Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Ciencias marinas
versão impressa ISSN 0185-3880
Cienc. mar vol.41 no.4 Ensenada Dez. 2015
https://doi.org/10.7773/cm.v41i4.2521
Articles
Potential use of two subtropical mangrove species (Laguncularia racemosa and Rhizophora mangle) for nutrient removal in closed recirculating systems
1 Centro Interdisciplinario de Investigación para el Desarrollo Integral Regional-Instituto Politécnico Nacional (CIIDIR-IPN), Blvd. Juan de Dios Bátiz Paredes 250, Guasave, Sinaloa, México.
2 Instituto de Ciencias del Mar y Limnología, Unidad Académica Mazatlán, Universidad Nacional Autónoma de México, Av. Joel Montes Camarena s/n, Mazatlán, Sinaloa 82040, México.
3 Instituto de Ciencias del Mar y Limnología, Universidad Nacional Autónoma de México, Apartado postal 70305, Av. Universidad 3000, Ciudad Universitaria, Coyoacán, DF 04510, México.
Six silvofishery systems were constructed to estimate the potential use of mangrove seedlings for nutrient removal. Two systems did not contain seedlings (i.e., control treatment), while the remaining systems were divided into separate treatments using two mangrove species (Laguncularia racemosa and Rhizophora mangle). Each system consisted of two water tanks linked by two hoses. The first tank contained 150 poeciliid fishes, while the second contained a biological filter of gravel and sand on the bottom, as well as a hydroponic arrangement of 34 seedlings. Water exchange between both tanks was performed over a 24-h period, every ten days for seven months, and the concentration of nutrients (NH4 +, NO2 -, NO3 -, and PO4 -3) was measured every 8 h. Laguncularia racemosa showed a higher growth rate compared to R. mangle, but there were no differences regarding the growth of fishes among the six systems. Final dissolved inorganic nitrogen removal was 42% in the control treatment and 90% in both treatments using mangroves. Dissolved inorganic phosphorus removal was 45% in the control treatment, 44% in the L. racemosa treatment, and 35% in the R. mangle treatment. Our results indicate that both mangrove species are capable of removing a considerable amount of nitrogen, but phosphorus removal was unsatisfactory.
Key words: poeciliid; Mexico; biological filter
Seis sistemas de cultivo fueron construidos para evaluar el uso potencial de plántulas de mangle en la remoción de nutrientes. Dos de los sistemas no contenían plántulas (i.e., control), mientras que los sistemas restantes fueron divididos en tratamientos por separado con dos especies de mangle (Laguncularia racemosa y Rhizophora mangle). Cada sistema consistió en dos tanques de almacenamiento unidos por dos tubos. El primer tanque contenía 150 peces poecílidos, mientras que el segundo tanque contenía 34 plántulas en forma hidropónica y un filtro biológico de grava y arena en el fondo. Se hicieron recirculaciones de agua entre ambos tanques cada diez días por un periodo de siete meses. Durante cada recirculación, se midió la concentración de nutrientes (NH4 +, NO2 -, NO3 - y PO4 -3) cada 8 h. Laguncularia racemosa presentó una tasa de crecimiento mayor comparada con R. mangle; sin embargo, el crecimiento de los peces fue igual entre los seis sistemas. El porcentaje final de nitrógeno inorgánico disuelto fue 42% en el tratamiento de control y 90% en los tratamientos con L. racemosa y R. mangle. El porcentaje de remoción de fósforo inorgánico disuelto fue 45% en el control, 44% en el tratamiento con L. racemosa y 35% en el tratamiento con R. mangle. Los resultados indican que ambas especies de mangle son capaces de remover una cantidad considerable de nitrógeno, pero la remoción de fósforo fue insatisfactoria.
Palabras clave: poecílidos; México; filtro biológico
Introduction
Globally, aquaculture-related activities have grown at an annual rate of 8.8% over the past 30 years as a result of the increasing demand for aquaculture products (Jensen et al. 2014) and improved production lines (Asche 2008). The annual average consumption of aquatic organisms increased from 16 kg per person in 2000 to 18.6 kg per person in 2010 for aquatic organisms is expected to be met by intensive farming systems, and by 2022, aquaculture is expected to provide an additional 22 million tons of fish (FAO 2012).
Culture systems produce several chemical compounds that can be harmful to the cultured organism and the environment. If these compounds are not eliminated, persistent nutrient accumulation occurs (i.e., eutrophication), particularly in semi-closed or closed recirculating systems with little or no water exchange (Bouwman et al. 2013). Cultured organisms convert about 10-35% of nitrogen and phosphorus into biomass and the rest is released as organic and inorganic nutrients directly into the aquaculture pond (Shimoda et al. 2007, Herbeck et al. 2014). Consequently, aquaculture effluents contain a considerable amount of nitrogen and phosphorus compounds (Schneider et al. 2005, Buhmann and Papenbrock 2013, Barraza-Guardado et al. 2014, Herbeck et al. 2014). Among these compounds, ammonium (NH4 +) and nitrite (NO2 -) are toxic to cultured organisms, and nitrate (NO3 -) is toxic only at high concentrations (Camargo et al. 2005). If the waters of these culture systems are discharged without prior treatment, ammonium, nitrite, nitrate, and phosphate (PO4 -3) can cause eutrophication of the receiving water bodies (Herbeck et al. 2014). Nitrate and phosphate are considered to be the limiting nutrients in freshwater and marine environments (Sundareshwar et al. 2003), and high levels of these nutrients can thus cause massive algal blooms that can affect the biochemical aspects and cycles of coastal environments.
