Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Ciencias marinas
versão impressa ISSN 0185-3880
Cienc. mar vol.41 no.4 Ensenada Dez. 2015
https://doi.org/10.7773/cm.v41i4.2560
Articles
Horizontal and vertical movements of the common dolphinfish (Coryphaena hippurus) in La Paz Bay, Mexico
1 Centro de Investigaciones Biológicas del Noroeste, Calle IPN 195, Playa Palo de Santa Rita Sur, CP 23096, La Paz, Baja California Sur, México.
2 Pelagios Kakunjá, A.C., La Paz, Baja California Sur, México.
The common dolphinfish (Coryphaena hippurus) is an important resource for the sport-fishing industry in Mexico. It is captured incidentally by the eastern Pacific purse-seine tuna fishery and in coastal areas by artisanal fisheries. Although it is considered a highly migratory species, only few studies of its movements are available. In this study, the behavior of the common dolphinfish was analyzed by means of acoustic telemetry at three locations within La Paz Bay (southern Gulf of California) from summer to autumn 2013. Acoustic transmitters were attached to six individuals and they were tracked for periods of up to 48 h. Every hour, vertical profiles of temperature and bathymetry were made. To determine the effect of biotic (sex) and abiotic (water temperature and depth, thermocline depth, hour, and bathymetry) variables on the horizontal and vertical movements of tagged organisms, generalized additive mixed models were applied. The results showed a tendency of horizontal movements to the southwestern zone of the bay; two individuals crossed from the eastern to the western side. The average speed was 0.51 m s-1. The vertical movements showed that the individuals spent 80% of the time above 34 m depth, of which around 45% was spent in the upper 10 m of the water column. During daylight hours they swam in shallow waters (upper 10 m) and at night they performed deeper dives (73 m maximum depth). Biotic and abiotic variables influenced both types of movements; however, bathymetry and temperature largely explained fish movements.
Key words: horizontal and vertical movements; Coryphaena hippurus; La Paz Bay; acoustic transmitters.
El dorado (Coryphaena hippurus) es un recurso importante para la pesca deportiva en México. Es capturado incidentalmente por la flota atunera de cerco en el Pacífico oriental y en áreas costeras por la pesca artesanal. Aunque es considerada una especie altamente migratoria, existen pocos estudios sobre sus movimientos. En este estudio se analizaron los movimientos del dorado por medio de telemetría acústica en tres localidades dentro de la bahía de La Paz, parte sur del golfo de California, entre verano y otoño de 2013. Se adhirieron transmisores acústicos a seis individuos y se siguieron durante periodos de hasta 48 h. Cada hora, se hicieron perfiles verticales de temperatura y batimetría. Para determinar el efecto de variables bióticas (sexo) y abióticas (temperatura y profundidad del agua, profundidad de la termoclina, hora y batimetría) sobre los movimientos horizontales y verticales de los organismos marcados, se aplicaron modelos aditivos generalizados mixtos. Los resultados mostraron una tendencia a movimientos horizontales a la zona suroeste de bahía de La Paz; dos movimientos atravesaron la bahía de sureste a suroeste. La velocidad promedio fue de 0.51 m s-1. Los movimientos verticales indicaron que los individuos estuvieron por encima de los 34 m de profundidad el 80% del tiempo, del cual aproximadamente 45% estuvieron en los 10 m superiores. En horas de luz los peces nadaron por arriba de los 10 m de profundidad y en la noche realizaron inmersiones de hasta 73 m. Las variables bióticas y abióticas influyeron ambos tipos de movimientos; sin embargo, la batimetría y temperatura explicaron en gran parte los movimientos de los peces.
