Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Ciencias marinas
versión impresa ISSN 0185-3880
Cienc. mar vol.41 no.3 Ensenada sep. 2015
https://doi.org/10.7773/cm.v41i3.2482
Articles
Reproductive pattern of the reef-building coral Pavona gigantea (Scleractinia: Agariciidae) off southwestern Mexico
1 Centro Universitario de la Costa, Universidad de Guadalajara, Av. Universidad No. 203, Puerto Vallarta, 48280 Jalisco, México.
2 Instituto de Investigaciones Oceanológicas, Universidad Autónoma de Baja California, Carretera Transpeninsular Ensenada-Tijuana #3917, Fraccionamiento Playitas, 22860 Ensenada, Baja California, México.
3 Universidad del Mar, Ciudad Universitaria, Distrito de San Pedro Pochutla, 70902 Puerto Ángel, Oaxaca, México.
4 Departamento de Hidrobiología, Universidad Autónoma Metropolitana, San Rafael Atlixco 186, 09340 Distrito Federal, México.
The reproductive biology of the massive coral Pavona gigantea has been studied along Mexico's Pacific coast, but basic aspects such as its relation to local environmental variables and its variation on a mesoscale level have not been addressed. The reproductive cycle of P. gigantea was monitored monthly over a two-year period (2010-2012) at four sites along the coast of Oaxaca (southwestern Mexico). Except for one hermaphroditic colony, P. gigantea was gonochoric and exhibited asynchronous development. The data suggest that, in the study area, the species may reproduce seasonally, with minor interannual differences (May-September 2010, April-August 2011, April-May 2012). There were spatial and temporal variations in the percentage of reproductively active colonies and the presence of mature sex cells. A multiple regression analysis revealed that the percentage of reproductively active colonies was significantly explained (69.6%) by mesoscale variations in environmental variables such as sea surface temperature, photosynthetically active radiation, diffuse attenuation coefficient, percentage of lunar illumination, and photoperiod, the latter being the most relevant variable in the model. The data suggest that temporal mesoscale variations can exert a meaningful influence on coral reproduction in the study area.
Key words: reproduction; gametogenesis; coral; spawning
La biología reproductiva del coral masivo Pavona gigantea en las aguas del Pacífico frente a las costas mexicanas ha sido estudiada; sin embargo, algunos aspectos básicos, como su relación con factores abióticos locales y su variación a nivel de mesoescala, no han sido resueltos. Se monitoreó el ciclo reproductivo de P. gigantea durante dos años (2010-2012) en cuatro localidades frente a la costa de Oaxaca, sudoeste de México, en el Pacífico. Pavona gigantea presentó colonias gonocóricas con desarrollo asincrónico y una colonia hermafrodita. Los datos sugieren que la especie podría reproducirse de manera estacional con variaciones interanuales (mayo-septiembre de 2010, abril-agosto de 2011, abril-mayo de 2012). Se observaron variaciones espaciotemporales en el porcentaje de colonias con actividad reproductiva y en la presencia de gametos maduros. El modelo de regresión lineal múltiple sugiere que las variaciones ambientales a mesoescala de temperatura superficial oceánica, radiación fotosintéticamente activa, coeficiente de atenuación difusa, porcentaje de iluminación lunar y fotoperiodo explicaron significativamente el 69.6% de la variación en el porcentaje de colonias con actividad reproductiva, y el fotoperiodo fue la variable más importante en el modelo. Los datos indican que las variaciones temporales a mesoescala podrían influenciar la reproducción de las especies de coral en el área de estudio.
Palabras clave: reproducción; gametogénesis; coral; desove
Introduction
Stony corals can reproduce asexually and sexually. In sexual reproduction, the gametes of both sexes exchange genetic material during fecundity and form a larva. Once competence has been attained, the larva settles and undergoes metamorphosis until a colony is formed. This process generally occurs annually (Harrison and Wallace 1990, Harrison 2011). This type of reproduction favors the dispersal of the species and maintains variation by the recombination of genetic material (Fadlallah 1983).
Coral reproduction involves processes such as gameto-genesis and reproductive activity that are driven by environmental conditions (Harrison 2011). During periods of stress, some reproductive processes can be affected or inhibited, with the consequent reduction in fecundity or reproductive success (Harrison et al. 1984, Tanner 1996, Cortés 1997). Temperature is one of the main abiotic factors controlling the onset of the reproductive cycle, but photoperiod, wind and current patterns, lunar cycle, and irradiance can also act as cues to trigger and synchronize reproductive activity (Harrison 2011). Conversely, the reproductive cycle can be inhibited by extreme temperatures that exceed the thermotol-erance of the species and by factors associated with light conditions, such as turbidity, sedimentation, and ultraviolet light.
The coral communities off the west coast of Mexico are considered among the most important of the Eastern Tropical Pacific (ETP). They have been described as small and patchy in distribution and can form sparse or dense assemblages (Glynn and Ault 2000, Glynn et al. 2000), composed mainly of species belonging to the genera Pocillopora (>90% coverage), Pavona (<5% coverage), and Porites (<1% coverage) (Glynn and Leyte-Morales 1997, Reyes-Bonilla and López-Pérez 1998, López-Pérez and Hernández-Ballesteros 2004).
