Introduction
Mycotoxin contamination is a problem affecting nearly 25% of the world's crop production, representing the most important category of biological toxins impacting human and animal health (CAST 2003). According to the annual BIOMIN Mycotoxin Survey (BIOMIN 2014), an even higher percentage of crops are affected by mycotoxins (a prevalence of 66% and average contamination level of 1,394 parts per billion in 2014).
These toxic secondary metabolites are produced by filamentous fungi that frequently contaminate agricultural commodities used for human and animal feed (Hussein and Brasel 2001, Sudakin 2003). Chemically, mycotoxins display a wide range of structures, differing also in biological effects: carcinogenic, teratogenic, mutagenic, estrogenic, neurotoxic, or immunotoxic (Abd-Allah et al. 1999, McKean et al. 2006, El-Sayed et al. 2009, Hooft et al. 2011). Aflatoxins are polycyclic aromatic hydrocarbons, produced mainly by Aspergillus flavus and Aspergillus parasiticus, but also to a lesser extent by Aspergillus nominus. The main aflatoxins commonly found in feedstuffs are aflatoxin B1 (AFB1), aflatoxin B2 (AFB2), aflatoxin G1 (AFG1), and aflatoxin G2 (AFG2) (Ottinger and Kaattari 1998, 2000; Huang et al. 2011). These mycotoxins occur especially in subtropical and tropical areas, contaminating mainly feedstuffs with high starch and lipid content, such as cottonseed, corn, peanut, wheat, and soybean (Ostrowski-Meissner et al. 1995).
AFB1 is known as the most potent carcinogen among aflatoxins, classified as a group I carcinogen by the International Agency for Research on Cancer (IARC 1993) and highly hepatocarcinogenic (Busby and Wogan 1984, Sharma and Salunkhe 1991, Miller and Trenholm 1994, Wang et al. 1998).
The toxicity and carcinogenicity of AFB 1 is thought to be directly linked to its bioactivation. After ingestion, AFB1 is converted in the liver, which results in a highly reactive AFB1-8,9-epoxide (AFBO). This bioactivation occurs primarily by a microsomal cytochrome P450 (CYP450) dependent epoxidation of the terminal furan ring of AFB1 and is responsible for binding to cellular macromolecules such as DNA, RNA, and other protein constituents (Massey et al. 1995, Smith et al. 1997, Wang and Groopman 1999). This will directly result in damage/necrosis of hepatocytes and other metabolically active cells, leading to reduction of body weight, behavioral abnormalities, yellowing of the body surface, and immune suppression (Jantrarotai and Lovell 1990, Chávez-Sánchez et al. 1994, Eaton and Groopman 1994, Sahoo and Mukherjee 2001, Manning et al. 2005, Deng et al. 2010).
In aquaculture production, considerable research has been carried out on the toxicity of AFB1 on fish and crustacean species, including rainbow trout, Oncorhynchus mykiss (Ngethe et al. 1993; Bailey et al. 1994; Ottinger and Kaattari 1998, 2000; Carlson et al. 2001; Hooft et al. 2011; Hanson et al. 2014); channel catfish, Ictaluruspunctatus (Plumb et al. 1986, Jantrarotai and Lovell 1990, Gallagher and Eaton 1995, Lumlertdacha et al. 1995, Manning et al. 2005); Nile tilapia, Oreochromis niloticus (Chávez-Sánchez et al. 1994, Anh Tuan et al. 2002, Deng et al. 2010, Hassan et al. 2010); rohu, Labeo rohita (Sahoo and Mukherjee 2001); seabass, Dicentrarchus labrax (El-Sayed and Khalil 2009, El-Sayed et al. 2009); gibel carp, Carassius auratus gibelio (Huang et al. 2011); and shrimps (Ostrowski-Meissner et al. 1995, Burgos-Hernández et al. 2005).