Palabras clave: movimientos horizontales y verticales; Coryphaena hippurus; bahía de La Paz; transmisores acústicos
Introduction
The movement of organisms is defined as a change in their spatial location as a result of a complex interaction between internal factors that promote movement and interpret why, how, when, and where to move and external factors (biotic and abiotic) that affect movement (Nathan et al. 2008). The movements of migratory pelagic fish such as tuna, billfish, and dolphinfish have been shown to be affected by environmental conditions (e.g., currents, changes in temperature and oxygen concentrations with depth), which in turn play a key role in determining their permanence and habitat preference (Brill and Lutcavage 2001, Furukawa et al. 2011, Schaefer et al. 2011). It is important to know the movements and habitat preferences of pelagic fish because their vulnerability to fishing is influenced by factors such as the depth they inhabit, travelling speed, residence time in certain areas, and school formation (Brill and Lutcavage 2001).
The common dolphinfish, Coryphaena hippurus Linnaeus, 1758, is a pelagic species distributed in tropical and subtropical seas (Palko et al. 1982). It is ecologically important because it is a top predator in the pelagic ecosystem (Varghese et al. 2013). It is opportunistic (Tripp-Valdez et al. 2010), highly voracious, and has a wide trophic spectrum, and its food habits are closely linked to the epipelagic environment (Aguilar-Palomino et al. 1998).
In Mexico, C. hippurus is distributed along the Pacific coast, from the Baja California Sur region, where it is found year-round (Aguilar-Palomino et al. 1998), to the Gulf of Tehuantepec, off Oaxaca (Alejo-Plata et al. 2011, Zúñiga-Flores et al. 2011). This species is reserved for sport fishing within 50 nautical miles measured from the territorial sea baseline (DOF 1995). It is also caught incidentally by the purse-seine and artisanal fisheries (Aguilar-Palomino et al. 1998, Madrid and Beltrán-Pimienta 2001, Martínez-Rincón et al. 2009).
Dolphinfish grow rapidly and have a short life span (Alejo-Plata et al. 2011). Maximum catch sizes of 192 cm and 30 kg have been reported for the northwestern coast of Mexico (Madrid and Beltrán-Pimienta 2001). Off Baja California Sur, dolphinfish reach maturity at 80 cm fork length and during the breeding season (summer-autumn) they are multiple spawners (Zúñiga-Flores et al. 2011).
Little information is available on dolphinfish movements. Studies have analyzed their movements around floating fish aggregating devices (Girard et al. 2007, Taquet et al. 2007) and described their movements in the open sea (Furukawa et al. 2011, 2014; Merten et al. 2014a, 2014b, 2014c). These studies indicate that dolphinfish tend to stay above the ther-mocline (Furukawa et al. 2011) and that they are able to perform directed movements and return to their point of origin (Girard et al. 2007). In coastal water bodies, however, many aspects relating to their movements, use of habitat, and environmental preferences are still unknown. The objective of this study was to identify the biotic and abiotic factors that influence the fine-scale horizontal and vertical movements (every 16 min) of dolphinfish in a short time frame (up to 48 h) in a coastal water body in the Gulf of California.
Materials and methods
Study area and tagging
This study was carried out in the southwestern part of La Paz Bay (LPB), Baja California Sur, Mexico (Fig. 1). Eight dolphinfish were caught by hook and line at three localities within the bay (Califín [north], Quelele, and Pichilingue) in September and October 2013. Two of these individuals were only used for a few hours to test the tracking equipment.

Figure 1: (a) Study area and tracking of six common dolphinfish (Coryphaena hippurus) at three locations in La Paz Bay (Mexico): (1) north of Califín, (2) Quelele, and (3) Pichilingue. (b-g) Tracks 1-6, respectively; circles indicate the start and triangles the end point.
Each captured individual was placed on a wet towel on the boat's deck and the eyes were covered with a wet cloth to reduce stress caused by illumination. A plastic hose connected to an underwater pump was inserted into the mouth to provide a continuous flow of seawater and facilitate oxygenation of the gills. Each fish was measured for fork length and total length, and sexed based on the sexual dimorphism described by Palko et al. (1982): the males are more distinctive than the females because they develop a bony ridge on the head.