Pavona gigantea Verrill, 1869 is a hermatypic coral endemic to the eastern Pacific (Glynn and Ault 2000), and it is one of the most common and widely distributed coral species off the west coast of Mexico (Reyes-Bonilla et al. 2005). The information available on the reproductive activity of this species is limited to reports for the equatorial Pacific Ocean and the Pacific coast of central and southern Mexico (Glynn et al. 1996, Carpizo-Ituarte et al. 2011, Rodríguez-Troncoso et al. 2011). Genetic studies (Saavedra-Soleto et al. 2011) suggest that the populations of P. gigantea along the west coast of Mexico are genetically isolated, which makes them vulnerable to local disturbances and extirpations and may even compromise the regional connectivity of the species. On a large scale, however, there is evidence that the southern populations can be relevant to the central populations and even to those that inhabit the Gulf of California (Saavedra-Soleto et al. 2011).
The reproductive activity of stony corals can vary spatially depending on the local environmental conditions and the life history of the species (Somero 2005). In Central America, some species can develop gametes throughout the year and present microscale spatial variations (i.e., tens of meters) mainly associated with fluctuations in temperature and nutrients caused by upwelling (Glynn et al. 1996). In Pacific waters off southern and central Mexico, the onset of gametogenetic activity is directly related to the increase in sea surface temperature (SST) during summer (Carpizo-Ituarte et al. 2011, Rodríguez-Troncoso et al. 2011); however, to date, there are no reports of the presence of mature gametes or any evidence of spawning and there is a lack of information regarding the mesoscale variations in reproduction in the region.
Information on reproductive success (i.e., from gamete formation and release to larval settlement) is essential for a better understanding of the development, permanence, and recovery of coral reefs and communities after natural or anthropogenic disurbances in the region. This paper contributes information on the reproductive cycle of P. gigantea off the coast of Oaxaca (southwestern Mexico), analyzes the spatial and temporal variations, and evaluates whether the reproductive activity is influenced by environmental variations in the area.
Materials and methods
Study area
Samples were collected from Montosa Island and three beaches (Estacahuite, Riscalillo, and La Entrega) along the coast of Oaxaca, located in the coastal corridor known as Mazunte-Bahías de Huatulco (15°39'N, 96°33'W; 15°45'N, 96°04'W) (Fig. 1). The maximum distance between sampling sites is 45 km; however, despite their proximity, the four sites have different characteristics and dimensions: at La Entrega, the reef covers an area of 3.51 ha and has a maximum depth of 13.3 m; at Riscalillo, the reef covers an area of 3.51 ha and has a maximum depth of 10 m; at Estacahuite, the reef covers an area of 2.30 ha and has a maximum depth of 20 m (López-López 2011); and at Montosa Island, the reef covers an area of 11.8 ha and has a maximum depth of 11.6 m (Glynn and Leyte-Morales 1997).
The region has a warm subhumid climate, and summer rains account for less than 5% of the annual precipitation (García 1973). The sampling sites are within the Gulf of Tehuantepec, characterized by scant river inputs, sandy sediments, and seasonal upwelling that is responsible for low temperature and dissolved oxygen values and high nutrient concentrations (Tapia-García et al. 2007). During winter and spring, northerly wind events known as Tehuanos occur in the region. These winds are produced when there is a difference in atmospheric pressure between the Gulf of Mexico and the tropical Pacific (Steenburgh et al. 1998). They generate mesoscale eddies in the Gulf of Tehuantepec that can affect tens of kilometers and cause a decrease in SST, which promotes the formation of a shallow thermocline and generates thermal anomalies and changes in the concentration of chlorophyll (Kessler 2002, Fernández-Álamo and Farber-Lorda 2006).
Survey
Monthly surveys were conducted from May 2010 to May 2012. At each site, fragments of approximately 3 cm2 were collected from five randomly selected colonies of P. gigantea by scuba divers. The selected colonies measured more than 30 cm in diameter and did not show signs of bleaching. The fragments were fixed in situ in 10% formaldehyde in seawater for 24 h, and then transferred to 70% ethyl alcohol and stored at room temperature until processing. Each fragment was decalcified individually in a solution of 10% acetic acid for 24 h. The tissue was dehydrated in an eight-stage ethyl alcohol series (70%, 80%, 90%, 90%, 96%, 96%, 100%, and 100%) and a two-step whitening treatment with Citrisol in a Leica TP102 automatic tissue processor. The tissues were embedded in Paraplast in a Leica EG1160 tissue embedding center, and 6- μm-thick sections were cut with a Leica RM2145 semiautomatic microtome. The sections were immediately stained with hematoxylin and eosin and then mounted with synthetic resin. The coral tissue was examined under a Carl Zeiss AxioScope optical miscroscope and photo-documented using a AxioCam ERc 5s digital camera. The maximum diameter of oocytes was measured using AxioVision Rel v2.0 for Windows. The reproductive stages were determined according to the criteria proposed by Glynn et al. (1996), Carpizo-Ituarte et al. (2011) and Rodríguez-Troncoso et al. (2011).
Environmental variables
At each site, SST was measured in situ using a Hanna HI9828 multiparameter meter (accuracy ±0.15 °C). The diffuse attenuation coefficient (Kd490) and photosynthetically active radiation (PAR) were derived from satellite images (Aqua/MODIS, http://oceancolor.gsfc.nasa.gov).
Owing to meteorological factors, the data for our sampling days were unavailable, so the images used to extract the data represent the average of the eight days prior to each survey. The Aqua/MODIS images (level 3 processing, resolution of 9 × 9 km) were processed using the Wam_statist program. Lunar illumination (%) and photoperiod data (resolution of 1 × 1 km) were obtained from the Weather Underground database (http://www.wunderground.com/) for each sampling day.