As a result of the environmental problems caused by high nutrient concentrations in aquaculture farms and the consequent restrictions implemented by federal agencies, engineers and ecologists have developed several types of treatments for the optimum use of effluents from culture systems. Some of the most widely used methods are reverse osmosis (Schoeman and Steyn 2003), ion exchange (Kim and Benjamin 2004), electrodialysis (Menkouchi Sahli et al. 2006), activated carbon (Sison et al. 1995), chemical denitri-fication (Hu et al. 2002), and treatments using microorganisms (Zaitsev et al. 2008). While these are efficient methods for removing nutrients from polluted waters, the operational cost is considerable and developing relatively inexpensive methods to counteract eutrophication is a practical necessity (Páez-Osuna et al. 2003, Huang et al. 2012). Natural and constructed wetlands have been proposed as alternative, cost-effective wastewater-treatment systems (Lin et al. 2002, 2003). Aquatic macrophytes in wetlands play an important role in nutrient removal. The processes involved in the transformation of nitrogen compounds in wetland soil are ammonification followed by nitrification and denitrification (Maltais-Landry et al. 2009, Buhmann and Papenbrock 2013). In wetland sediments, 80-90% of the phosphorus occurs in organic form, some is incorporated by the plants, and a small part occurs in the form of orthophosphate (Buhmann and Papenbrock 2013).
Mangrove wetlands are one of the most productive coastal ecosystems in tropical and subtropical regions (Kristensen et al. 2008). They are considered a tolerant group of plants for aquaculture effluents because of their huge demand for nutrients (Yang et al. 2008, Huang et al. 2012, Bao et al. 2013). Moreover, mangroves play a critical role in the removal and degradation of pollutants such as heavy metals, pesticides, and nitrogen and phosphate compounds (Kristensen et al. 2008, Reef et al. 2010, Adame and Lovelock 2011, Bayen 2012). Hence, the use of mangrove wetlands is considered a relatively simple, low-cost alternative for the treatment of aquaculture effluents (Ye et al. 2001, Huang et al. 2012, De-León-Herrera et al. 2015).
In recent decades, mangrove wetlands have been used as a viable option for removing nitrogen and phosphate compounds. For example, Wong et al. (1997) studied the rate of organic nitrogen and carbon removal at two intertidal sites dominated by Kandelia candel and Aegiceras corniculatum, and their results suggest that these two mangrove species have great potential for the removal of nutrients. Tam and Wong (1995) measured the rate of nutrient and heavy metal retention in two types of mangrove soil and found that mangroves act as traps for phosphorus and heavy metals but were less efficient at retaining nitrogen. Wu et al. (2008) studied the potential use of K. candel as a secondary treatment of municipal wastewater and their findings indicate that it is feasible to use this species to remove dissolved organic carbon, ammonium, and orthophosphates. In a pilot-scale constructed wetland using K. candel, A. corniculatum, and Sonneratia caseolaris for the removal of organic matter and nutrients, Yang et al. (2008) found that the treatment efficiency of S. caseolaris and A. corniculatum was higher than that of K. candel. In an experiment in which Sonneratia apetala was irrigated with three concentration levels of wastewater, Zhang et al. (2010) found that this species has great potential for the removal of nutrients and heavy metals. Moroyoqui-Rojo et al. (2012) studied the nutrient removal capacity of Laguncularia racemosa and Rhizophora mangle in shrimp culture ponds and found that both species presented similar nutrient removal rates. De-León-Herrera et al. (2015) measured the removal of inorganic nutrients derived from a population of Dormitator latifrons in a closed system containing Avicennia germinans, L. racemosa, and R. mangle, and reported that all three mangrove species can be used to remove nitrogen and phosphorus compounds.
Most studies have focused on wastewater treatment using K. candel that grows at tropical latitudes. In the case of the three mangrove species (A. germinans, L. racemosa, and R. mangle) typical of the American continent (subtropical latitudes), Moroyoqui-Rojo et al. (2012) and De-León-Herrera et al. (2015) examined nutrient removal in closed tanks without a biological filter (e.g., gravel and sand). The objective of the present study was to study the removal of fish-derived inorganic nutrients (ammonium, nitrite, nitrate, and phosphate) in tanks with periodic recirculation containing a biological filter (gravel and sand) as well as L. racemosa and R. mangle seedlings.
Materials and methods
Collection and monitoring of mangrove propagules and fruits
Red mangrove (R. mangle) propagules and white mangrove (L. racemosa) fruits were collected in August from Urías Lagoon, northwestern Mexico (23°13'-23°11'N, 106°23'-106°21'W). All the samples were transported in plastic boxes to the Coastal Ecosystem Laboratory of the Institute of Marine Sciences and Limnology (UNAM) at Mazatlán (Mexico). The red mangrove propagules were placed in plastic buckets containing freshwater for one month for the optimum development of the root system. The white mangrove fruits were sown in polyurethane trays (68 × 34 cm) using a mixture of dolomite and vermiculite as substrate (Sunshine Mix #3). After one month (i.e., September), the seedlings of both species were transported to a tank in which salinity was raised by 10 (practical salinity scale) every month until reaching a concentration of 35-40 in December. In this tank, all seedlings were arranged in a hydroponic wooden frame. In January, all the seedlings were transported to the experimental tanks. The height of both species was measured monthly until August (i.e., seven months). All seedlings were measured with a 1-m-long flexible ruler. The red mangrove seedlings were measured from the first growth ring to the apical part of the main shoot. The white mangrove seedlings were measured from the base of the shoot to the apical part of the main shoot.
