. The ΔF/Fm´ and ETR values were determined using a Water-PAM fluorometer according to Figueroa et al. (2003). Both parameters were calculated as explained in experiment 1. From the RLCs, different ETR parameters, such as ETRmax, saturation irradiance (EkETR), and the initial slope of ETR versus irradiance function (α ETR) as estimator of photosynthetic efficiency, were obtained from the tangential function reported by Eilers and Peeters (1988).
Statistical analyses
Interactive effects between physiological variables were analyzed using ANOVA. In the case of L. margaritae, site, depth, and presence of animals were included as fixed factors for photosynthetic variables. In the case of N. brassicaflorida associated with vermetid reefs, temperature and location were included as fixed factors for oxygen production rate. Homogeneity of variance was tested using Cochran's tests and by visual inspection of the residuals. Student-Newman-Keuls tests (SNK) were performed after significant ANOVA interactions (Underwood 1997). All data conformed to homogeneity of variance. Analyses were carried out using SPSS v21 (IBM, USA).
Results
Experiment 1
Seawater temperature during the experimental period was about 19.0 ± 2.3 oC in the T treatment and 21.5 ± 2.3 oC in the T+ treatment.
The ΔF/Fm´ values obtained for N. brassica-florida forming vermetid reefs and incubated in outdoor mesocosms decreased from the morning to noon and increased from noon to the afternoon (Fig. 4a). The decrease was greater on the days with high daily irradiances (Fig. 4b). ETRmax(dc), determined from daily cycle data, was similar in the T+ (32.5 ± 0.65 μmol photon m-2 s-1) and the T (29.4 ± 0.62 μ mol photon m-2 s-1) treatments. Oxygen production, estimated from laboratory ETRmax, ranged from 29 to 53 mmol O2 m-2 h-1 in T and from 24 to 55 mmol O2 m-2 h-1 in T+ (Table 1). However, oxygen production estimated from outdoor ETR values was higher in T+ than that in T (Table 1). The oxygen production estimated under solar radiation in the mesocosms (48-196 mmol O2 m-2 h-1) was about 2 times higher than that determined in the laboratory (31-108 mmol O2 m-2 h-1) under artificial illumination (halogen lamp, PAM-2000 fluorometer) (Table 1). Finally, oxygen production estimated from ETRin situ (using the Water-PAM fluorometer) under ambient temperature was 4 times higher (216-230 mmol O2 m-2 h-1) than that under laboratory conditions and about 2 times higher than that in the mesocosm (outdoor) trial (Table 1).
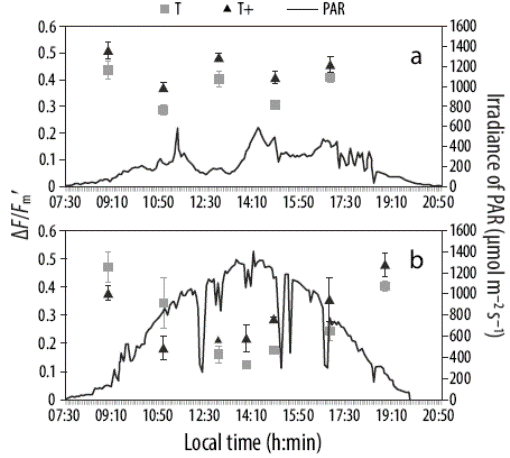
Figure 4 Effective quantum yield (ΔF/Fm´, symbols) of Neogoniolithon brassica-florida in the experimental tanks throughout the day (h:min) on 3 May (a) and 29 May 2013 (b). Data for ambient temperature (gray squares, T) and ambient temperature plus 2 oC (black triangles, T+) are presented. Dashed lines represent photosynthetically active radiation (PAR) irradiance (400-700 nm; μ mol photons m-2 s-1).
Table 1 Oxygen production rate (mmol O2 m-2 h-1) estimated from the electron transport rate values for Neogoniolithon brassica-florida in vermetid reefs in situ in the coastal area (field, 0 m depth) and incubated in outdoor mesocosms and under laboratory conditions at 2 different temperatures: ambient temperature (T) and ambient temperature plus 2 oC (T+). The data are compared to data from another study (Chisholm 2003) on free-living N. brassica-florida at 3 and 6 m depth.