Data analysis
We determined the sex ratio and the percentage of reproductively active male and female colonies. The data for all the environmental variables and the percentage of reproductively active colonies were subjected to tests for normality (Kolmogorov-Smirnov, P < 0.05) and homogeneity of variance. As the data were neither normally distributed nor homoscedastic, a nonparametric analysis of variance (Kruskal-Wallis) was performed to determine possible differences between sites. Since there were no significant differences in regard to the environmental variables (SST, H(3, 68) = 0.265, P = 0.966; PAR, H(3, 68) = 0.307, P = 0.959; Kd490, H(3, 68) = 2.499, P = 0.475), the data were averaged to obtain a monthly value for the study area. The relation between the averages of the environmental variables and the percentage of reproductively active male and female colonies was assessed by multiple linear regression analysis in order to examine whether the reproductive activity of P. gigantea was related to environmental conditions in the area and to determine the most important variable(s) in the reproductive process. The statistical analyses were performed using Statistica v.6.
Results
Reproduction
Pavona gigantea had three reproductive periods during the study: May-September 2010, April-August 2011, and April-May 2012. All the colonies were gonochoric (Fig. 2a) except for one, which was hermaphroditic (Fig. 2b). The species displayed asynchronous maturation and the sex ratio was 1:1.5 (male:female).
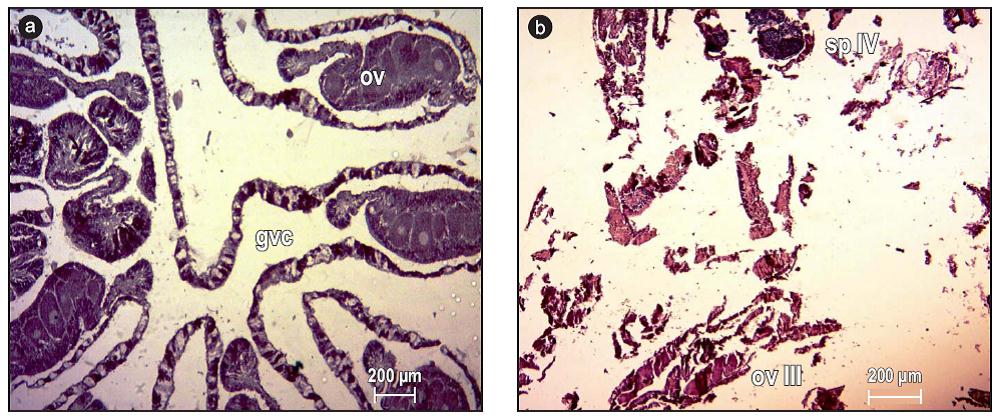
Figure 2: Reproductive activity of Pavona gigantea off the coast of Oaxaca (southwestern Mexico). (a) Entire gonochoric polyp (4x) and (b) hermaphroditic polyp (4x). Abbreviations: sp IV, stage IV spermaries; gvc, gastrovascular cavity; ov III, stage III oocyte.
The cord-like female germ cells extended from the mesentery to the gastric cavity of the polyp. Four stages of oocyte maturation were observed (Fig. 3). Stage I oocytes (5-30 μm in diameter) were observed in May 2010; they were oval in shape and the nucleus occupied most of the cytoplasm (Fig. 3a). Stage II oocytes (31-50 in diameter) were observed in May and June 2010 and presented a well-defined nucleus and nucleolus, as well as a cord-like arrangement of previtellogenic oocytes (Fig. 3b). Stage III oocytes (51-100 μm in diameter) occurred throughout the reproductive cycle; they were partially vitellogenic and presented a nucleus, nucleolus, and lipid vacuoles, as well as symbionts at the periphery of the cell (Fig. 3c). Stage IV oocytes (>100 μm in diameter) were observed in June 2010 and August 2011; they were considered mature gametes and were characterized by the migration and reduction of the nucleus, located on the opposite side of the cytoplasm (Fig. 3d).
