Introduction
Studies on seasonal cycles of zooplankton (e.g., Loots et al. 2009, Eloire et al. 2010, Grigor et al. 2014) provide critical information about the dynamics of the ecosystem, such as changes in biomass, size structure, species composition, distribution, growth, and reproduction (e.g., Mackas and Beaugrand 2010, Overland et al. 2010). They also address key questions such as the effects of climate change on plankton communities (Drinkwater et al. 2010, Perry et al. 2010). The demographic characteristics of marine mesozooplankton make them especially suitable for examining the variability of marine ecosystems (Mackas and Beaugrand 2010). This is particularly relevant in coastal areas, typically of considerable ecological, economic, and social importance (Calbet et al. 2001). In particular, polar and temperate seas are characterized by pronounced seasonal fluctuations in irradiance, temperature, phytoplankton abundance, and species composition, all factors that directly or indirectly influence the growth rate and production of copepods (Kiørboe and Nielsen 1994, Madsen et al. 2001).
Physical factors such as salinity and temperature also regulate the seasonal plankton succession. Solar radiation, in particular ultraviolet radiation (UVR, 280-400 nm), has overall negative effects on many aquatic organisms including zooplankton (Bancroft et al. 2007). UVR is known to affect both behavior and mortality rates in zooplankton communities (Gonçalves and Hylander 2014) and these in turn structure trophic interactions (Williamson et al. 2001, Gonçalves et al. 2010). Many copepod species are known to be susceptible to UVR exposure, which is reflected in suppressed reproduction and increased adult and juvenile mortality (Zagarese et al. 1994), among other effects.
In spite of the economic importance of Patagonian waters as nursery sites for Merluccius hubbsi, Engraulis anchoita, and Pleoticus muelleri (Hansen et al. 2001, Pájaro et al. 2004, De Carli et al. 2012), the area is still rather unexplored in terms of the annual cycles of plankton communities. Thus, there is an obvious need to understand the temporal and spatial dynamics of lower trophic levels that may help to explain the abundance and distribution of such important commercial species. Although the annual cycles of phytoplankton have been extensively reported (e.g., Villafañe et al. 2004, Halac et al. 2011, Villafañe et al. 2013), there are no comparable studies on the seasonal cycles of small copepods or other zooplankton groups. The aim of this work is to provide data on the seasonal succession of copepod species (calanoids, cyclopoids, and harpacticoids) in relation to the different fractions of available food throughout the year and to the fluctuations of environmental variables.
Materials and methods
Study area
This study was conducted at Engaño Bay (43°21'S, 65°01'W), Chubut, Argentina (Fig. 1), from August 2010 to February 2012. The study site is located in close proximity to the Chubut River estuary. The estuary receives nutrients (from anthropogenic activities) as river runoff, resulting in relatively high phytoplankton biomass (Helbling et al. 1992, 2010). It has an important diversity of phytoplankton species (Villafañe et al. 2004, 2008) with a characteristic winter bloom (Barbieri et al. 2002, Villafañe et al. 2004). This microplankton bloom (>20 µm) seems to be favored by the prevailing low wind conditions at this time of the year (Villafañe et al. 2004, Helbling et al. 2005), in contrast with the windy season (spring and summer) during which picoplanktonic and nanoplanktonic (<20 µm) cells dominate in terms of abundance (Barbieri et al. 2002; Villafañe et al. 2004, 2008).
Sample collection and analysis
Zooplankton samples were taken every ~30 days in the outer part of the estuary (~5 km offshore) by surface trawls (towing time: 2 min; towing rate: 60 m min-1) with a plankton net (67 µm mesh size, 30 cm mouth opening diameter). The mesh size was chosen to optimize retention of both adults and immature stages of zooplankton (Di Mauro et al. 2009, Antacli et al. 2010). The trawls were done from a small pneumatic boat with careful observation of the operator. No clogging or reflux during trawling of the nets was observed. The volume of filtered water was estimated by means of a mechanical flowmeter (General Oceanics). The samples were preserved in 5% formalin in seawater for abundance and species analysis. Zooplankton was quantified and the density (ind m-3) of each class/order was estimated from the volume of filtered seawater. The different taxa/groups were separated under a stereomicroscope (Zeiss 475052-9901) and those samples with >300 specimens were fractionated in 20-mL aliquots. For the least abundant taxa, the entire sample was analyzed. Copepods were identified to the species level according to Bradford-Grieve et al. (1999). Developmental stages (nauplii, copepodites I-III and IV-V) were also differentiated, and the individual size (prosome length) of 1,223 individuals of the three dominant species was measured with an optical microscope (Olympus BHS) equipped with an ocular micrometer. The remaining taxa were identified to the class level according to Boltovskoy (1999).