Each individual was fitted with an external V16-TP continuous acoustic transmitter (16 mm diameter, 71 mm wide, 11 g weight in water; VEMCO, Halifax, Canada) equipped with a temperature sensor (accuracy 0.12 °C) and depth sensor (accuracy 0.9 m). The transmitters were attached by passing a plastic strap using a stainless steel needle through 1 cm of muscle near the base of the caudal fin. The entire capture and tagging process did not take more than 7 min.
Acoustic monitoring
Each individual was actively tracked. The boat was outfitted with a VH110 directional hydrophone and a VR100 receiver (VEMCO) to detect the transmissions from the acoustic tags every 2000 ms. The receiver recorded water temperature, depth, signal strength (dB) from the transmitter, and the boat's geographic position. Range tests were performed before initiating the continuous tracking. The detection range of the receiver was tested relative to different distances and depths of the acoustic trasmitter. The range test revealed that the maximum detection distance was 1260 m at depths of 0.5 and 10.0 m. During each tracking period, the bathymetry was measured with a GPSMAP431s sounder (Garmin International, Olathe, KS), and hourly CTD casts (YSI CastAway 11B111032, YSI, Yellow Springs, OH) were conducted to collect water samples and construct vertical temperature profiles.
Treatment of data
The data recorded every 2000 ms by the receiver were subjected to the process proposed by Ketchum et al. (2014) to eliminate temporal autocorrelation and false data, and time series with 16 min intervals were obtained. Maps were then elaborated in ArcMap 10 (Esri, 2010) for each track. The minimum distance from shore was calculated in ArcView (2010) based on the geographic position of the fish along each track. The data used to statistically assess the horizontal and vertical movements were filtered to obtain 1-min values in order to use as much information as possible.
Thermocline depth was calculated based on the Brunt-Väisälä frequency. The thermocline is located at the depth where stabilization occurs in the vertical displacement frequency of a water mass over a density gradient in the water column (Cushman-Roisin and Beckers 2009).
Models
Generalized additive mixed models (GAMMs) were used to determine the factors that influence the horizontal and vertical movements of C. hippurus. Two models were constructed to assess the movements. In the first model for horizontal movements, the response variable was the minimum distance from shore of the tagged fish and the explanatory variables were bathymetry of the area, water temperature recorded by the accoustic transmitters, time of day (hour), and gender. In the second model for vertical movements, the response variable was depth and the explanatory variables were water temperature, bathymetry, thermocline depth, and gender. All the explanatory variables except for gender were continuous. For both models the random effect corresponds to each individual (Table 1). Considering the nature of the data for the response variables, the Poisson family was used for the statistical analysis (Zuur et al. 2009).
Table 1: Generalized additive mixed models to assess the effects of the explanatory variables on the movements of Coryphaena hippurus: R2, adjusted coefficient of determination; AIC, Akaike information criterion; BIC, Bayesian information criterion. The model that best fits the data is shown in bold.

To determine the model that best fit the data, the methodology used by Papastamatiou et al. (2013) was followed. The models were initially constructed considering the explanatory variables individually. These variables were all then incorporated depending on the contribution of each variable to the variability in the data. The best model was chosen based on standard selection criteria (adjusted coefficient of determination [R2], Akaike information criterion, and Bayesian information criterion). The model assumptions were verified in the residual plots.
To analyze the effect of the explanatory variables on the response variable after fitting the model, main-effects plots were generated. The effect line is interpreted as follows: values >0 on the y-axis have an effect on the response variable regarding the specific values of the explanatory variable. The GAMMs were built using the R 3.0.3 software (R Core Team 2014) and the mgcv package (Wood 2006).
Results
Six individuals of C. hippurus (three males and three females) were tracked for a period of more than 20 h. They ranged in fork length from 67.0 to 83.5 cm and, based on the size at sexual maturity reported by Zúñiga-Flores et al. (2011), they were juveniles. Monitoring of three of them (tracks 2, 5, and 6; Table 2) concluded when the position of the fish did not change and the depth sensor reading coincided with the echosounder seafloor depth reading. The average distance covered per hour was 1.59 km (±0.106, standard error), at a mean speed of 0.51 m s-1 (±0.51) (Table 2). The mean depth of the thermocline, when detected, was 34 m (±0.12).