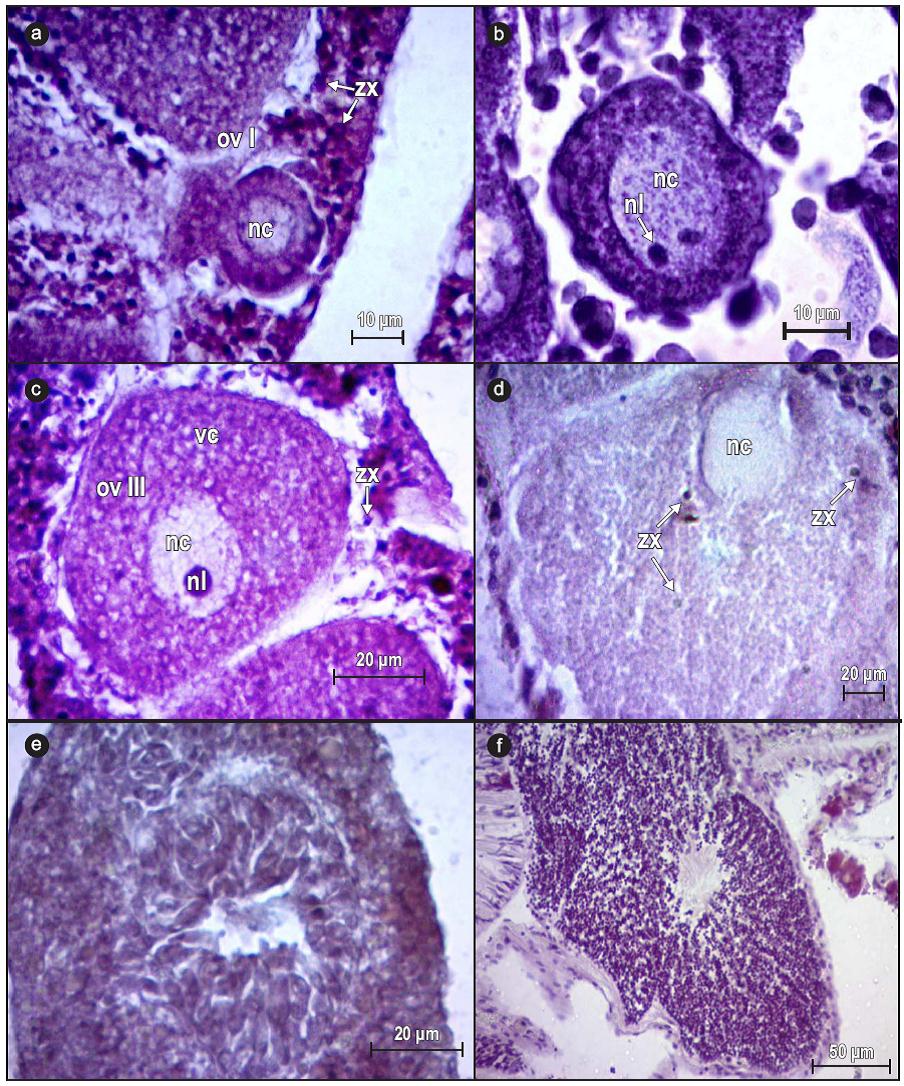
Figure 3: Developmental stages of Pavona gigantea reproductive cells off the coast of Oaxaca (southwestern Mexico). (a) Stage I oocyte (ovI), (b) stage II oocyte, (c) stage III oocyte (ov III), (d) stage IV oocyte, (e) stage II spermaries, and (f) stage IV spermaries (100x). Abbreviations: zx, symbiont; nc, nucleus; nl, nucleolus; vc, vacuoles.
Spermaries were found in sacs that extended from the mesentery to the gastric cavity. Mature cells were observed in 85.7% of the male colonies. Stage II spermatocytes were observed only in June 2011 and 2012, and presented prominent nuclei that migrated from the periphery of the sac and formed clusters (Fig. 3e). During stage IV, the spermatids were clearly reduced in size as a result of the elimination of the cytoplasm during spermiogenesis, and the clusters migrated towards the periphery of the spermaries, where fla-gella aligned towards the central region were observed (Fig. 3f).
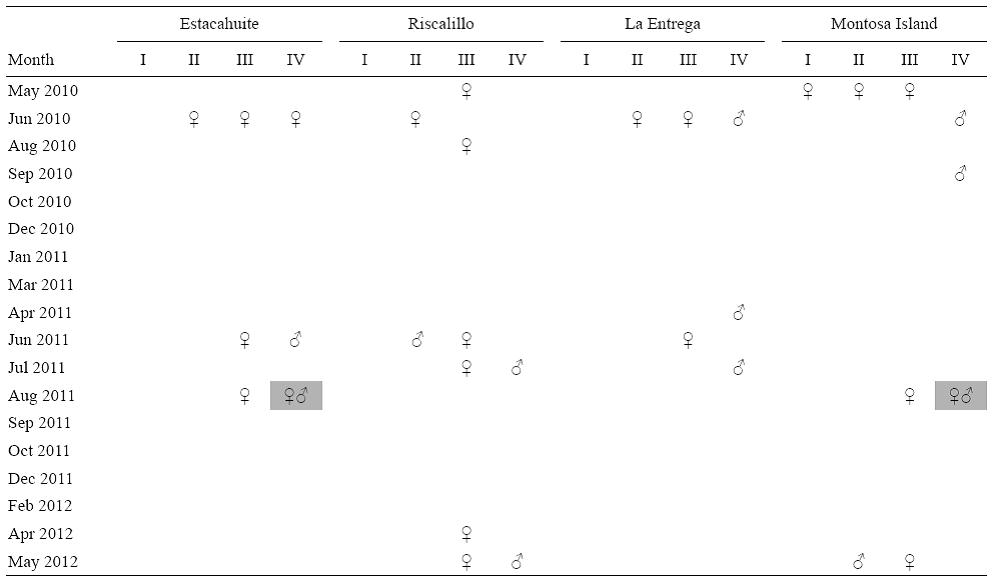
The Riscalillo colonies exhibited the highest reproductive activity (13.63%, n = 85) and a sex ratio of 1:3 (M:F), whereas the La Entrega colonies exhibited the lowest reproductive activity (7.78%, n = 90) and a sex ratio of 1:0.75 (M:F). The colonies from Estacahuite and Montosa Island were characterized by the presence of mature oocytes and spermaries in August 2011 (Table 1). Nonetheless, the analysis of variance indicated that there were no significant differences in the reproductive activity of P. gigantea between sites (H(3 68) = 4.029, P = 0.2583).
Table 1: Presence of male and female sex cells and developmental stages (Roman numerals) in Pavona gigantea samples collected at four sites along the coast of Oaxaca (Mexico) between May 2010 and May 2012. The grey boxes indicate the presence of both mature male and female gametes in the same place at the same time.
Temporal variation in reproductive activity
During the three reproductive periods recorded in this study, more than 20% of the P. gigantea colonies presented reproductive activity (June 2010, August 2011, and May 2012). Peak reproductive activity occurred in June 2010 and there were periods of inactivity between the reproductive periods.