Several methods have been tested in an attempt to decrease the bioavailability of aflatoxin. The addition of mycotoxin binders to contaminated diets has been considered the most promising dietary approach to reduce effects of some mycotoxins (Galvano et al. 1996, 2001). Binders decontaminate aflatoxins by binding them strongly enough to prevent toxic interactions with the animal and to prevent mycotoxin absorption across the digestive tract. Several potential adsorbent materials have been tested, including activated carbon, aluminosilicates (clay, bentonite, mont-morillonite, zeolite, phyllosilicates, etc.), complex indigestible carbohydrates (cellulose, polysaccharides from cell walls of yeast and bacteria such as glucomannans and peptidogly-cans, and others), and synthetic polymers such as cholestry-amine and polyvinylpyrrolidone and derivatives. However, their binding efficacy strongly differs according to the specific binder, its source, and the chemical structure of the mycotoxin. Additionally, some binders can have negative effects on growth due to their influence on nutrient utilization and mineral absorption (Chestnut et al. 1992, Kubena et al. 1993).
The yellow catfish, Pelteobagrus fulvidraco, is an important commercial species in China. This teleost fish species belongs to the order Siluriformes and has a high market value (Tan et al. 2009, Luo et al. 2011); however, as it is an omnivorous freshwater fish from a subtropical area, the probability of being affected by aflatoxin contaminated feedstuffs is large. Therefore, the objectives of this study were to investigate the sensitivity of yellow catfish to dietary AFB1 contamination, and to evaluate the efficiency of an aflatoxin binder in offsetting the negative effects of AFB1.
Materials and methods
Experimental conditions
Yellow catfish weighing 2.02 ± 0.10 g (mean ± SD) each were obtained from a local commercial fish hatchery and transported to the experimental facilities. Prior to the start of the experiment, the fish were stocked in net cages (3.0 × 3.0 × 3.0 m), and fed the control diet for 2 weeks in order to acclimate to the experimental diet and facility conditions.
At the start of the experiment, fish were fasted for 24 h and weighed after being anesthetized. Fish with similar sizes were randomly distributed into 24 net cages (2.0 × 2.0 × 2.0 m). Each cage was stocked with 40 fish. Fish were randomly hand-fed to apparent satiation twice a day (08:00 and 17:00) for approximately 5 min in each cage. As a voracious species, all feed given was estimated to be the same as eaten. No feed rejection, due to mycotoxin content, was observed. The feeding trial lasted for 12 weeks. The amount of feed given was adjusted every two weeks, according to fish growth (10 fish were bulk weighed), mortality, and feed conversion rate. Feeding was maintained equivalent between treatments with different survival rates. Water parameters (temperature, 28 ± 1 °C; dissolved oxygen, 5 ± 0.5 mg/L; NO3-N, 0 ± 0.2 mg/L; and NO2-N, 0 ± 0.1 mg/L) were checked daily and a natural photoperiod was provided.
Preparation of experimental diets
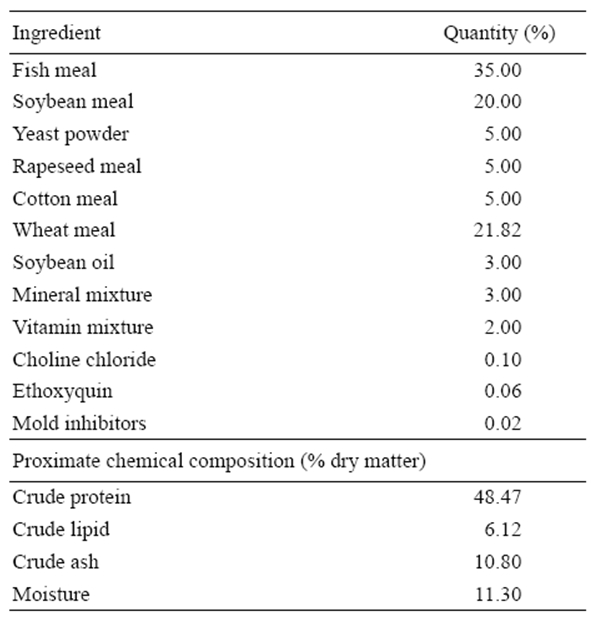
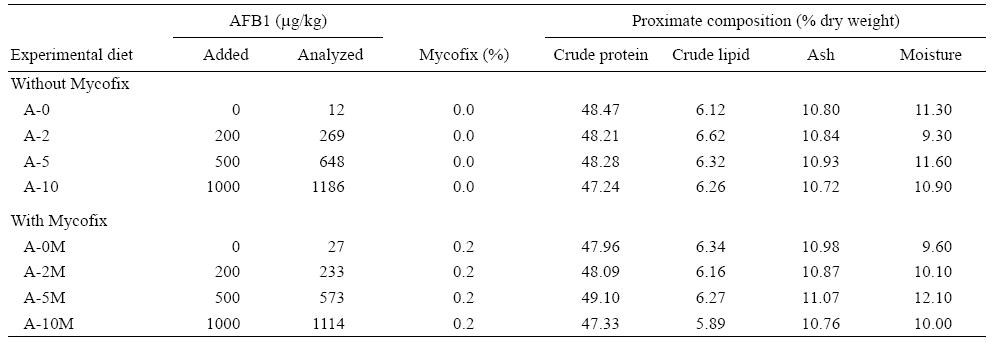