Water samples (50 mL) for phytoplankton identification and abundance were preserved with buffered formaline (0.4% final formaldehyde concentration in the sample) and analyzed using the Utermõhl (1958) technique, under an inverted microscope (Leica DM IL). Water samples were also collected for the determination of chlorophyll a (Chla). In the laboratory, Chla concentration was determined by filtering 500 mL of the collected water onto Whatman GF/F filters (25 mm) and extracting photosynthetic pigments in absolute methanol (Holm-Hansen and Riemann 1978). Chla concentration was determined by fluorometric techniques using a fluorometer (TD-700, Turner Designs). Chla in the pico- and nanoplankton fraction (2-20 µm) was estimated as the difference between total Chla and that of a pre-filtered (20-um mesh) 500-mL aliquot.
Water temperature and salinity were registered using a multiparametric probe (YSI 600 XLM, Yellow Springs Instruments, Inc., USA). Solar UVR was continuously measured with a broadband radiometer (Eldonet, Real Time Computers, Inc., Germany), which measures ultraviolet B (UVB, 280-320 nm), ultraviolet A (UVA, 320-400 nm), and photosynthetically active radiation (PAR, 400-700 nm) with a frequency of one datum per minute.
Data analysis
Based on copepod abundance, ecological diversity indices were calculated to estimate changes in taxonomic composition. The Shannon-Weaver diversity index (H') (Shannon and Weaver 1949) was used for the estimation of community diversity. Species richness was calculated with the Margalef index (d) (Margalef 1958), and the evenness of the samples was estimated using the Pielou index (J') (Pielou 1969). Principal component analysis (PCA) was applied to evaluate the relationships among the abundance of each phytoplankton group (diatoms, flagellates, and dinoflagellates), the concentration of Chla (total and <20 µm), the abundance of copepod species, the diversity indices (H', d, and J'), and abiotic variables (temperature and UVR [UVB + UVA] and PAR). A multivariate analysis of numerical classification was used to define sample groups with distinct copepod species composition (cluster). Prior to analysis, data were transformed using log(x + 1). Hierarchical agglomerative clustering was carried out using the Bray-Curtis similarity index (Bray and Curtis 1957) coupled with group average. The sample groups resulting from the similarity analysis (Euclidean distance index) of the selected environmental variables were ordinated by a nonmetric multidimensional scaling (MDS) analysis. To examine the contribution of each taxon to the similarity within a group and dissimilarity between groups, a similarity percentage analysis (SIMPER) was applied using the Bray-Curtis similarity index. The PRIMER 5.0 software package (Clarke and Warwick 1994) and InfoStat software package (Di Rienzo et al. 2013) were used for data analysis.
Results
During the study period, daily doses of solar UVR varied from 45 to 2,200 kJ m-2 and PAR from 350 to 13,900 kJ m-2, both with maximum values in summer, mainly in December and January (Fig. 2). The water column was considered mixed throughout the study period, temperature and salinity always being homogeneous. The surface water temperature ranged from 7 to 17 °C, while salinity oscillated between 30.0 and 32.4 (data not shown).
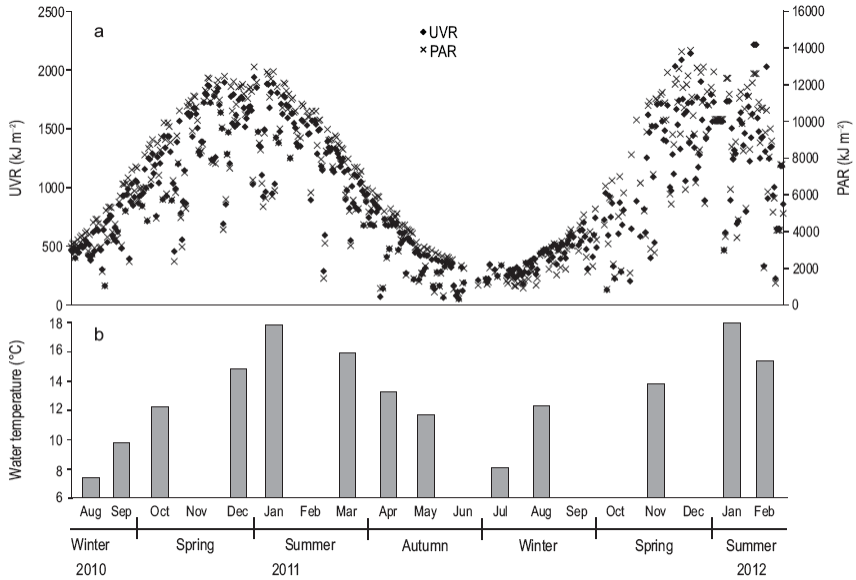
Figure 2: (a) Surface dose (kJ m 2) of solar ultraviolet radiation (UVR, 280-100 nm, black diamonds) and photosynthetically active radiation (PAR, 400-700 nm, black crosses) and (b) surface water temperature (°C) at the study site during the period August 2010-February 2012.