Reproductive activity occurred when SST was between 28 and 31 °C (Fig. 4), PAR exceeded 48 mol photons m-2 day (Fig. 5), Kd490 was less than 0.1490 nm m-1 (Fig. 6), and the photoperiod was longer than 12 h (Fig. 7). There was no relation between the percentage of lunar illumination on the day of sampling and reproductive activity (Fig. 8); however, the months when mature oocytes were observed (June 2010 and August 2011) coincided with high percentages of illumination.
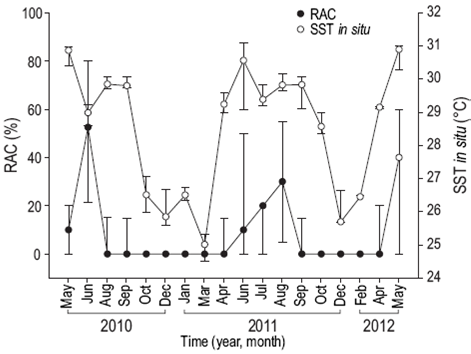
Figure 4: Relationship between in situ sea surface temperature (SST) and the percentage of reproductively active colonies (RAC) of Pavona gigantea (median and standard deviation) off the coast of Oaxaca (southwestern Mexico).
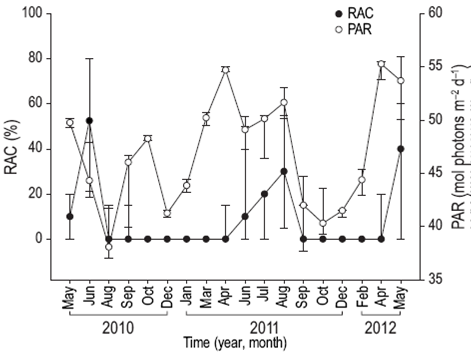
Figure 5: Relationship between photosynthetically active radiation (PAR) and the percentage of reproductively active colonies (RAC) of Pavona gigantea (median and quartiles) off the coast of Oaxaca (southwestern Mexico).
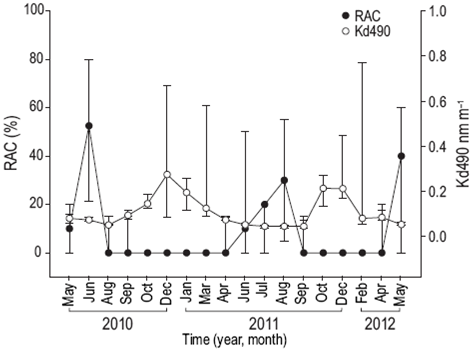
Figure 6: Relationship between the diffuse attenuation coefficient (Kd490) and the percentage of reproductively active colonies (RAC) of Pavona gigantea (median and quartiles) off the coast of Oaxaca (southwestern Mexico).
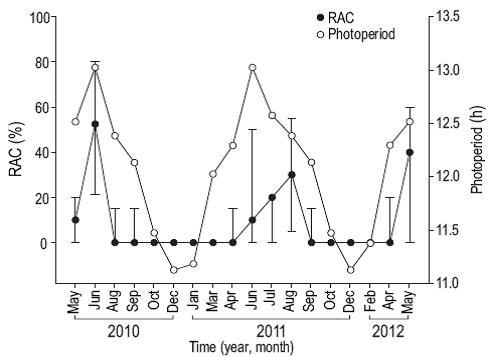
Figure 7: Relationship between photoperiod and the percentage of reproductively active colonies (RAC) of Pavona gigantea (median and standard deviation) off the coast of Oaxaca (southwestern Mexico).
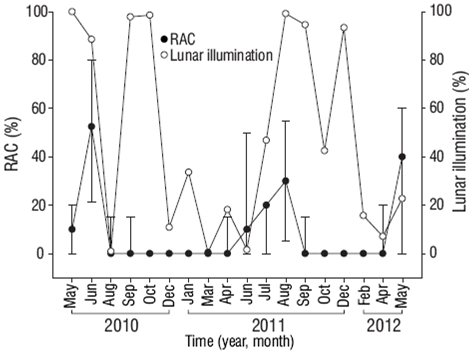
Figure 8: Relationship between the percentage of lunar illumination and the percentage of reproductively active colonies (RAC) of Pavona gigantea (median and standard deviation) off the coast of Oaxaca (southwestern Mexico).
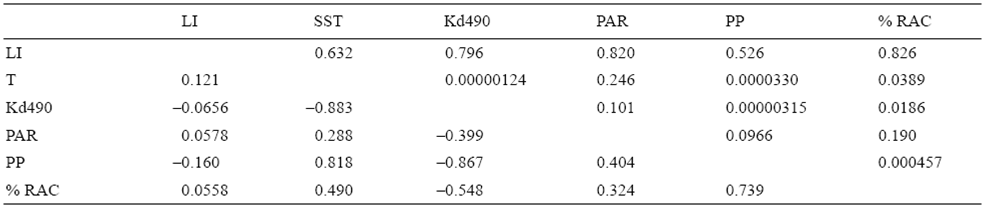
The multiple linear regression model explained 69.6% of the variation in the percentage of reproductively active colonies when both sexes were considered (R = 0.834, n = 18, P = 0.007), 67% when only females were considered (R = 0.818, n = 18, P = 0.012), and 51% when only males were considered (R = 0.714, n = 18, P = 0.09). Hence, the model adequately explained the variation in the reproductive activity of P. gigantea when both sexes or only females were considered in the analysis, but was not adequate when only males were considered. This indicates that oocyte development of this coral species is significantly influenced by changes in the environmental conditions of this region. The analysis revealed that the percentage of reproductively active colonies was highly correlated with photoperiod (R = 0.739), and was inversely related to Kd490 (R = -0.548), SST (R = 0.49), PAR (R = 0.324), and percentage of lunar illumination (R = 0.056). This suggests that, in the study area, the reproductive pattern of P. gigantea is mainly related to the length of the photoperiod and to a lesser extent to lunar illumination (Table 2).