A basal diet was prepared using fish meal and soybean meal as main protein sources. Formulation and proximate chemical composition of the basal diet (%) are listed in Table 1. Eight experimental diets were then prepared and the basal diet was spiked with different concentrations of pure AFB1 with or without the addition of an aflatoxin binder (Mycofix Secure). The four treatments with AFB1 were named A-0, A-2, A-5, and A-10, and contained 0, 200, 500, and 1,000 μg/kg of AFB1, respectively. The treatments with Mycofix Secure were named A-0M, A-2M, A-5M, and A-10M, and contained 0, 200, 500, and 1,000 μg/kg of AFB1, respectively, plus 0.2% of Mycofix Secure in each diet (BIOMIN recommended inclusion level).
Ingredients were ground into fine powder through a 250 μm mesh. All ingredients were mixed with fish oil and water was added to produce a stiff dough. The dough was then pelleted with an experimental feed mill (F-26(II), South China University of Technology, China) and dried in a ventilated oven at 40 °C. After drying, diets were broken up and cut into 2.0 × 5.0 mm pellets. Diets were stored at -20°C until use. The amounts of AFB1 and Mycofix Secure, and the proximate composition of the experimental diets after addition of AFB1 are given in Table 2.
Growth performance
Feed consumption was recorded daily. At the end of the experiment, fish were counted and individually weighed to calculate the survival rate and weight gain. Specific growth rate (SGR) was calculated according to the formula SGR = 100(lnW2 - lnW1)/(t2 - t1) where W1 and W2 are the weights of the fish measured at times t1 and t2. The feed conversion ratio (FCR) for each treatment was calculated by the following equation: FCR = F/(W2 - W1), where F is the weight of food supplied to fish during the study period, W1 is the live weight of fish at the beginning of the study period, and W2 is the live weight of the fish at the end of the study period. The FCR was measured every 2 weeks; the FCR presented in the manuscript is an average of the 6 weeks of measurements.
Blood sampling
At the end of the trial, 10 fish from each net cage were collected for blood samples. Blood was drawn from the caudal vein of the fish and was allowed to clot at room temperature for 4 h. The serum was removed and frozen at -80 °C until use.
Immunosuppressive effects
Bactericidal activity
Aliquots of the collected serum were used to study bactericidal activity (Kajita 1990). Serum samples from each group were pooled into three aliquots and diluted three times with 0.1% gelatin veronal buffer (GVB2+) (pH 7.5, containing 0.5 mM mL-1 Mg2+ and 0.15 mM mL-1 Ca2+). Live, washed bacterial cells of Edwardsiella tarda were suspended in the same buffer to 1 × 105 CFU per milliliter. The diluted serum and bacteria were mixed at a proportion of 1:1, incubated for 90 min at 25 °C, and shaken. The number of viable bacteria was calculated by counting the colonies from the resultant incubated mixture on TSA plates, in duplicate, after 24 h of incubation.
Albumin/globulin ratio
The rest of the serum from the 10 fish of each cage was analyzed for the albumin/globulin (A:G) ratio (Sahoo et al. 1999). Serum samples were analyzed for total protein following the dye-binding method of Bradford (1976) and using bovine serum albumin as standard. Albumin was measured by the bromocresol green method and globulin by subtracting the albumin value from the total protein value. Finally, the A:G ratio was calculated.