Table 2: Fork length, age, and sex of six tagged individuals of Coryphaena hippurus and tracking data. SE, standard error.

Horizontal movements
The majority of the horizontal movements occurred in a shallow zone (1-80 m depth) in the southwestern part of LPB (Fig. 1). Two fish tagged in the southeastern part crossed almost the entire bay to the opposite side (tracks 5 and 6). The other four moved in different directions, two of them northwestwards (tracks 2 and 3), one southwards (track 1), and one remained close to where it was captured (track 4).
The model that best fit the data for the horizontal movements was:
DistShore = α + f1 (bathymetry) + f2 (water temperature) + f3 (hour) + gender + (1/track)
where DistShore is the minimum distance from shore, a is the intercept, fn is the smooth function for each explanatory variable, and (1/track) is the random effect of the individuals. All the explanatory variables were significant at P < 0.05. The model explained 45% (R2 = 0.45, Table 2) of the total deviance. Bathymetry and water temperature were the most important variables, followed by hour and gender (Table 1).
The plots showing the effect of the explanatory variables on the minimum distance from shore indicate that there is greater likelihood of the fish moving away from the shore when the bathymetry is deep (40-120 m) and the water temperature is 27-30 °C, and during the night (23:00 and 6:00 h). Females moved further away from the shore than males (Fig. 2).

Figure 2: Generalized additive mixed models for the horizontal movements of Coryphaena hippurus. Plots show the effect of the explanatory variables (bathymetry, water temperature, hour, and gender) on the response variable (distance from shore). In (a-c), black tick marks above the x-axes indicate the distribution of data and the y-axes show the response variable on the scale of the linear predictor; the shaded areas indicate the standard error. In (d), the bars above the x-axis indicate the cumulative data for each gender, the thin horizontal lines indicate the value for the response variable (distance from shore) on the linear predictor scale, and the horizontal dashed lines indicate the standard error.
Vertical movements
The depth range for all the tracks was 1 to 73 m. The tagged fish remained 80% of the time in the mixed layer, that is, above 34 m depth, but 45% of the time was spent in the upper 10 m (Fig. 3a). The fish spent 70% of their time in areas where water temperature was 27-28 °C (Fig. 3b). Differences were observed between the diurnal and nocturnal movement patterns. The individuals remained closer to the surface during daylight hours than during the night, as shown by the fragment of the depth time series for track 4 (Fig. 4), and a greater number of vertical immersions from the surface to depths of more than 70 m occurred during the night.
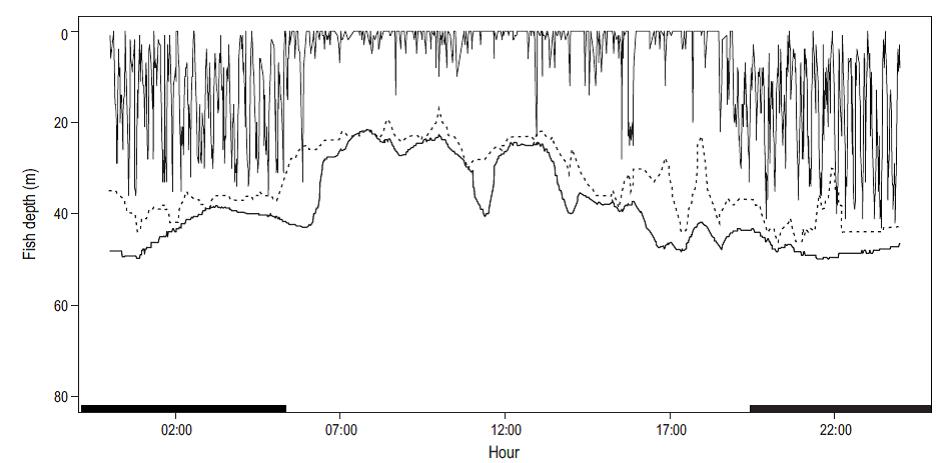
Figure 4: Fragment (24 h) of the time series of vertical movements along track 4: vertical lines, fish depth; bold line, bathymetry; dotted line, thermocline depth. The thick black bars on the x-axis indicate hours of darkness.