Total Chla concentration (Fig. 3) had the highest values in winter, particularly in August of 2010 and 2011 (6.2 and 6.6 µg L-1, respectively). Chla in the <20-µm fraction was high in spring and summer, representing about 80% of the total Chla. Flagellates were the most abundant group throughout the study period, especially in the summer of 2011 (~1,400 cells mL-1) and 2012 (1,900 cells mL-1, ~90% of cells), except in August 2011 when the abundance of diatoms was the highest (~2,000 cells mL-1, 83% of cells). The abundance of dinoflagellates was very low throughout the study period, with a maximum value of 67 cells mL-1 in December 2010, corresponding to 3.5% of the total abundance.
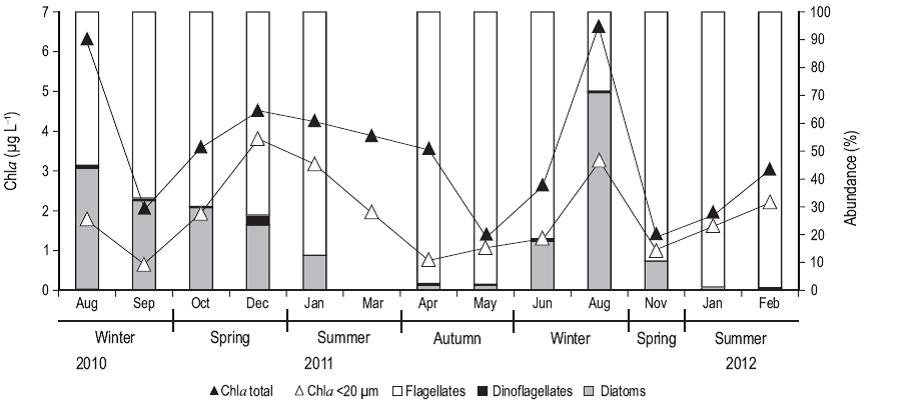
Figure 3: Concentration (ug L-1) of chlorophyll a (Chla; total fraction [black triangles] and pico- and nanoplankton [<20 µm, white triangles]), and percent abundance of flagellates (in white), dinoflagellates (in black), and diatoms (in gray) from samples collected from August 2010 to February 2012 at the northern Patagonian coastal station. Data are not available for September, October, and December 2011.