Lysozyme assay
Lysozyme activity in serum of 5 fish from each cage was determined as described by Ellis (1990). Results were expressed in units of lysozyme per millimeter of serum. One unit is defined as the amount of sample causing a decrease in absorbance of 0.001 min-1 at 530 nm compared to the control (Micrococcus lysodeikticus suspension without serum).
Alternative complement pathway activity
Alternative complement pathway (ACP) activity was analyzed in serum of 5 fish per cage according to Yano (1996). Briefly, a series of volumes of the diluted serum ranging from 0.1 to 0.25 mL were dispensed into test tubes and the total volume made up to 0.25 mL. Then, with barbitone buffer in the presence of ethylene glycol-bis (2-amino-ethoxy)-tetraacetic acid (EGTA) and Mg2+, 0.1 mL of rabbit red blood cells (RaRBC) was added to each tube. After incubation during 2 h at 22 °C, 3.15 mL of 0.9% NaCl was added. Following this, the sample was centrifuged at 836 × g for 5 min at 4 °C to eliminate unlysed RaRBC. The optical density of the supernatant was measured at 414 nm. The volume of serum producing 50% hemolysis (ACH50) was determined and the number of ACH50 units per milliliter was obtained for each group.
Serum pathology parameters
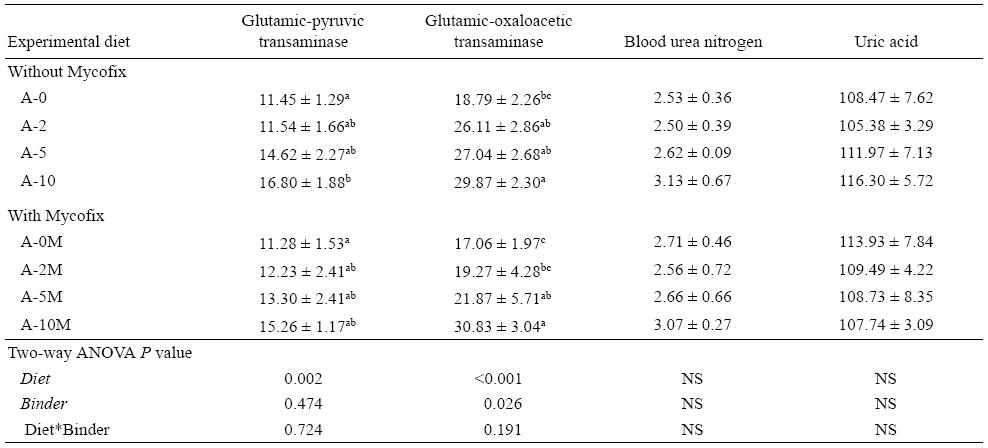
Glutamic-pyruvic transaminase (GPT), glutamic-oxalo-acetic transaminase (GOT), blood urea nitrogen (BUN), and uric acid were analyzed using the respective assay kit (Nanjing Jiancheng Bioengineering Institute, China).
Mycotoxin analysis
Feed samples were collected according to Richard (2000). The analyses were carried out as described by Binder et al. (2007). All samples were analyzed with HPLC. For the purpose of data analysis, non-detect levels are based on the limits of detection of the HPLC. For AFB1, AFB2, AFG1, and AFG2, the detection limits are 0.3, 0.1, 0.1, and 0.1 μ/kg, respectively.
Statistical analysis
All data were subjected to analysis of variance in SPSS 19.0 for Windows. One-way ANOVA was performed and differences between the means were tested by Tukey's multiple range test. Additionally, a two-way ANOVA was performed to analyze the effects of the binder and AFB1 concentration on the response variables. The level of significance was chosen at P < 0.05 and the results are presented as mean ± SD (standard deviation of the mean).
Results
Growth performance and survival
Feed analysis showed that AFB1 contamination was successfully achieved although observed levels were higher than expected (Table 2).