Collection of poeciliid fishes
Poeciliid fishes (Poeciliidae) were collected in December from the mangrove area within Urías Lagoon with a spoon net (0.5 cm mesh size). The individuals were transported in plastic containers (100 × 35 cm) and placed in a 450-L tank with salinity of 35. They were offered a balanced commercial feed once a day at a rate of 2.56 g per day. In January, the individuals were placed in the experimental tanks and the length of all specimens was measured monthly until the end of the experiment (i.e., seven months) using a standardized biometric ruler for fishes.
Design and construction of the experimental systems
Six recirculating water systems were constructed (Fig. 1). Each system consisted of two 450-L polyvinyl tanks (Polyplas). One tank contained 150 poeciliid fishes and the other tank contained a biological filter consisting of a 14-cm layer of gravel (θ = 5 mm) and a 14-cm layer of sand. Two of the six tanks with the biological filter did not contain mangrove seedlings (i.e., control treatment). The other four contained an arrangement of 34 seedlings on a floating 1-cm-thick polyurethane sheet. Two of them contained red mangrove seedlings and two contained white mangrove seedlings. Salinity in the tanks was kept at 35.
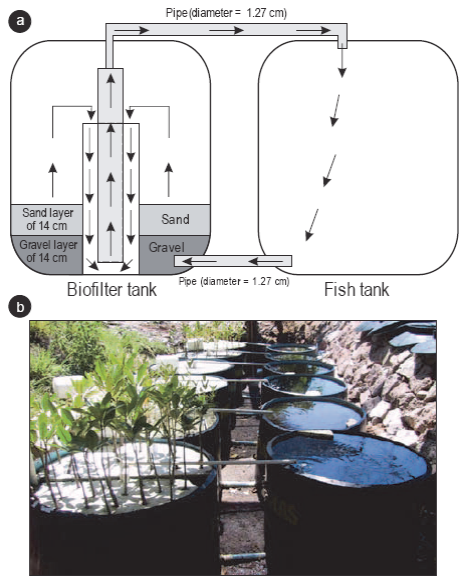
Figure 1: Diagram (a) and photograph (b) of the water recirculating systems. The six tanks on the right-hand side held poeciliid fishes. The six tanks on the left contained a biological filter and either Laguncularia racemosa or Rhizophora mangle seedlings or no mangrove seedlings (i.e., control treatment). The black arrows indicate the direction of water circulation.
The two tanks of each system were connected by two plastic pipes (1.27 cm in diameter), one at the top and one at the bottom, through which the water exchange occurred (Fig. 1). Three Elite-802 air pumps (115 V) were used to circulate the water between the tanks. There were two air ducts in the tank containing the biological filter. Each duct supplied air to a recirculating system via a hose (0.64 cm in diameter) that reached the middle section of the system. The water flowed from the bottom of the fish tank into the bottom of the seedling tank, where it circulated vertically through the sand and gravel biofilter to the middle section of the tank. A 10.16-cm-diameter PVC tube was installed in the center of the biofilter, which in turn contained another PVC tube (1.91 cm in diameter) that was connected to a horizontal tube (1.27 cm in diameter) through which the water returned to the fish tank.
Water-quality sampling and nutrient measurements
Samples to analyze water quality in the tanks were taken from January to August. The fish were fasted for 24 h prior to taking the water samples so that the food would not influence the nutrient measurements. The water exchanges between tanks were performed every 10 days over a 24-h period, during which four samples were taken. The first sample was taken before initiating the water exchange (0 h), the second sample was taken after 8 h (first recirculation), the third sample after 16 h (second recirculation), and the fourth sample after 24 h (third recirculation). The monthly values were then grouped by seasons: winter (January to March), spring (April to June), and summer (July to August). Duplicate water samples (i.e., pseudo-replicates) were taken in 125-mL plastic bottles from each tank containing the biofil-ter. All samples were analyzed for ammonium, nitrite, nitrate, and phosphate content using the spectrophotometric techniques described by Strickland and Parsons (1972). Water temperature and salinity were measured weekly with a thermometer (Brannan) and a refractometer (Atago #80-124), respectively. The efficiency of nutrient removal in the experimental system was calculated using the equation described by Paniagua-Michel and García (2003):
R = ((E-S)/E)100
where R is the percent nutrient removal, E is the mean concentration of nutrients at the beginning of the recirculation period (i.e., 0 h), and S is the mean concentration of nutrients at the end of the period (i.e., 24 h).
Statistical analysis
The growth of mangrove seedlings was analyzed by linear regressions. The linear association between seedling height and time was examined using the coefficient of determination (R 2) and analysis of variance. The Kruskal-Wallis nonparametric test was used to analyze differences in seedling growth as well as differences in temperature, salinity, and nutrient content between the control and mangrove treatments. The growth of the poeciliid fishes in the six tanks was analyzed by logarithmic regressions.
Results
Growth of mangrove seedlings and poeciliid fishes
There was a significant difference (P < 0.05) in seedling growth between treatments (Fig. 2a), with L. racemosa showing a greater slope (1.85 cm/month) than R. mangle (0.85 cm/month). There were no significant differences (P > 0.05) in fish length between treatments (Fig. 2b).
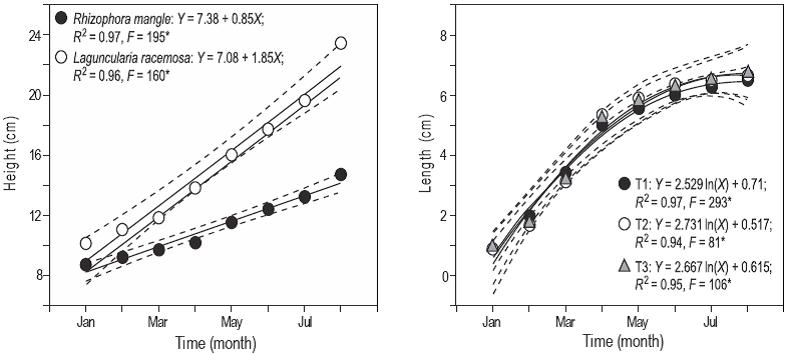
Figure 2: Mangrove seedling height (a) and poeciliid fish growth (b) from January to August. T1 represents the Rhizophora mangle treatment, T2 the Laguncularia racemosa treatment, and T3 the control treatment. Each plot shows the linear or logarithmic equation, the coefficient of determination (R 2), and the F-test value. Asterisks indicate significant F values at a = 0.05. Sixty-eight seedlings and 300 fish were measured monthly per treatment.