Experiment 2
The photosynthetic activities estimated as in vivo chlorophyll a fluorescence of rhodoliths (L. margaritae) collected in Magdalena Bay (Mexico) are shown in Figures 5 and 6. ETRmax in rhodoliths with attached epifauna collected from 1 m depth was higher than in rhodoliths collected from 2 m, but no significant differences were observed between the 2 collection sites (Fig. 5a, Table 2). Pooled data show that ETRmax was higher in rhodoliths collected at 1 m depth in Pedregoso than at 1m depth in CFE regardless of the presence or absence of animals attached to them (Fig. 5b, Table 2), whereas no significant differences were observed between sites for the rhodoliths collected at 2 m depth. This indicates spatial heterogeneity in productivity expressed as ETR of rhodoliths. As expected, productivity was significantly (P < 0.05) higher in Pedregoso, the site with better water quality and rocky substrate, than at CFE, an embayment that is closer to urban and industrial activities, and has a greater percentage of muddy sediments in comparison to Pedregoso (Fig. 1b). ETRmax was 2 times higher at Pedregoso than at CFE (Fig. 5b, Table 2), meaning that the Pedregoso rhodoliths have a higher photosynthetic capacity. In rhodoliths from Pedegroso (representing a pristine area) with animals attached to them, saturation irradiance (Ek) was much higher in algae collected at 1 m compared to 2 m depth (Fig. 5c, Table 2). This confirms that rhodoliths growing at 1 m depth presented a full sunlight photosynthetic pattern compared to that of rhodoliths collected at 2m depth (dimmed sunlight). However, no significant differences were observed in Ek in rhodoliths collected from both depths at CFE (Fig. 5c, Table 2). The presence of animals attached to rhodoliths did not show significant differences by site and depth. There were no significant differenes in photosynthetic efficiency (α ETR) by depth (Fig. 6a, Table 2), but α ETR was higher in the CFE rhodoliths with animals attached to them than in those without epifauna (Fig. 6b, Table 2).

Figure 5 Maximum electron transport rate (ETRmax, μ mol electrons m-2 s-1) (a), ETRmax pooled data (b), and saturation irradiance (Ek, μ mol photons m-2 s-1) (c) for rhodoliths (Lithophyllum margaritae) with or without epifauna attached collected at the Pedregoso (white bars) and CFE (black bars) sampling stations in Magdalena Bay at 2 different depths (1 and 2 m). Treatments with significant differences are shown with different letters (P < 0.05).
Table 2 ANOVA results for the experiments using Lithophyllum margaritae collected in Magdalena Bay at 2 sites (S, Pedregoso and CFE), at 2 depths (D, 1 and 2 m), and with or without epifauna (E), with respect to photosynthesis parameters: maximal electron transport rate (ETRmax), ETR saturation irradiance (EkETR), and photosynthetic efficiency (α ETR). Significant differences at P < 0.01 and P < 0.05 are shown in bold.

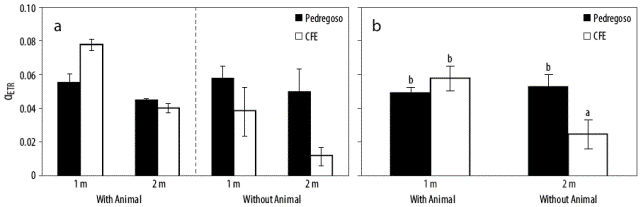
Figure 6 Photosynthetic efficiency (α ETR, relative units) for Lithophyllum margaritae with or without epifauna attached collected at 2 different depths (1 and 2 m) at the Pedregoso (white bars) and CFE (black bars) sampling stations in Magdalena Bay (a), and pooled data of the 2 stations and different depths (b). Treatments with significant differences are shown with different letters (P < 0.05).
Discussion
In the present study, ETR values in daily cycles of N.brassica-florida, considered as an estimator of algal productivity, were higher under temperature conditions increased by 2 oC (T+) compared to those obtained under ambient temperature conditions (T). The increase in photosynthetic activity could be explained by the increase in the available CO2 due to the increase in respiration of the mollusc D. petraeum in the vermetid reefs exposed to the 2 oC increase in temperature.