Results after the 12-week feeding trial showed that yellow catfish are sensitive to AFB1 contamination in the feed. The presence of AFB1 in the diet at levels of 500 μg/kg or higher led to a significant (P = 0.014) increase in FCR. There was also a statistical significant interaction between binder and AFB1 in the diet (P = 0.019), representing a less accentuated increase in FCR when using the binder in the diet (Fig. 1). Survival decreased significantly (P = 0.002) with the increase of AFB1 in the diet; however, Mycofix had no effect on this parameter (P = 0.093). Although there was no statistical difference in binder efficacy, when the diet contained 1,000 μg/kg of AFB1, survival was reduced 22% in the A-10 treatment, while in the equivalent treatment with the binder, A-10M, survival was reduced only 13.5%, when compared with the control group (Table 3).

Figure 1: Effect of dietary aflatoxin B1 (AFB1) on feed conversion ratio during the experimental trial (12 weeks). Significant differences were found regarding the efficacy of Mycofix Secure offsetting the negative effects of AFB1 (diet*binder P = 0.019). Differences were also found for the level of mycotoxin in the diet (P < 0.001) and the binder (P = 0.014). Superscript letters show differences for the mycotoxin level and the asterisks indicate differences in the efficacy of the binder. Points sharing the same superscript letter are not significantly different according to Tukey's honestly significant difference. Data are presented as mean ± standard deviation.
Table 3: Summary of growth parameters for yellow catfish during the experimental period.

Data are presented as mean ± SD. Values in the same column with different letters are significantly different (P < 0.05). NS = not significant.
Growth performance was also affected by AFB1 at levels of 200 μ/kg or higher (Table 3). A negative relationship between the presence of AFB1 in the diet and fish growth performance (weight gain, SGR) was observed; however, this negative response was less pronounced when the aflatoxin binder was supplemented to the contaminated feed (Table 3). As observed for growth performance and survival rate, the negative impact of AFB 1 on fish fed diets supplemented with the binder was less pronounced.
Proximate carcass composition of fish
Regarding carcass composition of fish, no significant differences were found between treatments (Table 4).
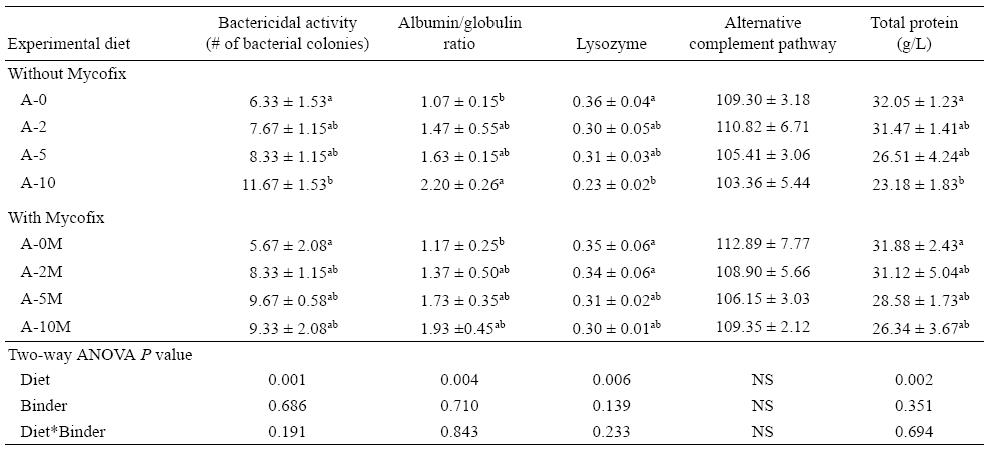
Serum immunological parameters
Table 5 shows a summary of serum immunological parameters (bactericidal activity [number of bacterial colonies], A:G ratio, lysozyme, ACP, and total protein) for yellow catfish fed the experimental diets for 12 weeks. It is clear that the presence of AFB1 in the diets affected these parameters, with the exception of ACP for which no significant differences between the diets were found. For the bactericidal activity, the higher the amount of AFB1 in the diets, the higher the number of bacterial colonies counted (P = 0.001). As such, the number of bacterial colonies in A-10 was significantly higher (P = 0.001) when comparing diets A-0 and A-0M. The A:G ratio had a similar pattern to that of the number of bacterial colonies, with A-10 presenting the highest A:G ratio and A-0 and A-0M the lowest. Values for lysozyme and total protein decreased throughout the treatments with the increase in AFB1 concentration. Groups supplemented with Mycofix had less pronounced reduction compared to non-supplemented ones, but no significant statistical effect of the binder was detected.