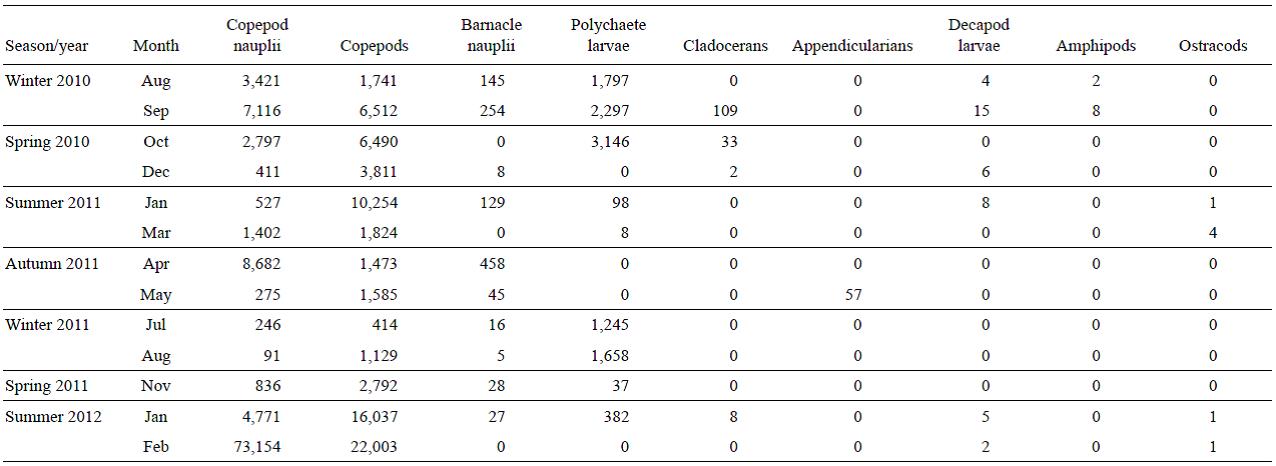
Copepods (juvenile and adults) were the most abundant group (Table 1) (>72% of total zooplankton in all samples, except for July and August of 2011). Copepod abundance varied between 380 ind m-3 (July 2011) and 20,700 ind m-3 (February 2012) for adults, and between 90 ind m-3 (August 2011) and 73,000 ind m-3 (February 2012) for nauplii. Poly-chaete larvae were abundant mainly in the winter and spring months, with a maximum abundance in October 2010 (3,150 ind m-3). Appendicularians were only found in May 2012 with a density of 57 ind m-3. The abundance of barnacle nauplli was high mainly in autumn and winter, with a maximum abundance in April 2011 (460 ind m-3), while cla-docerans were rather scarce in general, with a maximum abundance in September 2010 (100 ind m-3). The other groups (decapod larvae, amphipods, and ostracods) were found in a few samples and in very low abundances.
Table 1: Abundance (ind m-3) of mesozooplankton during the period August 2010-February 2012 at a northern Patagonian station (43°21'S, 65°01'W), Chubut, Argentina.
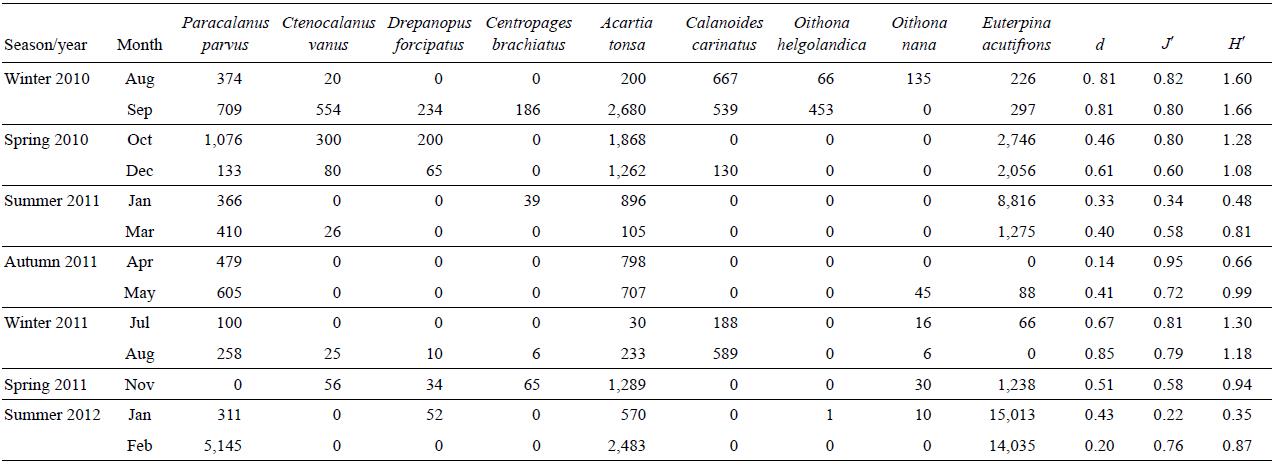
Among the copepods, harpacticoids and calanoids were more abundant than cyclopoids (Fig. 4). Calanoids were present throughout the cycle; however, harpacticoids dominated in the summer months and their densities were higher than those of calanoids. In particular, during January, the adults were more abundant, while in February the densities of adults and copepodites were similar. A total of 9 species were recorded (Table 2), showing a marked tendency throughout the seasonal cycle. Most species were observed in winter, mainly the calanoids Paracalanus parvus, Calanoides carinatus, Drepanopus forcipatus, Centropages brachiatus, Ctenocalanus vanus, and Acartia tonsa, and the cyclopoids Oithona nana and Oithona helgolandica. In summer, A. tonsa, P. parvus, and Euterpina acutifrons were observed, the last one being dominant (~90%). In autumn, A. tonsa and P parvus were dominant. The highest richness (Margalef index, d) and Shannon-Wiener index (H') values were recorded in the winter and spring months, whereas the lowest values were recorded in the summer months; the equitability index (J') showed a similar pattern.