On the other hand, α ETR in L. margaritae from the CFE site was higher in rhodoliths with animals attached to them than in rhodoliths without epifauna. In the case of the CFE site, animals attached to rhodoliths can probably provide CO2 through respiration for photosynthetic activity, as also reported by Mercado et al. (1998) for another red alga, Gelidium sesquipedale, with epifauna (bryozoans) attached to thalli. Mercado et al. (1998) showed that the presence of animals (Electra pilosa) attached to G. sesquipedale increased photosynthetic activity by about 40% compared to algae from which the bryozoan had been removed. Mercado et al. (1998) attributed this result to the higher amount of CO2 available for the red algae. In the case of the rhodolith populations in Magdalena Bay, the attached epifauna mainly consisted of sponges (Ávila and Riosmena-Rodríguez 2011). Ávila et al. (2012) found 13 species of sponges living associated with these non-geniculate macroalgal beds, forming a complex and diverse habitat. They found that sponges function as a connectivity tissue that contributes to bed stability.
The ETR and consequently oxygen production was higher in N. brassica-florida under in situ conditions in coastal waters compared to vermetids incubated both in the outdoor mesocosms or under laboratory conditions. Probably, both natural light and nutrient natural conditions favored photosynthetic production when compared to the results obtained with samples maintained in mesocosms or under laboratory conditions. Oxygen production in N. brassica-florida forming part of the vermetid reef was higher than that reported by Chisholm (2003) for free-living N. brassica-florida (Table 1). Most studies about calcareous algae have used artificial radiation (Burdet et al. 2012); however, in recent years an increased number of studies have combined both laboratory and in situ measurements (Irving et al. 2004, Burdett et al. 2012). According to Chisholm (2003), net photosynthesis was variable between different tropical coralline algae: the highest in situ values corresponded to species of the genus Hydrolithon (24-28 mmol O2 m-2 h-1) and N. brassica-florida (28-14 mmol O2 m-2 h-1), whereas the lowest values corresponded to N. brassicaflorida (13.6-12.8 mmol O2 m-2 h-1), Neogoniolithum conicum (10.5 mmol O2 m-2 h-1), and Hydrolithon spp. (16.9-14.4 mmol O2 m-2 h-1) under laboratory conditions. The oxygen production values obtained by Chisholm (2003) and in the present study are higher than those reported for calcareous algae in previous studies (Littler and Littler 1980, Littler et al. 1983).
The ETR in rhodoliths was affected by the collection site due to the different environmental characteristics of the sites, the depth, and the presence of animals attached to rhodoliths. The ETRmax values (photosynthetic capacity) were 2 times higher at Pedregoso than at CFE, which could be associated with a greater transparency of the water column and consequently enhanced light availability for photosynthesis. The same result was found in ultraoligotrophic waters (Cabo de Gata-Níjar Natural Park, Spain) by Celis-Plá et al (2014). They recorded high ETRmax values for Ellisolandia elongata collected at 2 m depth in full light conditions. Photosynthetic efficiency (α ETR) in rhodoliths from Pedregoso was higher than in those collected at CFE but only in rhodoliths without epifauna. Multivariate ANOVA confirms that spatial variability was the major factor influencing photosynthetic yield, the highest values corresponding to Pedregoso. Sunlight exposure (full sunlight vs shade) and the presence of attached epifauna (epibionts vs no epibionts) had an equally significant effect on photosynthetic efficiency; the least significant factor in this analysis was depth. Ávila and RiosmenaRodríguez (2011) and Ávila et al. (2012) also reported that rhodolith beds at CFE, compared to those at Pedregoso, have higher densities per square meter, higher species diversity, greater branching of thalli, and higher sphericity, which are indicators of a dynamic zone, with strong tidal currents at the entrance of Banderitas Estuary (where the Pedregoso and CFE sites are located, Fig. 1b).