Table 2: Correlation of environmental variables with the percentage of reproductively active colonies (% RAC) in Pacific coastal waters off Oaxaca (Mexico). Upper diagonal, P values; lower diagonal, correlation values. LI, lunar illumination; SST, sea surface temperature; Kd490, diffuse attenuation coefficient; PAR, photosynthetically active radiation; PP, photoperiod.
Discussion
In the study area, the P. gigantea colonies were gonochoric. Only one hermaphroditic colony was found at Estacahuite in July 2011. This type of reproductive behavior, known as sequential cosexuality, has been reported for the study area and other areas of the ETP (Glynn et al. 1996, Rodríguez-Troncoso et al. 2011). Corals can begin their reproductive life as males and then become hermaphroditic and generate protandrous hermaphrodite colonies (Fadlallah 1983, Harrison 2011). It has been suggested that sequential cosexuality can guarantee sexual reproduction and increase connectivity among populations (Fadlallah 1983, Harrison and Wallace 1990), but it can also decrease genetic variability and increase the probablity of local or regional extinction of the species in question. In view of the small number of hermaphrodyte samples in the present and previous studies, it has not been possible to accurately determine whether P. gigantea is capable of developing sequential cosexuality and more studies are needed.
In our study area, P. gigantea displayed asynchronous maturation. This concurs with that reported for the southwestern coast of Mexico (Rodríguez-Troncoso et al. 2011), but differs from the synchronous maturation observed for the same species off the Pacific coast of Central America (Glynn et al. 1996) and central Mexico (Carpizo-Ituarte et al. 2011). A variation in reproductive behavior can be advantageous because it allows the species to undergo multiple spawns during the reproductive period. Moreover, asynchronous maturation among colonies of a different sex promotes the exchange of genetic material among sites (Harrison and Wallace 1990).
Unlike other studies conducted off the west coast of Mexico (Carpizo-Ituarte et al. 2011, Rodríguez-Troncoso et al. 2011), we observed the four stages of oocyte development as well as interstitial cells, which is indicative of oogenesis and spermatogenesis (Szmant-Froelich et al. 1985). The presence of the four maturation stages suggests that the environmental conditions in the study area were appropriate for successful gametogenesis, but may not have been sufficiently favorable for the completion of oocyte maturation and spawning.
The presence of mature male and female gametes at Estacahuite and Montosa Island in August 2011 suggests that both the final phase of gametogenesis and spawning may have occurred during this month, since the period of rest began in September 2011 at both sites. To date, it had not been possible to observe mature male and female sex cells in the same place at the same time, so these findings may be the first indication of the successful sexual reproduction of P. gigantea off the west coast of Mexico. Further studies are clearly needed, but August could be one of the months when gamete release occurs in the study area. The environmental conditions recorded in August were SST of 29.88 °C, photo-period of 12.41 h, and 99% lunar illumination, and these conditions may thus be favorable for oocytes to reach maturity stage IV. Numerical experiments have shown that particle transport occurs close to shore in August (Lara-Hernández 2012), and this could guarantee auto-recruitment as well as the connectivity with reef systems at higher latitudes (e.g., Zihuatanejo) (López-Pérez et al. 2012).
Our results suggest that P. gigantea reproduces seasonally. Reproductive activity initiates at the end of the dry season (winter), during which northerly winds occur, and continues during the rainy season (summer). While this behavior has been observed along the Pacific coast of Mexico (Carpizo-Ituarte et al. 2011) and Central America (Glynn et al. 1996), previous studies in our study area indicate that the species can have both seasonal and monthly periods of game-togenesis (Glynn et al. 1996, Carpizo-Ituarte et al. 2011, Rodríguez-Troncoso et al. 2011). Overall, the results indicate that the reproductive activity of P. gigantea in the ETP occurs mainly during the warm season (Glynn et al. 1996, Carpizo-Ituarte et al. 2011, Rodríguez-Troncoso et al. 2011, this study), though it is sufficiently dynamic to vary in space and time possibly in response to oscillations in local and regional oceanographic conditions modulated by interannual variations. This statement is supported by the significant relationship between the percentage of reproductively active colonies (i.e., both sexes and females) of P. gigantea and the mesoscale seasonal and interannual variation in the study area, which indicates that the reproduction of this coral species in the region is linked to local environmental variations.
Contrary to that documented for other species from other areas (Levy et al. 2007), the relationship between reproductive activity and percentage of lunar illumination was the least significant. Several studies have reported a relationship between the lunar cycle and the reproductive cycle of corals (Stoddart and Black 1985); in fact, the perception of moonlight is fundamental for coral spawning (Harrison and Wallace 1990). On the other hand, the Kd490 values were inversely related to reproductive activity, contrary to the relationship between PAR and reproductive activity; that is, when the reproductive peaks were higher, the Kd490 values were close to zero and the PAR values were high. This indicates that light is likely important in determining the percentage of reproductively active P. gigantea colonies in the study area. Numerous studies have indicated that light is the main source of energy for hermatypic corals because symbionts use solar energy to fix carbon dioxide and metabolize it into oxygen and carbon compounds, which are then translocated to the coral cells (Yellowlees et al. 2008). Thus, symbionts contribute to the nutrition, respiration, growth, and reproduction of corals (Stat et al. 2008, Yellowlees et al. 2008, Stambler 2011). This contribution can be affected by turbidity levels, because when they increase they can also decrease, among other things, the symbiont's photosynthetic capacity. An increase in PAR, therefore, translates into greater energy for the host's metabolism.