The best-fit model for the vertical movements was:
Fish depth = α + f 1 (water temperature) + f 2 (bathymetry) + f3 (hour) + f4 (thermocline depth) + gender + (1/track)
The explanatory variables were significant at P < 0.05. The model explained 64% of the deviance (R 2 = 0.6, Table 1). The most important explanatory variables were water temperature and bathymetry, followed by hour, thermocline depth, and gender.
The individuals are likely to remain at a greater depth when water temperature is less than 28 °C, when bottom depth is greater than 30 m, between 15:00 and 3:00 h, and when the thermocline depth is between 40 and 60 m. Females dove deeper than males (Fig. 5).

Figure 5: Generalized additive mixed models for vertical movements of Coryphaena hippurus. Plots show the effect of the explanatory variables (water temperature, bathymetry, hour, thermocline depth, and gender) on the response variable (fish depth). In (a-d), black tick marks above the x-axes indicate the distribution of data and the y-axes show the response variable on the scale of the linear predictor; the shaded areas indicate the standard error. In (e), the bars above the x-axis indicate the cumulative data for each gender, the thin horizontal lines indicate the value for the response variable (fish depth) on the linear predictor scale, and the horizontal dashed lines indicate the standard error.
Discussion
The horizontal and vertical movements of C. hippurus in LPB were identified in relation to biotic (sex) and abiotic (water temperature recorded by the acoustic transmitters, bathymetry, thermocline depth, and hour) factors. Eight juvenile dolphinfish were tagged, but only six were tracked for a maximum period of 48 h in summer. All the tagged individuals remained inside LPB and most of their horizontal movements occurred in a shallow area (1-80 m) in the southwestern part where water temperature was 27-29 °C. These temperatures coincide with other reported preferential temperatures (Palko et al. 1982, Campos et al. 1993, Massutí and Morales-Nin 1995, Farrell et al. 2014).
Two individuals tagged in the southeastern part of LPB, one male and one female, showed similar movements, both crossing the bay to the opposite side. The other four individuals moved in different directions but did not stray far from their capture site. Merten et al. (2014a, 2014b) reported that movement directions and rates of dolphinfish tagged in the western Atlantic depend on the latitude, longitude, and distance from shore released, but do no elucidate further on this behavior.
In our study, the tagged dolphinfish exhibited vertical movements in the upper 30 m of the water column, above the thermocline (when it was detected). This coincides with the epipelagic nature of these fish and with other reports that mention that they spend most of their time at the surface (Furukawa et al. 2011, Merten et al. 2014c). This habitat preference has been linked to the distribution of their food in the water column (Furukawa et al. 2011, Merten et al. 2014c). Several studies on the feeding habits of this species have reported that epipelagic prey distributed in the surface strata of the water column are an important component of the diet (Aguilar-Palomino et al. 1998, Olson and Galván-Magaña 2002, Tripp-Valdez et al. 2010). Moreover, thermal stratification in the sea can act as a physical barrier that prevents the prey from escaping. Gray and Kingsford (2003) suggested that the most important stratum for trophic interactions among fish larvae and zooplankton is the upper 30 m of the water column, regardless of the position of the thermo-cline. In the present study, the tagged dolphinfish spent most of their time above 30 m depth.
Furukawa et al. (2011, 2014) suggested that dolphinfish spend most of their time in shallow waters to avoid rapid changes in temperature and thus minimize energy use by remaining in isothermal conditions above the thermocline. Our findings indicate that the dolphinfish occasionally crossed the thermocline (when it was detected), a behavior also reported by Furukawa et al. (2011) and Merten et al. (2014c).