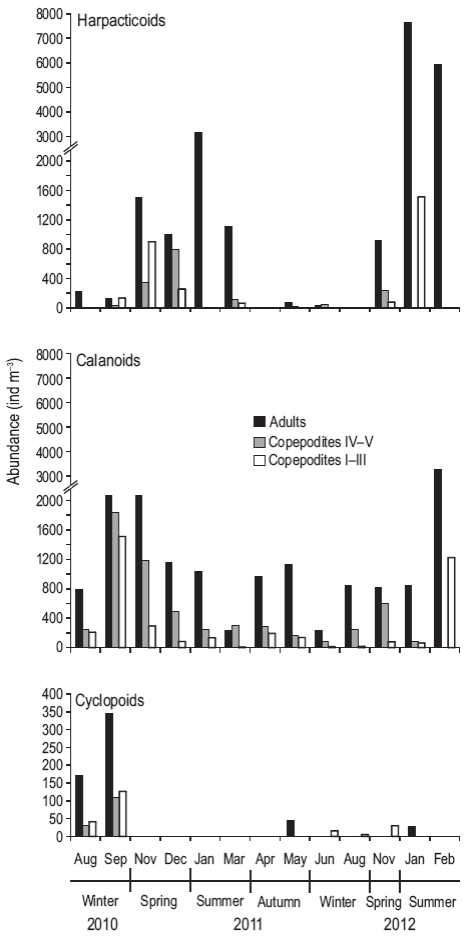
Figure 4: Abundance (ind m-3) of adults (black bars) and copepodites IV-V (gray bars) and I-III (white bars) of harpacticoids (a), calanoids (b), and cyclopoid copepods (c) during August 2010-February 2012 at the northern Patagonian coastal station. Note the different scales on the y axis for cyclopoids.
Table 2: Abundance of copepod species (ind m-3) and Margalef (d), Pielou (J'), and Shannon-Wiener (H') ecological indices during the period August 2010-February 2012 at a northern Patagonian coastal station (43°21'S, 65°01'W), Chubut, Argentina.
The dominant calanoid species during the study period were E. acutifrons, P. parvus, and A. tonsa. The mean prosome length of E. acutifrons varied between 170 and 950 µm (526 ± 200; n = 355) (Fig. 5), with the largest individuals found in summer (i.e., mostly mature individuals, especially egg-carrying females). Similarly, the mean prosome length of P. parvus varied from 190 to 1,190 µm (540 ± 234; n = 380), while the sizes of A. tonsa (copepodites and adults) oscillated between 150 and 1700 µm (688 ± 293; n = 488), being larger in winter.
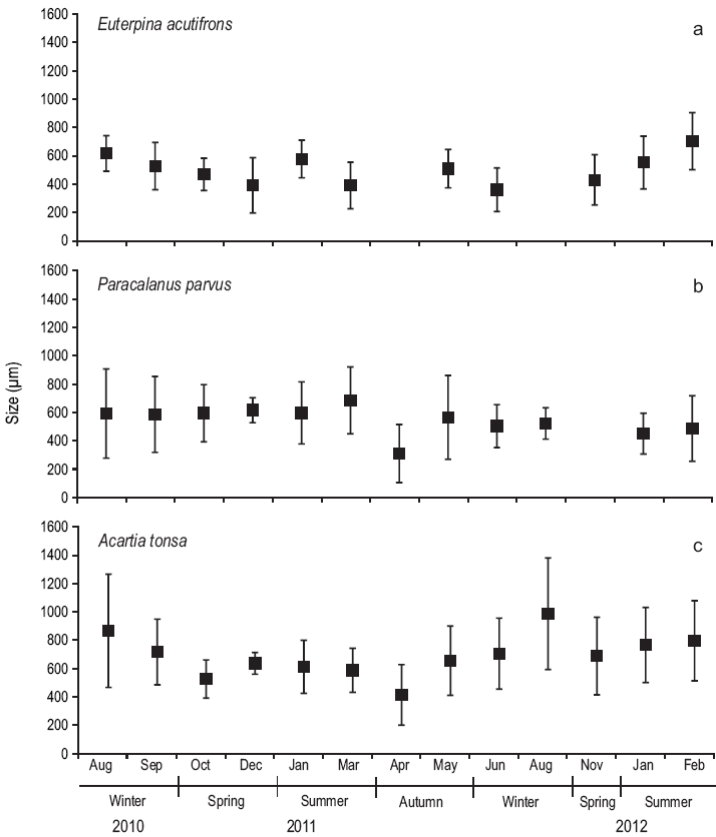
Figure 5: Size structure (um) of Euterpina acutifrons (a), Paracalanus parvus (b), and Acartia tonsa (c) during August 2010-February 2012 at the northern Patagonian coastal station. The lines on top of the mean (black squares) indicate the standard deviation.