In the present study, gametogenesis occurred when SST was between 28 and 31 °C. Similarly, Rodríguez-Troncoso et al. (2011) and Carpizo-Ituarte et al. (2011) reported that gametogenesis occurred when SST was 27 °C in La Entrega Bay (Oaxaca, southwest Mexico) and 28.5 °C in Banderas Bay (Nayarit, west-central Mexico), respectively.
We found that photoperiod was the variable that most influenced the reproductive activity of P. gigantea. Symbi-onts are the main energy source for stony corals (Muscatine 1990, Stambler 2011); in fact, the energy transferred by Symbiodinium to the coral cells covers the host's daily energetic requirements and contributes to the reproductive activity. Photoperiod, together with other environmental conditions in the Gulf of Tehuantepec, could act as a synchronizer of the gametogenic cycle, as suggested by Benítez-Villalobos and Martínez-García (2012) for other invertebrates. Moreover, photoperiod in combination with warm temperatures could modulate gamete maturation in the region.
Our findings show that the reproductive activity of P. gigantea is driven by seasonal and interannual changes that occur in the Gulf of Tehuantepec, as has also been documented for other coral species and other regions (Harrison and Wallace 1990). Whether this environmental pattern drives the reproductive activity of other reef-building corals in the region (i.e., Pocillopora spp., Porites spp.) has yet to be determined. This type of information is relevant at both a local (i.e., Oaxaca) and regional scale because the connectivity among coral reef populations of the ETP depends on good management practices.
Acknowledgments
This study was supported by the National Council for Science and Technology (CONACYT, Mexico, project 80228 headed by ALP). JDSV was supported by a scholarship from CONACYT (No. 18410). We thank Denise Zavala for her help in processing the samples.
REFERENCES
Benítez-Villalobos F, Martínez-García M. 2012. Reproductive biology of the starfih Pharia pyramidatus (Echinodermata: Asteroidea) from the Mexican Tropical Pacific. J. Mar. Biol. Assoc. UK 92(6): 1409-1418. [ Links ]
Carpizo-Ituarte E, Vizcaíno-Ochoa V, Chi-Barragán G, Tapia-Vázquez O, Cupul-Magaña AL, Medina-Rosas P. 2011. Evidence of sexual reproduction in the hermatypic corals Pocillopora damicornis, Porites panamensis, and Pavona gigantea in Banderas Bay, Mexican Pacific. Cienc. Mar. 37: 97-112. http://dx.doi.org/10.7773/cm.v37i1.1773 [ Links ]
Cortés J. 1997. Biology and geology of eastern Pacific coral reefs. Coral Reefs 16: S39-S46. [ Links ]
Fadlallah YH. 1983. Sexual reproduction, development and larval biology in scleractinian corals. Coral Reefs 2: 129-150. [ Links ]
Fernández-Álamo MA, Farber-Lorda J. 2006. Zooplankton and the oceanography of the eastern tropical Pacific: A review. Prog. Oceanogr. 69: 318-359. [ Links ]
García E. 1973. Modificaciones al sistema de clasificación climática de Köpen (para adaptarlo a las condiciones de la República Mexicana). Instituto de Geografía, Universidad Nacional Autónoma de México, 264 pp. [ Links ]
Glynn PW, Leyte-Morales GE. 1997. Coral reefs of Huatulco, West Mexico: Reef development in upwelling Gulf of Tehuantepec. Rev. Biol. Trop. 45(3): 1033-1047. [ Links ]
Glynn PW, Ault JS. 2000. A biogeographic analysis and review of the far eastern Pacific coral reef region. Coral Reefs 19: 1-23. [ Links ]
Glynn PW, Colley SB, Gassman NJ, Black K, Cortés J , Mate JL. 1996. Reef coral reproduction in the eastern Pacific: Costa Rica, Panamá and Galápagos Islands (Ecuador). III. Agariciidae (Pavona gigantea and Gardineroseris planulata)Mar. Biol. 125: 579-601. [ Links ]
Glynn PW, Colley SB, Ting JH, Maté JL, Guzmán HM. 2000. Reef coral reproduction in the eastern Pacific: Costa Rica, Panamá, and Galápagos Islands (Ecuador). IV. Agariciidae recruitment and recovery of Pavona varians and Pavona spp. Mar. Biol. 136: 785-805. [ Links ]
Harrison PC, Wallace CC. 1990. Reproduction, dispersal and recruitment of scleractinian corals. In: Dubinsky Z (ed.), Ecosystems of the World 25. Coral Reefs. Elsevier, Amsterdam, pp. 133-207. [ Links ]
Harrison PC, Babcock RC, Bull GD, Oliver JK, Valle CC, Willis BL. 1984. Mass spawning in tropical reef corals. Science 464: 221-224. [ Links ]