The tagged dolphinfish undertook deep vertical immersions at night most likely to feed. Merten et al. (2014c) reported that the vertical immersions of adult male dolphin-fish are related to the search and capture of epipelagic and mesopelagic prey, but they stress that their data may not be representative of the vertical movement strategy of females or juveniles. In the present study, however, juvenile males showed this behavior and juvenile females dove deeper than juvenile males. Given the limited information obtained in our study and that there are no feeding behavior data for C. hippurus in LPB, further research is needed to corroborate this relationship between vertical immersions and feeding behavior.
One limitation of this study was the number of individuals tagged. We recommend tagging a greater number of fish in future studies in order to be able to generate a database covering the annual cycle of C. hippurus and in particular the sport fishing season. Future research in LPB should contemplate studying the feeding habits of this species.
In summary, the majority of the movements of juvenile dolphinfish in LPB occurred in shallow near-shore areas, mainly between the surface and 30 m depth. While only a few individuals were tracked over a short period of time, we consider that this information could be taken into account to regulate dolphinfish catches in shallow areas of LPB in order to allow juveniles to reach sexual maturity and thus ensure that fishing activities are conducted in a sustainable manner in the region.
Acknowledgments
This study was supported by the National Council for Science and Technology (CONACYT, Mexico, project 133541). CHT is a recipient of a fellowship (CONACYT, no. 487113). We thank Mauricio Hoyos for lending the acoustic telemetry equipment; Armando Trasviña-Castro for lending the Cast-Away CTD; César Augusto Salinas-Zavala for lending the Ruskin XR-420 CTD; Jesús Bautista-Romero and Salvador Lluch-Cota for lending the GPSMAP 431s sounder; Guillermo García-Cortés, Jorge Cobos-Anaya, and Carlos Soto-Carrasco for building the hydrophone platform and developing other tagging and tracking material; Ira Fogel for editing services; and the captains Jorge Ángulo-Calvillo, Enrique Calvillo-Espinoza, Mario Cota-Castro, and Oswaldo Rodríguez-García and all the other people who helped to track the fish. The input of Raúl Martínez-Rincón (data analysis and R software use) and Patricia González-Zamorano (map depelopment and data analysis) is gratefully acknowledged. Finally, we thank the anonymous reviewers for their valuable comments that helped to improve this article.
REFERENCES
Aguilar-Palomino B, Galván-Magaña F, Abitia-Cárdenas LA, Muhlia-Melo AF, Rodríguez-Romero J. 1998. Feeding aspects of the dolphin Coryphaena hippurus Linnaeus, 1758 in Cabo San Lucas, Baja California Sur, Mexico. Cienc. Mar. 24(3): 253-265. [ Links ]
Alejo-Plata C, Díaz-Jaimes P, Salgado-Ugarte IH. 2011. Sex ratios, size at sexual maturity, and spawning seasonality of dolphinfish (Coryphaena hippurus) captured in the Gulf of Tehuantepec, Mexico. Fish. Res. 110: 207-216. http://dx.doi.org/10.1016/j.fishres.2011.04.008 [ Links ]
Brill RW, Lutcavage ME. 2001. Understanding environmental influences on movements and depth distributions of tunas and billfishes can significantly improve population assessments. Am. Fish. Soc. Symp. 25: 179-198. [ Links ]