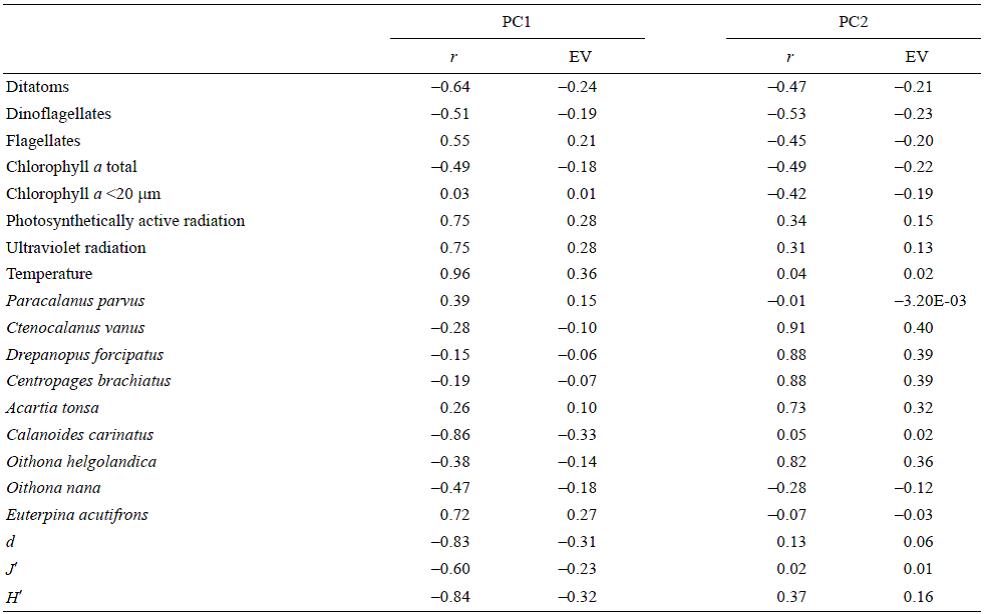
The PCA results are shown in Figure 6. The abundances of the copepods C. vanus, D. forcipatus, C. brachiatus, A. tonsa, and O. helgolandica were correlated, mainly in the winter months. The abundance of C. carinatus was positively correlated with that of diatoms (r = 0.73; P < 0.05) and negatively correlated with sea surface temperature and UVR (r = -0.77 and r = -0.73, respectively; P < 0.05). The abundance of E. acutifrons was positively correlated with UVR, PAR, temperature, and the concentration of flagellates (r = 0.61, r = 0.58, r = 0.64, and r = 0.72, respectively; P < 0.05). The abundance of P. parvus was positively correlated with the concentration of flagellates (r = 0.67; P < 0.05) and with the abundance of E. acutifrons (r = 0.71; P < 0.05). The diversity indices (d, J', and H) were negatively correlated with temperature (r = -0.73, r = -0.69, and r = -0.82, respectively; P < 0.05). The PCA showed that UVR, PAR, and temperature were mainly correlated with principal component 1 (Table 3), separating the samples according to the seasons.

Figure 6: Principal component analysis (PCA) between species of copepods, phytoplankton groups (diatoms, flagellates, and dinoflagellates), chlorophyll a (Chla, total fraction and pico-nanoplankton fraction), abiotic variables (temperature, ultraviolet radiation [UVR], photosynthetically active radiation [PAR]) and diversity indices (d, J', and H) during August 2010-February 2012 at the northern Patagonian coastal station.
Table 3: Results of the principal components analysis. Eigenvectors (EV), eigenvalues, percent of the variance explained by the original dataset (r2), and correlation (r) of the original variables with the first two principal components (PC1 and PC2).