Harrison PL. 2011. Sexual reproduction of scleractinian corals. In: Dubinsky Z , Stambler N (eds.), Coral Reefs: An Ecosystem in Transition. Springer, New York, pp. 59-85. [ Links ]
Kessler WS. 2002. Mean three-dimensional circulation in the Northeast Tropical Pacific. J. Phys. Oceanogr. 32: 2457-2471. [ Links ]
Lara-Hernández JA. 2012. Transporte larvario y conectividad potencial de corales pétreos en el Pacífico Mexicano: Estudio mediante simulaciones numéricas. BSc thesis, Universidad del Mar, México, 100 pp. [ Links ]
Levy O, Appelbaum L, Leggat W, Gothlif Y, Hayward DC, Miller DJ, Hoegh-Guldberg O. 2007. Light-responsive cryptochromes from a simple multicellular animal, the coral Acropora milleporaScience 318: 467-70. [ Links ]
López-López DA. 2011. Bioerosión provocada por el erizo Diadema mexicanum A. Agassiz, 1863 en localidades de Oaxaca y Guerrero. BSc thesis, Universidad del Mar, México, 65 pp. [ Links ]
López-Pérez RA, Hernández-Ballesteros LM. 2004. Coral community structure and dynamics in the Huatulco area, western Mexico. Bull. Mar. Sci. 75: 453-472. [ Links ]
López-Pérez RA, Calderón-Aguilera LE, Reyes-Bonilla H, Carriquiry JD, Medina-Rosas P, Cupul-Magaña AL, Herrero-Pérezrul MD, Hernández-Ramírez HA, Ahumada-Sempoal MA, Luna-Salguero BM. 2012. Coral communities and reefs from Guerrero, southern Mexican Pacific. Mar. Ecol. 33(4): 407-416. http://dx.doi.org/10.1111/j.1439-0485.2011.00505.x [ Links ]
Muscatine L. 1990.The role of symbiotic algae in carbon and energy flux in reef corals. In: Dubinsky Z (ed.), Coral Reefs. Elsevier, Dordrecht, pp. 75-87. [ Links ]
Reyes-Bonilla H, López-Pérez RA. 1998. Biogeography of the stony corals (Scleractinia) of the Mexican Pacific. Cienc. Mar. 24: 211-224. [ Links ]
Reyes-Bonilla H, Calderón-Aguilera LE, Cruz-Piñon G, Medina Rosas P, López-Pérez RA, Herrero-Pérez MD, Leyte-Morales GE, Cupul-Magaña AL, Carriquiry-Beltrán JD. 2005. Atlas de los Corales Pétreos (Anthozoa: Scleractinia) del Pacífico Mexicano. CICESE, CONABIO, CONACYT, DBM/UABCS, CUC/Univ. de Guadalajara, Univ. del Mar, México, 124 pp. [ Links ]
Rodríguez-Troncoso AP, Carpizo-Ituarte E, Leyte-Morales GE, Chi-Barragán G, Tapia-Vázquez O. 2011. Sexual reproduction of three coral species from the Mexican South Pacific. Mar. Biol. 158: 2673-2683. http://dx.doi.org/10.1007/s00227-011-1765-9 [ Links ]
Saavedra-Sotelo N, Calderón-Aguilera L, Reyes-Bonilla H, López-Pérez A, Medina-Rosas P , Rocha-Olivares A. 2011. Limited genetic connectivity of Pavona gigantea in the Mexican Pacific. Coral Reefs, 30: 677-686. [ Links ]
Somero GN. 2005. Linking biogeography to physiology: Evolutionary and acclimatory adjustments of thermal limits. Front. Zool. 2: 1. http://dx.doi.org/10.1186/1742-9994-2-1 [ Links ]
Stambler N. 2011. Zooxanthellae: The yellow symbionts inside animals. In: Dubinsky Z , Stambler N (eds.), Coral Reefs. An Ecosystem in Transition. Springer, New York , pp. 87-106. http://dx.doi.org/10.1007/978-94-007-0114-4 [ Links ]
Stat M, Loh WKW, Hoegh-Guldberg O, Carter DA. 2008. Symbiont acquisition strategy drives host-symbiont associations in the southern Great Barrier Reef. Coral Reefs 27: 763-772. [ Links ]
Steenburgh WJ, Schultz DM, Colle BA. 1998. The structure and evolution of gap outflow over the Gulf of Tehuantepec, Mexico. Mon. Weather Rev. 126: 2673-2691. [ Links ]
Stoddart JA, Black R. 1985. Cycles of gametogenesis and planulation in the coral Pocillopora damicornis Mar. Ecol. Prog. Ser. 23: 153-164. [ Links ]
Szmant-Froelich A, Reutter M, Riggs S. 1985. Sexual reproduction of Favia fragum (Esper): Lunar patterns of gametogenesis, embryogenesis and planulation in Puerto Rico. Bull. Mar. Sci. 37: 880-892. [ Links ]
Tanner JE. 1996. Seasonality and lunar periodicity in the reproduction of Pocilloporid corals. Coral Reefs. 15: 59-66. [ Links ]
Tapia-García M, García-Abad MC, Carranza-Edwards A, Vázquez-Gutiérrez F. 2007. Environmental characterization of the continental shelf of the Gulf of Tehuantepec, Mexico. Geo. Int. 46(4): 249-260. [ Links ]
Yellowlees D, Rees TAV, Leggat W. 2008. Metabolic interactions between algal symbionts and invertebrate hosts. Plant Cell Environ. 31: 679-694. [ Links ]
Received: October 2014; Accepted: April 2015











 texto en
texto en 