Campos JA, Segura A, Lizano O, Madrigal E. 1993. Ecología básica de Coryphaena hippurus (Pisces: Coryphaenidae) y abundancia de otros grandes pelágicos en el Pacífico de Costa Rica. Rev. Biol. Trop. 41: 783-790. [ Links ]
Cushman-Roisin B, Beckers JM. 2009. Introduction to Geophysical Fluid Dynamics. Physical and Numerical Aspects. Under contract with Academic Press, 773 pp. [ Links ]
[DOF] Diario Oficial de la Federación. 1995. Norma Oficial Mexicana NOM-017-PESC-1994 para regular las actividades de pesca deportiva en las Aguas de Jurisdicción Federal de los Estados Unidos Mexicanos. Tomo No. 15-19. México, DF. [ Links ]
Farrell ER, Boustany AM, Halpin PN, Hammond DL. 2014. Dolphinfish (Coryphaena hippurus) distribution in relation to biophysical ocean conditions in the northwest Atlantic. Fish. Res. 151: 177-190. http://dx.doi.org/10.1016/j.fishres.2013.11.014 [ Links ]
Furukawa S, Kawabe R, Ohshimo S, Fujioka K, Nishihara GN, Tsuda Y, Aoshima T, Kanehara H, Nakata H. 2011. Vertical movement of dolphinfish Coryphaena hippurus as recorded by acceleration data-loggers in the northern East China Sea. Environ. Biol. Fish. 92: 89-99. http://dx.doi.org/10.1007/s10641-011-9818-y [ Links ]
Furukawa S, Tsuda Y, Nishihara GN, Fujioka K, Ohshimo S, Tomoe S, Nakatsuka N, Kimura H, Aoshima T, Kanehara H, Kitagawa T, Chiang W-C, Nakata H, Kawabe R. 2014. Vertical movements of Pacific bluefin tuna (Thunnus orientalis) and dolphinfish (Coryphaena hippurus) relative to the thermocline in the northern East China Sea. Fish. Res. 149: 86-91. http://dx.doi.org/10.1016/j.fishres.2013.09.004 [ Links ]
Girard C, Dagorn L, Taquet M, Aumeeruddy R, Peignon C, Benhamou S. 2007. Homing abilities of dolphinfish (Coryphaena hippurus) displaced from fish aggregating devices (FADs) determined using ultrasonic telemetry. Aquat. Living Resour. 20: 313-321. http://dx.doi.org/10.1051/alr:2008005 [ Links ]
Gray CA, Kingsford MJ. 2003. Variability in thermocline depth and strength, and relationships with vertical distributions of fish larvae and mesozooplankton in dynamic coastal waters. Mar. Ecol. Prog. Ser. 247: 211-224. [ Links ]
Ketchum JT, Hearn A, Klimley AP, Espinoza E, Peñaherrera C, Largier JL. 2014. Seasonal changes in movements and habitat preferences of the scalloped hammerhead shark (Sphyrna lewini) while refuging near an oceanic island. Mar. Biol. 161: 755-767. http://dx.doi.org/10.1007/s00227-013-2375-5 [ Links ]
Madrid JV, Beltrán-Pimienta R. 2001. Length, weight and sex of the dolphin fish Coryphaena hippurus (Perciformes: Coryphaenidae) of the littoral of Sinaloa, Nayarit and Baja California Sur, Mexico. Rev. Biol. Trop. 49: 931-938. [ Links ]
Martínez-Rincón RO, Ortega-García S, Vaca-Rodríguez JG. 2009. Incidental catch of dolphinfish (Coryphaena spp.) reported by the Mexican tuna purse seiners in the eastern Pacific Ocean. Fish. Res. 96: 296-302. http://dx.doi.org/10.1016/j.fishres.2008.12.008 [ Links ]
Massutí E, Morales-Nin B. 1995. Seasonality and reproduction of dolphinfish (Coryphaena hippurus) in the western Mediterranean. Sci. Mar. 59:357-364. [ Links ]
Merten W, Appeldoorn R, Hammond D. 2014a. Movements of dolphinfish (Coryphaena hippurus) along the U.S. east coast as determined through mark and recapture data. Fish. Res. 151: 114-121. http://dx.doi.org/10.1016/j.fishres.2013.10.021 [ Links ]