Physical and chemical properties of water and nutrient content
Water temperature in the experimental tanks was 24.5 °C in January, February, and March, and ranged from 35 to 37 °C between April and August (Fig. 3a). There were no significant differences (P > 0.05) in water temperature between treatments. Salinity was higher in January, July, and August (~35), and lowest in March (~27), but no significant differences (P > 0.05) were observed between treatments (Fig. 3b).
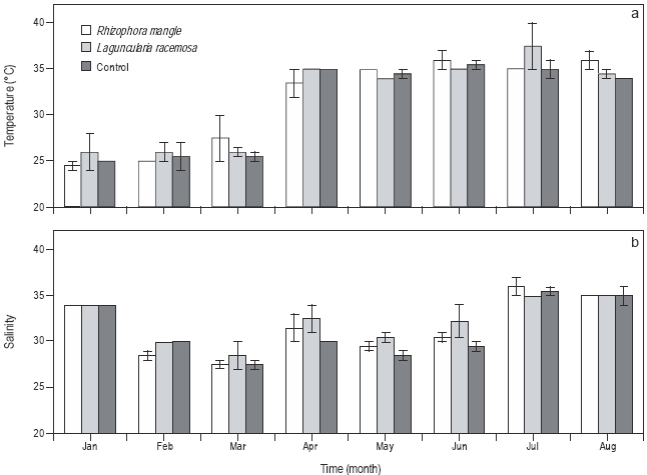
Figure 3: Time series of water temperature and salinity in the control, Laguncularia racemosa, and Rhizophora mangle treatments. Error bars represent one standard deviation. Eight temperature and salinity measurements were taken monthly per treatment.
There were significant differences in the percent removal and temporal variability (January to August) of dissolved inorganic nitrogen (NH4 +, NO2 -, and NO3 -) in the three treatments during the 24-h recirculation period (Fig. 4). During the first three months (i.e., winter), dissolved inorganic nitrogen removal in the control treatment was very low (32 ± 10%) relative to the treatments using L. racemosa (78 ± 10%) and R. mangle (75 ± 7%) (Fig. 4a). In spring, it decreased in the control treatment (29 ± 8%) as well as in the R. mangle treatment (70 ± 15%), but increased in the L. racemosa treatment (82 ± 10%) (Fig. 4b). During the last two months of the experiment (i.e., summer), it increased to 42 ± 8% in the control treatment, but increased considerably in the L. racemosa and R. mangle treatments to 90 ± 13% and 90 ± 11%, respectively (Fig. 4c).
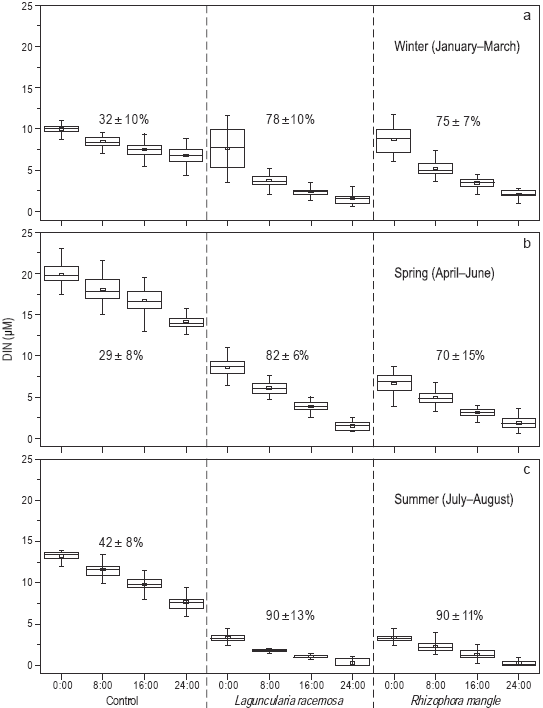
Figure 4: Percent removal (mean and standard deviation) and temporal variability in winter (a), spring (b), and summer (c) of dissolved inorganic nitrogen (DIN: NH4 +, NO2 -, and NO3 -) in the three treatments during the 24-h water recirculation periods. Each box diagram depicts the mean (small box), minimum sample, lower quartile (lower part of the box), median (middle part of the box), upper quartile (upper part of the box), and maximum sample. The total number of monthly samples per nutrient was 24.
Significant differences were observed in the percent removal of dissolved inorganic phosphorus (PO4 -3). In winter it was lower (48 ± 12%) in the control treatment than in the L. racemosa (62 ± 18%) and R. mangle (55 ± 21%) treatments (Fig. 5a). In spring it decreased slightly in all three treatments, to 45 ± 16% in the control treatment, 41 ± 24% in the L. racemosa treatment, and 53 ± 25% in the R. mangle treatment (Fig. 5b). In summer it remained similar in the control treatment (45 ± 14%), but increased to 44 ± 37% in the L. racemosa treatment and decreased to 35 ± 39% in the R. mangle treatment (Fig. 5c).