Serum pathological parameters
Fish displayed no significant differences in BUN and uric acid among all treatments, although A-10 tended to have higher values when compared with the other diets. There was a significant increase in GPT (P = 0.002) and GOT (P < 0.001) with the increase of AFB1 in the diets. In the Mycofix treated groups, GPT values showed no difference between treatments; however, GOT levels significantly increased (P = 0.026) with the increase of aflatoxin levels in the diets (Table 6).
Discussion
The levels of AFB1 obtained in the experimental diets were higher than expected, and this is linked to natural contamination of ingredients used in the basal diets (12 and 27 μg/kg for diets A-0 and A-0M, respectively). This fact highlights the risk of AFB1 contamination in feedstuffs used in aquafeeds. It is well known that some plant sources used in aquaculture feeds, especially in subtropical and tropical areas, have a high risk of aflatoxin contamination, which adversely affects fish health and welfare, resulting in high production losses (Ciegler et al. 1981, Cole and Cox 1981, CAST 2003, McKean et al. 2006, Bryden 2011, Hooft et al. 2011).
Throughout the experimental period it was possible to confirm that the yellow catfish, P. fulvidraco, is sensitive to AFB1. Based on growth performance parameter results, levels of 200 μg/kg or higher of AFB1 in the diet are toxic to yellow catfish. Looking at other species, Deng et al. (2010) reported no significant differences in survival among tilapia exposed to AFB1 concentrations ranging from 19 to 1,641 μg/kg diet in a 20-week trial. Tuan et al. (2002) also found no significant differences in tilapia survival when fish were fed levels of 10 mg AFB1/kg, and Chávez-Sánchez et al. (1994) found the same conclusions at levels up to 30 mg AFB 1/kg. These data suggest that the yellow catfish is more sensitive to AFB1 than tilapia, which might be related to the respective feeding habits. Several authors (El-Banna et al. 1992, Zaki et al. 2008, Deng et al. 2010) have demonstrated that AFB1 exposure could cause adverse effects on growth performance of different fish species. Normally, a reduction in weight gain and increased FCR are observed (Jantrarotai and Lovell 1990, Deng et al. 2010, Huang et al. 2011) and confirmed by the present study on yellow catfish. Nonetheless, discrepancies between results, even for the same species, are considerable (Deng et al. 2010, Zychowski et al. 2013). For example, in a 20-week trial using tilapia, Deng et al. (2010) showed that just after 15 weeks, fish exposed to 1,641 μg AFB1/kg diet had significantly lower weight gain. However, Zychowski et al. (2013) concluded that AFB1 had a significant impact on tilapia over 10 weeks, with decreased weight gain and lower feed efficiency, at a lower level of AFB1 (3 μg/kg). These discrepancies can be attributed to differences in sex, age, and locality, or to different environmental or nutritional conditions (Eaton and Groopman 1994), and to exposure time which changes the way the animal copes with the toxin.
In the current study, the diets containing Mycofix Secure showed a tendency to decrease the negative effects on survival and growth performance (weight gain, SGR, and FCR) when compared with the diets lacking the binder. The diets containing Mycofix showed a slower decrease in weight gain when compared with the diets without the binder. It is also very interesting to perceive an accentuated drop in weight gain, between 500 and 1,000 μg/kg of AFB1, suggesting that this is the level at which this species starts having difficulties coping with biological damage from the toxins. The presence of AFB1 in the diet negatively affected the FCR of yellow catfish. In the A-10M treatment Mycofix improved the FCR by 24% when compared with the respective treatment without the binder (A-10). In general, Mycofix allowed improvements in FCR when compared with the relative control group. While the differences are not statistically significant, from an economic perspective, the improvement obtained in FCR (e.g., 24% for 1,000 μg/kg of AFB1 level) represents genuine revenue for aquaculture producers.