Merten W, Appeldoorn R, Hammond D. 2014b. Spatial differentiation of dolphinfish (Coryphaena hippurus) movements relative to the Bahamian archipelago. Bull. Mar. Sci. 90: 849-864. http://dx.doi.org/10.5343/bms.2013.1078 [ Links ]
Merten W, Appeldoorn R, Rivera R, Hammond D. 2014c. Diel vertical movements of adult male dolphinfish (Coryphaena hippurus) in the western central Atlantic as determined by use of pop-up satellite archival transmitters. Mar. Biol. 161: 1823-1834. http://dx.doi.org/10.1007/s00227-014-2464-0 [ Links ]
Nathan R, Getz WM, Revilla E, Holyoak M, Kadmon R, Saltz D, Smouse PE. 2008. A movement ecology paradigm for unifying organismal movement research. Proc. Natl. Acad. Sci. USA 105: 19052-19059. http://dx.doi.org/10.1073/pnas.0800375105 [ Links ]
Olson RJ, Galván-Magaña F. 2002. Food habits and consumption rates of common dolphinfish (Coryphaena hippurus) in the Eastern Pacific Ocean. Fish. Bull. 279-298. [ Links ]
Palko BJ, Beardsley GL, Richards WJ. 1982. Synopsis of the biological data on dolphinfishes, Coryphaena hippurus and Coryphaena equiselis Linnaeus. FAO Fish Synop. 130: 1-28. [ Links ]
Papastamatiou YP, Meyer CG, Carvalho F, Dale JJ, Hutchinson MR, Holland KN. 2013. Telemetry and random-walk models reveal complex patterns of partial migration in a large marine predator. Bull. Ecol. Soc. Am. 94: 2595-2606. [ Links ]
R Core Team. 2014. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria, http://www.R-project.org/ [accessed May 2013]. [ Links ]
Schaefer KM, Fuller DW, Block BA. 2011. Movements, behavior, and habitat utilization of yellowfin tuna (Thunnus albacores) in the Pacific Ocean off Baja California, Mexico, determined from archival tag data analyses, including unscented Kalman filtering. Fish. Res. 112: 22-37. http://dx.doi.org/10.1016/j.fishres.2011.08.006 [ Links ]
Taquet M, Dagorn L, Gaertner JC, Girard C, Aumerruddy R, Sancho G, Itano D. 2007. Behavior of dolphinfish (Coryphaena hippurus) around drifting FADs as observed from automated acoustic receivers. Aquat. Living Resour. 20: 323-330. http://dx.doi.org/10.1051/alr:2008008 [ Links ]
Tripp-Valdez A, Galván-Magaña F, Ortega-García S. 2010. Feeding habits of dolphinfish (Coryphaena hippurus) in the southeastern Gulf of California. Mexico. J. Appl. Ichthyol. 26: 578-582. http://dx.doi.org/10.1111/j.1439-0426.2010.01483.x [ Links ]
Varghese SP, Somvanshi VS, John ME, Dalvi RS. 2013. Diet and consumption rates of common dolphinfish, Coryphaena hippurus, in the eastern Arabian Sea. J. Appl. Ichthyol. 29: 1022-1029. http://dx.doi.org/10.1111/jai.12166 [ Links ]
Wood SN. 2006. Generalized Additive Models: An Introduction with R. CRC Texts in Statistical Science, 384 pp. [ Links ]
Zúñiga-Flores MS, Ortega-García S, Rodríguez-Jaramillo MC, López-Martínez J. 2011. Reproductive dynamics of the common dolphinfish Coryphaena hippurus in the southern Gulf of California. Mar. Biol. Res. 7: 7677-7689. http://dx.doi.org/10.1080/17451000.2011.554558 [ Links ]
Zuur AF, Ieno EN, Walker NJ, Saveliev AA, Smith GM. 2009. Mixed Effects Models and Extensions in Ecology with R. Springer Science+Business Media, New York, 574 pp. [ Links ]
Received: July 2015; Accepted: October 2015











 texto em
texto em 