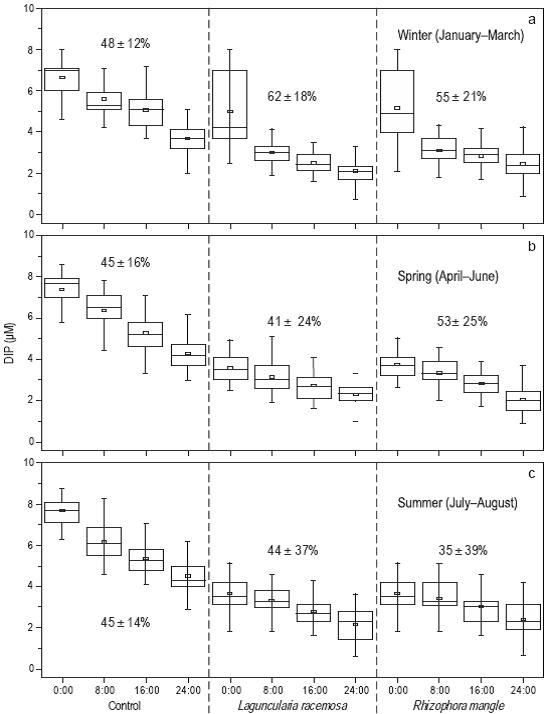
Figure 5: Percent removal (mean and standard deviation) and temporal variability in winter (a), spring (b), and summer (c) of dissolved inorganic phosphorus (DIP: PO4 -3) in the three treatments during the 24-h water recirculation periods. Each box diagram depicts the mean (small box), minimum sample, lower quartile (lower part of the box), median (middle part of the box), upper quartile (upper part of the box), and maximum sample. The total number of monthly samples was 24.
Discussion
In view of the constant expansion of aquaculture activities, it is necessary to understand to what extent mangrove wetlands can be used as biofilters to reduce the eutrophic conditions in culture ponds. In this study we examined the removal of nutrients (NH4 +, NO2 -, NO3 -, and PO4 -3) in closed recirculating systems by two mangrove species typical of semi-arid areas of Mexico.
The linear growth of the mangrove seedlings indicated the removal of dissolved inorganic nutrients (nitrogen and phosphorus) in the experimental tanks. Nitrogen removal rates increase according to plant biomass and coverage (Tanner et al. 1995, Yang et al. 2008, De-León-Herrera et al. 2015). The nitrogen cycle in wetlands is complex and is discussed in detail by Reed et al. (1995). In our experiment, largest nitrogen loss occurred because of denitrification. Denitrification is a form of anaerobic respiration by certain bacteria, whereby nitrate (or nitrite) is used as the terminal electron acceptor for the oxidation of organic compounds and is reduced to gaseous products such as N2O and N2 (Gersberg et al. 1983). However, the formation of intermediate products during nitrification and denitrification is not fully understood (Tam et al. 2009). In general, nitrates can be removed through denitrification and plant uptake. Mangrove seedlings not only absorb nitrate for their growth, but also improve the efficiency of the nitrification and denitrification processes (Wu et al. 2008). Our results indicate that the growth of the L. racemosa and R. mangle seedlings was associated with the absorption of nitrogen and phosphorus from the metabolic residues of the poeciliid fishes and the excess food.
Mangrove growth rates depend on the nutrients in the system (Saenger 2002); however, L. racemosa seedlings present a more rapid increase in height relative to R. mangle under a variety of experimental conditions (e.g., Cardona-Olarte et al. 2006, Krauss et al. 2006, Moroyoqui-Rojo et al. 2012, Monroy-Torres et al. 2014, De-León-Herrera et al. 2015). In the present study, the mangrove seedlings showed this same growth pattern, but our results indicate that there were no differences in nutrient removal between both species. This suggests that L. racemosa and R. mangle have different metabolic adaptations depending on the concentration of nutrients. We suggest that the difference in height increase but similar nutrient uptake of both species is attributable to their typical morphological patterns. For example, the nutrients assimilated by R. mangle seedlings can be used to develop new leaves, which are larger than those of L. racemosa (Flores-de-Santiago et al. 2012). Also, the stem density of R. mangle seedlings is greater than that of L. racemosa (Flores-Verdugo et al. 1990). One advantage of this difference in growth is that R. mangle could be used in bioremediation processes that are restricted by the physical dimensions of the culture systems such as in our experiment, whereas in large culture areas (e.g., farms) L. racemosa could be used because of its rapid growth.
Another problem that can limit the removal of nitrogen compounds is oxygen insufficiency. Oxygen is needed to accelerate the mineralization process and is essential for the nitrification process (Bowmer 1987). Unlike closed systems with no oxygenation (e.g., De-León-Herrera et al. 2015), our experimental design had two air pumps and a gravel and sand filter. We believe that 90% nitrogen removal was obtained as a result of the increase in oxygen level and presence of the filter. Microalgae were observed in all the culture systems during the experiment. These microalgae, typical of subtropical climates, can affect the nutrient removal measurements because they require inorganic nutrients in order to grow. Despite the presence of microalgae in all the systems, the difference in nutrient removal between the control and mangrove treatments is clear and we do not believe that they greatly affected the results obtained in this study. Moreover, the variability in temperature and salinity was not a limiting factor for nutrient removal and fish growth since there were no differences among the three treatments.
The uptake of phosphate compounds by wetlands involves several pathways and is considered a complex biogeochemical cycle. The main removal mechanisms include the sedimentation of phosphorus-containing particles, the adsorption of soluble phosphorus onto clay particles, and phosphate assimilation by aquatic macrophytes (Greenway and Woolley 1999). Even though the concentrations of phosphate are lower than those of nitrogen compounds in aquacul-ture ponds, it is considered a limiting nutrient in mangrove forests (Ye et al. 2001), and uptake by mangrove plants could be a major phosphate removal mechanism (Greenway and Woolley 1999).