In addition, Steller et al. (2007) reported that biological stressors and physical variables such as temperature had an effect on the photosynthetic and calcification rates of L. margaritae; rhodolith photosynthetic, calcification, and growth rates showed wide fluctuations as a result of changes in laboratory or field temperatures. Maximum photosynthetic and respiratory rates both increased 5-fold as incubation temperature increased from 25 to 30 oC (Steller et al. 2007). Laboratory data suggest that rhodolith growth is regulated seasonally by seawater temperature. Field growth rates were significantly higher in summer (5.02 ± 1.16 mm yr-1) than in winter (0.83 ± 0.16 mm yr-1), supporting the laboratory results (Steller et al. 2007). According to Steller et al. (2007), the strong effects on the photosynthetic, calcification, and growth rates of L. margaritae in the Gulf of California suggest that changes in sea surface temperature directly regulate bed production.
Studies on the effect of climate change on aquatic organisms have mainly been conducted with 1 or 2 variables, and studies considering interactions of 3 or more factors are scarce (Bischof et al. 2006, Häder et al. 2007). The responses of photoautotrophs to a single factor depend on other factors (Breitburg et al. 1998, Stengel et al. 2014). This is probably the reason for the high variability of responses to high CO2 found among algae (Koch et al. 2013). The combination of an increase in both CO2 and light exposure negatively impacts photosynthesis and growth of marine primary producers (Gao et al. 2012). Photosynthetic activity increases as a result of an increase in CO2 both in calcified (Reiskind et al. 1988, Semesi et al. 2009) and non-calcified macroalgae (Connell and Russell 2010, Russell et al. 2011). On the other hand, under elevated CO2 levels, the calcification rate decreased and affected the growth of Corallina pilulifera (Gao et al. 1993). In contrast, increases in CO2 may enhance photosynthesis in calcifying algae such as Halimeda discoidea (De Beer and Larkum, 2001) and C. pilulifera (Gao et al. 1993), but these effects will be offset against those of decreased calcification as a result of decreased carbonate saturation state; however, this effect is difficult to predict.
If nutrient availability increases due to higher terrestrial inputs, there is a possibility of synergistic increases in the growth of turf algae (due to increased CO2 and nutrients), which promotes an increase in turf algae in relation to calcifying macroalgae. For example, little is known about how calcifying algae respond to solar UV radiation in combination to acidification. UV radiation may act synergistically, antagonistically or independently with ocean acidification (high CO2/low pH of seawater) to affect the calcification process (Gao and Zheng 2010). In Corallina sessilis, the presence of UV radiation inhibited growth, photosynthetic O2 evolution, and calcification rates, reflecting a synergistic effect of CO2 enrichment with UV radiation (Gao and Zheng 2010). Nevertheless, UV-induced inhibition of photosynthesis increased when the ratio of particulate inorganic carbon to particulate organic carbon decreased under the influence of CO2-acidified seawater. Thus, coralline algae can suffer more damage from UVB rays as they calcify less and less with progressing ocean acidification. In combination with other stress factors, such as global warming, increased storm frequencies, and pollution, the impact on littoral seaweeds will increase even at an exponential rate, so that several species might become endangered or be pushed to seek shelter in deeper waters from near-surface UV radiation (Wahl et al. 2004, Connell and Russell 2010, Figueroa et al. 2014b). Most research has been conducted at species level and there is still scarce information on the interactive effect of climate change variables on the structure, diversity, and primary production of the algal and aquatic macrophyte communities (Wahl et al. 2004, Bischof et al. 2006). Consequently, identification of how alternate conditions modify resource availability and limitations may facilitate anticipation of the future sustainability of major ecosystem components and the communities they support (Falkenberg et al. 2013). According to Harley et al. (2012), relative to present-day conditions, future warming will favor grazers and have direct and indirect negative effects on canopy-forming kelps. Future increases in CO2 will have strong negative effects on crustose coralline algae and positive effects on non-calcifying seaweeds both directly via improved growth and indirectly via reduced consumption by calcified herbivores.
In order to evaluate the vulnerability and acclimation of red calcareous macroalgae to climate change factors (IPCC 2014), it is necessary to conduct multifactorial experiments under controlled conditions simulating climate change scenarios (Martin and Gattuso 2009, McElhany and Busch 2013, Stengel et al. 2014) and using functional indicators for the non-intrusive estimation of photosynthesis such as in vivo chlorophyll a fluorescence techniques (Figueroa and Korbee 2010).











 texto en
texto en