Four groups of samples were determined at 80% level of similarity, corresponding to winter, spring, summer, and autumn (Fig. 7a). The MDS analysis confirmed the assemblages with a stress value of 0.11 (Fig. 7b). In summer and spring (groups 2 and 3), E. acutifrons and A. tonsa were the dominant species, the former being the most abundant (86%) mainly in January, February, and March (summer). In group 1 (winter: August and July), C. carinatus and P. parvus were the dominant species (62% and 30%, respectively), while in group 4 (autumn: April and May), A. tonsa and P. parvus were the dominant species (59.60% and 40.40%, respectively). However, when considering the abiotic variables (temperature and UVR), the MDS separated the assemblages into two groups (autumn/winter and spring/summer), mainly because there is a very pronounced gradient in these variables during these two periods of the year (Fig. 7c).
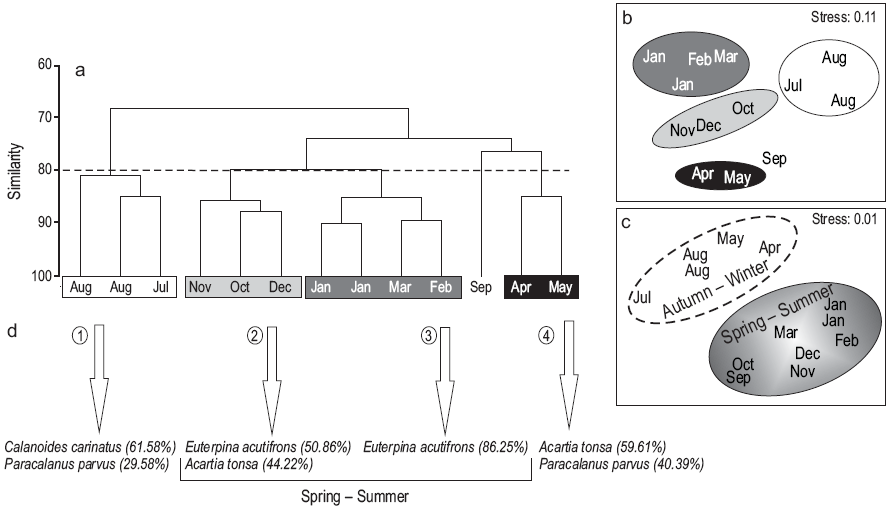
Figure 7: Analysis of similarity among the sampling dates. Cluster analysis (Bray-Curtis index) (a), and multidimensional scaling of samples using (b) biological and (c) abiotic variables (Euclidean distance index). The copepod species representative of each of the groups according to the SIMPER analysis are indicated with arrows (d).
Discussion
Copepods were the dominant metazooplankton group at the study site, as observed in other temperate coastal regions (Hopcroft et al. 2001, Aguirre et al. 2012). Copepods play a key role in the pelagic carbon flux because of their capability of taking up energy from the microbial food web and transferring it to higher trophic levels (Turner 2004, Viñas et al. 2013). All the copepod species found in this study have previously been reported for the Valdés Peninsula, Chubut Province (Sabatini and Martos 2002, Spinelli et al. 2012), except for E. acutifrons. This species is a small pelagic harpacticoid that often numerically dominates the meso-planktonic community in coastal and estuarine ecosystems (Sautour and Castel 1995, Eskinazi-Sant'Anna and Björnberg 2006).
Our results highlight the strong temporal variation in species composition and copepod abundance. Two different periods were found throughout the cycle, spring/summer and autumn/winter, with differences clearly marked by biotic and abiotic variables. The highest diversity of copepod species and abundance of polychaete larvae occurred in winter, likely related to the relatively high concentration of total Chla (i.e., bloom onset period) that favored the co-existence of more species of copepods. In spring/summer, under high radiation and temperature levels, flagellates (potential prey for copepods) were dominant in terms of abundance; therefore, the populations of small species such as the harpacticoid E. acutifrons became the most abundant. In agreement with our results, Villafañe et al. (2004, 2013) recorded maximum Chla values in winter; we were unable to collect stratification data (CTD profiles) during winter, but these authors observed a phytoplankton bloom, associated with favorable stratification, lower radiation, and temperature conditions. On the other hand, E. acutifrons, A. tonsa, and P. parvus were associated with flagellates, and this concurs with the observations made by Uye and Shibuno (1992) and Guisande et al. (2000). Similar results were also obtained experimentally by Vargas and González (2004). They detected that A. tonsa feeds on diatoms and flagellates and that P. parvus feeds on flagellates. The abundance of C. carinatus was positively correlated with that of diatoms and it is known that this species has typically been associated with this group of phytoplankton (Lopes et al. 1999). Taking into consideration that the calanoid species found were primarily herbivorous, a classical food chain can be suggested for winter, with large copepods such as C. carinatus associated with a higher concentration of diatoms.