While several studies have demonstrated the use of mangroves to reduce nutrients in coastal environments (e.g., Wong et al. 1997, Chu et al. 1998, Boonsong et al. 2003), few have compared different mangrove species (Ye et al. 2001, Yang et al. 2008), particularly in semi-arid areas. Nutrient removal by L. racemosa and R. mangle in semi-arid environments may be lower than that of tropical species such as A. corniculatum, K. candel, Bruguiera gymnorrhiza, and Rhizophora stylosa (De-León-Herrera et al. 2015); however, in our experiment using a gravel and sand filter in the experimental tanks, nitrogen removal by L. racemosa and R. mangle seedlings was 90%, similar to that of tropical species such as K. candel (92.7%) and B. gymnorrhiza (98%) (Ye et al. 2001, Huang et al. 2012). On the other hand, phosphorus removal by L. racemosa and R. mangle was unsatisfactory (35-44%). Therefore, understanding the processes related to mangroves as exporters or importers of phosphate depends on the location and environmental factors that affect the biological responses.
The differences observed in the removal of dissolved nitrogen by L. racemosa and R. mangle seedlings indicate that these semi-arid species are capable of removing a considerable amount of inorganic nitrogen in tanks with water recirculation. It is, however, necessary to understand the nutrient transformation processes in the mangrove ecosystems to optimize the operational parameters, including the physical and biological design of the treatment systems.
Acknowledgments
The first author received financial support from the Instituto Politécnico Nacional (Mexico). Funding for the field work was provided by the Instituto de Ciencias del Mar y Limnología, Universidad Nacional Autónoma de México (UNAM). The fourth author acknowledges financial support from UNAM (Dirección General de Asuntos del Personal Académico). We thank MN Herrera-Moreno for assistance during the laboratory analysis.
REFERENCES
Adame MF, Lovelock CE. 2011. Carbon and nutrient exchange of mangrove forests with the coastal ocean. Hydrobiologia 663(1): 23-50. http://dx.doi.org/10.1007/s10750-010-0554-7 [ Links ]
Asche F. 2008. Farming the sea. Mar. Resour. Econ. 23(4): 527-547. [ Links ]
Bao H, Wu Y, Unger D, Du J, Herbeck LS, Zhang J. 2013. Impact of the conversion of mangroves into aquaculture ponds on the sedimentary organic matter composition in a tidal flat estuary (Hainan Island, China). Cont. Shelf Res. 57: 82-91. http://dx.doi.org/10.1016/j.csr.2012.06.016 [ Links ]
Barraza-Guardado RH, Martínez-Córdoba LR, Enríquez-Ocaña LF, Martínez-Porchas M, Miranda-Baeza A, Porchas-Cornejo MA. 2014. Effect of shrimp farm effluent on water and sediment quality parameters off the coast of Sonora, Mexico. Cienc. Mar. 40(4): 221-235. http://dx.doi.org/10.7773/cm.v40i4.2424 [ Links ]
Bayen S. 2012. Occurrence, bioavailability and toxic effects of trace metals and organic contaminants in mangrove ecosystems: A review. Environ. Int. 48: 84-101. http://dx.doi.org/10.1016/j.envint.2012.07.008 [ Links ]
Boonsong K, Piyatiratitivorakul S, Patanaponpaiboon P. 2003. Potential use of mangrove plantation as constructed wetland for municipal wastewater treatment. Water Sci. Technol. 48(5): 257-266. [ Links ]
Bouwman L, Beusen A, Glibert PM, Overbeek C, Pawlowski M, Herrera J, Mulsow S, Yu R, Zhou M. 2013. Mariculture: Significant and expanding cause of coastal nutrient enrichment. Environ. Res. Lett. 8(4): 044026. http://dx.doi.org/10.1088/1748-9326/8/4/044026 [ Links ]
Bowmer KH. 1987. Nutrient removal from effluents by an artificial wetland: Influence of rhizosphere aeration and preferential flow studied using bromide and dye tracers. Water Res. 21(5): 591-599. http://dx.doi.org/10.1016/0043-1354(87)90068-6 [ Links ]
Buhmann A, Papenbrock J. 2013. Biofiltering of aquaculture effluents by halophytic plants: Basic principals, current uses and future perspectives. Environ. Exp. Bot. 92: 122-133. http://dx.doi.org/10.1016/j.envexpbot.2012.07.005 [ Links ]
Camargo JA, Alonso A, Salamanca A. 2005. Nitrate toxicity to aquatic animals: A review with new data for freshwater invertebrates. Chemosphere 58(9): 1255-1267. http://dx.doi.org/10.1016/j.chemosphere.2004.10.044 [ Links ]
Cardona-Olarte P, Twilley RR, Krauss KW, Rivera-Monroy V. 2006. Responses of neotropical mangrove seedlings grown in monoculture and mixed culture under treatments of hydroperiod and salinity. Hydrobiologia 569(1): 325-341. http://dx.doi.org/10.1007/s10750-006-0140-1 [ Links ]