AFB1 is also a potent immune modulator leading to the suppression of the immune system (Ottinger and Kaattari 1998) and consequently affecting the normal values of total protein, albumin, globulin, and A:G ratio. Abnormal levels of these proteins have been associated with hemolysis or increased breakdown of red blood cells and/or liver damage (Islam et al. 2004). The liver is responsible for clearing the blood of bilirubin, so liver damage can result in the pale yellow color presented by sick animals and described as a possible sympton of mycotoxicosis in fish. In our study, immunosuppression of catfish was confirmed from the reduced total protein and increased A:G ratio. Both globulin and albumin are produced by the liver and if the liver is damaged, it can no longer produce these proteins. So we can conclude that the reduction in total protein level may be associated with liver and kidney disorders due to the ingestion of AFB1. The increased A:G ratio suggests underproduction of immunoglobulins, indicating a suppression of the fish immune system. Further, the immunosuppressive nature of AFB1 in yellow catfish can also be confirmed by the reduced bactericidal activity, which can be associated with the poor bacteria-destroying capacity of serum antimicrobial factors in fish fed the highest concentration of AFB1. In the case of the diets without Mycofix, the number of bacterial colonies in the A-10 diet was significantly higher than in the control treatment, showing a clear impact of AFB1 on serum antimicrobial factors. In general, values of fish ACP activity are considerably higher than those for mammals, suggesting that this activation pathway of the complement system is of particular relevance for fish as a non-specific immune mechanism (Yano et al. 1988, Sunyer and Tort 1995). In this study it was not possible to evaluate the relation between the AFB1 ingested and the ACP values.
Similarly to the ACP activity, fish lysozyme appears to be more active than that of higher vertebrates and is able to eliminate a variety of bacterial pathogens (Yousif et al. 1994). The innate immune system of fish is considered to be the main defense against a broad spectrum of pathogens and is more important for fish in comparison to mammals, with lysozyme activity being an important index of innate immunity of fish. In fish, lysozyme is found in phagocytic cells, serum, mucus, and ova. It is a hydrolytic enzyme able to cleave the cell wall of Gram-positive and some Gram-negative bacteria. By measuring this parameter, it is possible to analyze the immunosuppression exerted by the exposure of catfish to AFB1. We were able to identify a decrease of lysozyme activity in both treatments; however, in the group supplemented with Mycofix, the decrease of lysozyme activity was less pronounced. For instance, the diet containing 200 μg/kg of AFB1 had lysozyme activity values equivalent to the diet containing 1,000 μg/kg of AFB1 with Mycofix, showing that Mycofix had a protective effect against AFB1.
As in mammals, in fish some enzymes can be used as specific "liver-guiding enzymes" that can be a sensitive indicator of hepatotoxic effects, GPT and GOT being two of those (Krajnovic-Ozretic and Ozretic 1987). These enzymes are found in small concentrations in plasma, derived probably from the regular physiological shedding of cells (Schmidt and Schmidt 1974). Therefore, any detectable increase of their activity in plasma can be a reliable indicator of not only changed metabolic functions but also of structural damage at tissue level, so the interpretation of results from these enzymes should be done carefully (Krajnovic-Ozretic and Ozretic 1987). In the present trial we observed an increasing value of GPT and GOT in response to the increasing values of AFB1 in the diets, suggesting tissue damage. These parameters confirm that Mycofix effectively protected the fish against structural damage at tissue level, caused by AFB1. Regarding BUN and uric acid, no strong conclusions could be obtained from these parameters; however, values increased when animals were fed diets containing increasing quantities of mycotoxins. This suggests a change in metabolism function or/and structural damage at tissue level, that probably could not be detected by macroscopic examination in such a short-term trial.
In conclusion, the yellow catfish is sensitive to the presence of AFB1 in the diet. A negative relationship between the AFB 1 level in the diet and fish survival, growth performance, feed efficiency, and immunological parameters was observed. Diets containing 1,000 μg/kg of AFB1 were highly toxic to yellow catfish. The use of the AFB1 binder (Mycofix) decreased the negative impact of AFB1 toxicity on the fish. While not statistically different in some cases, the results obtained represent an important step for counteracting AFB1 in aquaculture.











 texto en
texto en