A similar result was found in coastal waters of the northern Argentinean Sea (EPEA station, 38°28'S, 57°41'W), where the zooplankton succession also exhibited two main periods throughout the year: a cold winter/spring period characterized by a dominant classical herbivorous food web (with high Chla concentrations) and a warm summer period, dominated by picophytoplankton, in which smaller species such as O. nana and Paracalanus spp. were present (Viñas et al. 2013). However, temporal variations in copepod abundance may be explained not only by the availability of phytoplankton (bottom-up mechanism) but also by predation. In the study area, potential predators such as ctenophores and chaetognaths are very abundant (Mianzán and Guerrero 2000), and there is a high abundance of anchovy larvae (Hansen et al. 2001, Spinelli et al. 2012). On the other hand, Calbet et al. (2001) studied the seasonal succession of copepods in the coastal zone off Blanes (northwestern Mediterranean) and found differences in succession when compared to other coastal areas of the Mediterranean. These authors attribute the differences to the particularity of coastal areas. Coastal environments are highly variable and complex, and exposed to different intensities of anthropogenic and land-related influences (sewage discharges, rivers, etc.). The particular conditions of each location (oceanic currents, degree of enclosure, presence of submarine canyons, eddies, etc.) may strongly influence the annual distribution of phytoplankton and zooplankton (Calbet et al. 2001 and references therein).
Temperature and food quantity and quality are among the most important factors affecting egg production rates of marine copepods (Hirst and Bunker 2003, Gislason et al. 2008). Intensive reproduction of the small copepods E. acutifrons and P. parvus took place in summer, as indicated by the peaks of nauplii. This is probably related to the already known reproductive cycle of small copepod species in temperate seas (Pittois et al. 2009), which is positively controlled by temperature. The egg production rate increases exponentially with an increase in temperature (Vidal 1980, Uye and Shibuno 1992). Also, the high abundance of copepodites of E. acutifrons found in our study during the summer indicates active reproduction at this time of the year, in agreement with Viñas and Gaudy (1996), who observed mature organisms during the period of highest temperature in the San Matías Gulf (also in northern Patagonia). At low temperatures, the adults of this species remain in a state of quiescence (D'Apolito and Stancyk 1979, Viñas and Gaudy 1996) , which would explain the low abundances during the winter time. This shows that even a small variation in temperature may considerably affect the population dynamics of E. acutifrons. Moreover, the larger sizes of A. tonsa were found when sea temperature was lower. This is consistent with observations from other studies in which prosome length was negatively correlated with temperature (Chinnery and Williams 2004, Hansen et al. 2010).
Finally, in our study, a high diversity of species was found in winter when the UVR was low. Since UVR has, in general, negative effects on phytoplankton, it may alter the quantity and quality of food, which in turn may reduce survival, growth, and reproduction of zooplankton (Hessen et al. 1997) . Al-Aidaroos et al. (2015) recently reported that in the Red Sea the mortality rates of zooplankton increased greatly under UVR but declined when UVR was removed. Previous research in our study area addressing the effects of UVR has only been carried out on crab larvae (Hernández-Moresino et al. 2011, 2014; Gonçalves et al. 2014). Our work is the first study that considers the zooplankton community, especially the copepods, and natural variations with environmental factors. This highlights the potential importance of the effects of solar radiation on marine zooplankton from this area, and the need of performing longer-term studies considering natural communities and seasonal variations in solar radiation. Hence, the low diversity found in summer could be due to species-specific effects of detrimental solar radiation. This is the first study that reports the annual cycle of zooplankton in this productive area and our observations regarding the variation of copepod species at this northern Patagonian coastal station suggest different life-history strategies for this area. Evidently, more studies with a better temporal resolution are required to gain a deeper understanding about the interannual variability of this community, which in turn will help to interpret the effects of global climate change that are particularly relevant in high-latitude environments.











 texto en
texto en