Chu HY, Chen NC, Yeung MC, Tam NFY, Wong YS. 1998. Tide-tank system simulating mangrove wetland for removal of nutrients and heavy metals from wastewater. Water Sci. Technol. 38(1): 361-368. [ Links ]
De-León-Herrera R, Flores-Verdugo F, Flores-de-Santiago F, González-Farías F. 2015. Nutrient removal in a closed silvofishery system using three mangrove species (Avicennia germinans, Laguncularia racemosa, and Rhizophora mangle) Mar. Pollut. Bull. 91(1): 243-248. http://dx.doi.org/10.1016/j.marpolbul.2014.11.040 [ Links ]
[FAO] Food and Agriculture Organization. 2012. The State of World Fisheries and Aquaculture 2010. FAO Fisheries and Aquaculture Department, Rome, 209 pp. [ Links ]
Flores-de-Santiago F, Kovacs JM, Flores-Verdugo F. 2012. Seasonal changes in leaf chlorophyll a content and morphology in a subtropical mangrove forest of the Mexican Pacific. Mar. Ecol. Prog. Ser. 444: 57-68. http://dx.doi.org/10.3354/meps09474 [ Links ]
Flores-Verdugo F, González-Farías F, Ramírez-Flores O, Amezcua-Linares A, Yáñez-Arancibia M, Alvarez-Rubio M, Day JW. 1990. Mangrove ecology, aquatic primary productivity, and fish community dynamics in the Teacapán-Agua Brava lagoon-estuarine system (Mexican Pacific). Estuaries 13(2): 219-230. http://dx.doi.org/10.2307/1351591 [ Links ]
Gersberg RM, Elkins BV, Goldman CR. 1983. Nitrogen removal in artificial wetlands. Water Res. 17(9): 1009-1014. http://dx.doi.org/10.1016/0043-1354(83)90041-6 [ Links ]
Greenway M, Woolley A. 1999. Constructed wetlands in Queensland: Performance efficiency and nutrient bioaccumulation. Ecol. Eng. 12(1-2): 39-55. http://dx.doi.org/10.1016/S0925-8574(98)00053-6 [ Links ]
Herbeck LS, Sollich M, Unger D, Holmer M, Jennerjahn TC. 2014. Impact of pond aquaculture effluents on seagrass performance in NE Hainan, tropical China. Mar. Pollut. Bull. 85(1): 190-203. http://dx.doi.org/10.1016/j.marpolbul.2014.05.050 [ Links ]
Hu HY, Goto N, Fujie K, Kasakura T, Tsubone T. 2002. Reductive treatment characteristics of nitrate by metallic iron in aquatic solution. J. Chem. Eng. Japan 34(9): 1097-1102. http://dx.doi.org/10.1252/jcej.34.1097 [ Links ]
Huang Q, Liu Y, Zheng X, Chen G. 2012. Phytoplankton community and the purification effect of mangrove in the mangrove plantation-aquaculture coupling systems in the Pearl River Estuary. Procedia Environ. Sci. 15: 12-21. http://dx.doi.org/10.1016/j.proenv.2012.05.004 [ Links ]
Jensen F, Nielsen M, Nielsen R. 2014. Increased competition for aquaculture from fisheries: Does improved fisheries management limit aquaculture growth? Fish. Res. 159: 25-33. http://dx.doi.org/10.1016/j.fishres.2014.05.004 [ Links ]
Kim J, Benjamin MM. 2004. Modeling a novel ion exchange process for arsenic and nitrate removal. Water Res. 38(8): 2053-2062.http://dx.doi.org/10.1016/j.watres.2004.01.012 [ Links ]
Krauss KW, Doyle TW, Twilley RR, Rivera-Monroy VH, Sullivan JK. 2006. Evaluating the relative contributions of hydroperiod and soil fertility on growth of south Florida mangroves. Hydrobiologia 569(1): 311-324. http://dx.doi.org/10.1007/s10750-006-0139-7 [ Links ]
Kristensen E, Bouillon S, Dittmar T, Marchand C. 2008. Organic carbon dynamics in mangrove ecosystems: A review. Aquat. Bot. 89(2): 201-219. http://dx.doi.org/10.1016/j.aquabot.2007.12.005 [ Links ]
Lin YF, Jing SR, Lee DY, Wang TZ. 2002. Nutrient removal from aquaculture wastewater using a constructed wetlands system. Aquaculture 209: 169-184. http://dx.doi.org/10.1016/S0044-8486(01)00801-8 [ Links ]
Lin YF, Jing SR, Lee DY. 2003. The potential use of constructed wetlands in a recirculating aquaculture system for shrimp culture. Environ. Pollut. 123(1): 107-113. http://dx.doi.org/10.1016/S0269-7491(02)00338-X [ Links ]
Maltais-Landry G, Maranger R, Brisson J, Chazarenc F. 2009. Nitrogen transformations and retention in planted and artificially aerated constructed wetlands. Water Res. 43(2): 535-545. http://dx.doi.org/10.1016/j.watres.2008.10.040 [ Links ]
Menkouchi Sahli MA, Tahaikt M, Achary I, Taky M, Elhanouni F, Hafsi M, Elmghari M, Elmidaoui A. 2006. Technical optimization of nitrate removal for groundwater by ED using a pilot plant. Desalination 189: 200-208. http://dx.doi.org/10.1016/j.desal.2005.06.025 [ Links ]
Monroy-Torres M, Flores-Verdugo F, Flores-de-Santiago F. 2014. Growth of three subtropical mangrove species in response to varying hydroperiod in an experimental tank. Cienc. Mar. 40(4): 263-275. http://dx.doi.org/10.7773/cm.v40i4.2455 [ Links ]
Moroyoqui-Rojo L, Flores-Verdugo F, Hernández-Carmona G, Casas-Valdez M, Cervantes-Duarte R, Nava-Sánchez EH. 2012. Nutrient removal using two species of mangrove (Rhizophora mangle and Laguncularia racemosa) in experimental shrimp (Litopenaeus vannamei) culture ponds. Cienc. Mar. 38(2): 333-346. [ Links ]
Páez-Osuna F, Gracia A, Flores-Verdugo F, Lyle-Fritch LP, Alonso-Rodríguez R, Roque A, Ruiz-Fernández AC. 2003. Shrimp aquaculture development and the environment in the Gulf of California ecoregion. Mar. Pollut. Bull. 46(7): 806-815. http://dx.doi.org/10.1016/S0025-326X(03)00107-3 [ Links ]
Paniagua-Michel J, Garcia O. 2003. Ex-situ bioremediation of shrimp culture effluent using constructed microbial mats. Aquacult. Eng. 28: 131-139. http://dx.doi.org/10.1016/S0144-8609(03)00011-6 [ Links ]
Reed SC, Crites RW, Middlebrooks EJ. 1995. Wetland Systems. Natural Systems of Waste Management and Treatment. 2nd ed. McGraw-Hill, New York, pp. 173-284. [ Links ]
Reef R, Feller IC, Lovelock CE. 2010. Nutrition of mangroves. Tree Physiol. 30(9): 1148-1160. http://dx.doi.org/10.1093/treephys/tpq048 [ Links ]
Saenger P. 2002. Mangrove Ecology, Silviculture and Conservation. Kluwer Academic Publisher, Dordrecht, 360 pp. [ Links ]
Schneider O, Sereti V, Eding EH, Verreth JAJ. 2005. Analysis of nutrient flows in integrated intensive aquaculture systems. Aquacult. Eng. 32: 379-401. http://dx.doi.org/10.1016/j.aquaeng.2004.09.001 [ Links ]
Schoeman JJ, Steyn A. 2003. Nitrate removal with reverse osmosis in a rural area in South Africa. Desalination 155(1): 15-26. http://dx.doi.org/10.1016/S0011-9164(03)00235-2 [ Links ]
Shimoda T, Fujioka Y, Srithong C, Aryuthaka C. 2007. Effect of water exchange with mangrove enclosures based on nitrogen budget in Penaeus monodon aquaculture ponds. Fish. Sci. 73(2): 221-226. http://dx.doi.org/10.1111/j.1444-2906.2007.01327.x [ Links ]
Sison NF, Hanaki K, Matsuo T. 1995. High loading denitrification by biological activated carbon process. Water Res. 29(12): 2776-2779. http://dx.doi.org/10.1016/0043-1354(95)00119-6 [ Links ]
Strickland JD, Parsons TR. 1972. A Practical Handbook of Seawater Analysis. Fisheries Research Board of Canada Bulletin 167, 310 pp. [ Links ]
Sundareshwar PV, Morris JT, Koepfler EK, Fornwalt B. 2003. Phosphorus limitation of coastal ecosystem processes. Science 299(5606): 563-565. http://dx.doi.org/10.1126/science.1079100 [ Links ]
Tam NFY, Wong YS. 1995. Mangrove soils as sinks for wastewater-borne pollutants. Hydrobiologia 295(1): 231-241. http://dx.doi.org/10.1007/bf00029130 [ Links ]
Tam NFY, Wong AHY, Wong MH, Wong YS. 2009. Mass balance of nitrogen in constructed mangrove wetlands receiving ammonium-rich wastewater: Effects of tidal regime and carbon supply. Ecol. Eng. 35(4): 453-462. http://dx.doi.org/10.1016/j.ecoeng.2008.05.011 [ Links ]
Tanner CC, Clayton JS, Upsdell MP. 1995. Effect of loading rate and planting on treatment of dairy farm wastewaters in constructed wetlands. II. Removal of nitrogen and phosphorus. Water Res. 29(1): 27-34. http://dx.doi.org/10.1016/0043-1354(94)00140-3 [ Links ]
Wong YS, Tam NFY, Chen GZ, Ma H. 1997. Response of Aegiceras corniculatum to synthetic sewage under simulated tidal conditions. Asia-Pacific Conference on Science and Management of Coastal Environment. Dev. Hydrobiol. 123: 89-96. http://dx.doi.org/10.1007/978-94-011-5234-1_10 [ Links ]
Wu Y, Chung A, Tam NFY, Pi N, Wong MH. 2008. Constructed mangrove wetlands as secondary treatment system for municipal wastewater. Ecol. Eng. 34(2): 137-146. http://dx.doi.org/10.1016/j.ecoleng.2008.07.010 [ Links ]
Yang Q, Tam NFY, Wong YS, Luan TG, Su WS, Lan CY, Shin PKS, Cheung SG. 2008. Potential use of mangroves as constructed wetland form municipal sewage treatment in Futian, Shenzhen, China. Mar. Pollut. Bull. 57: 735-743. http://dx.doi.org/10.1016/j.marpolbul.2008.01.037 [ Links ]
Ye Y, Tam NFY , Wong YS. 2001. Livestock wastewater treatment by a mangrove pot-cultivation system and the effect of salinity on the nutrient removal efficiency. Mar. Pollut. Bull. 42(6): 512-520. http://dx.doi.org/10.1016/S0025-326X(00)00196-X [ Links ]
Zaitsev G, Mettanen T, Langwaldt J. 2008. Removal of ammonium and nitrate from cold inorganic mine water by fixed-bed biofilm reactors. Miner. Eng. 21(1): 10-15. http://dx.doi.org/10.1016/j.mineng.2007.08.014 [ Links ]
Zhang JE, Liu JL, Ouyang Y, Liao BW, Zhao BL. 2010. Removal of nutrients and heavy metals from wastewater with mangrove Sonneratia apetala Buch-Ham. Ecol. Eng. 36(6): 807-812. http://dx.doi.org/10.1016/j.ecoleng.2010.02.008 [ Links ]
Received: March 2015; Accepted: September 2015











 texto em
texto em 


