Introduction
Mangroves and seagrass beds constitute highly productive ecosystems that are known to represent suitable fish habitats, acting as shelters, nurseries or foraging areas (Beck et al. 2001, Nagelkerken et al. 2008). The protection against predation, the interception of planktonic fish larvae, and the abundance of food sources are among the main assumptions explaining the high abundance of juvenile reef fishes in these habitats (Beck et al. 2001, Laegdsgaard and Johnson 2001). In the Caribbean, mangroves are often interlinked with sea-grass beds and the low tidal influence enables permanent exchanges between these habitats through diurnal or nocturnal fish migrations (Nagelkerken et al. 2000, Kopp et al. 2007). Mangrove ecosystems can be classified into several categories including fringing mangroves that occur along sheltered coastlines or mangrove islets (Gilmore and Snedaker 1993, Ewel et al. 1998). The configuration and structure of these fringing mangroves can vary substantially among locations and have a direct influence on the function and role of these coastal habitats (Ewel et al. 1998, Heithaus et al. 2011).
Feeding activities of transient fishes, comprising species that are present in mangroves during their juvenile life stages, can represent linkages between mangroves and adjacent coastal habitats (Heithaus et al. 2011). Many fish species are characterized as opportunistic species because their diets may change according to the availability and abundance of prey items (Laegdsgaard and Johnson 2001).
Stable isotopes have been used as proxies for contributions of mangrove and seagrass prey to fish diets and have shown marginal contribution of mangrove-derived organic matter (Nagelkerken et al. 2008). Transient fishes in mangroves primarily feed on allochthonous organic matter derived from plankton, algae, or seagrass beds (Marguillier et al. 1997, Kieckbusch et al. 2004, Nyunja et al. 2009). Yet, mangrove and seagrass food source contributions to fish diets seem to be site-specific and depend on hydrological processes, including tides and currents, or habitat structure (Lugendo et al. 2007, Granek et al. 2009, Vaslet et al. 2012). In Tanzania, Lugendo et al. (2007) found that fishes remaining in enclosed mangrove ponds during low tides derived most of their food from this habitat, whereas fishes from open fringing mangroves migrated and fed in adjacent inundated seagrass beds. Tidal range is therefore an important factor in the Indo-Pacific area because it drives trophic migration of fish species from subtidal habitats to inundated mangroves (Sheaves and Molony 2000, Lugendo et al. 2007). However, this environmental descriptor is less relevant in the Lesser Antilles region, where tides are generally lower than 1 m and mangroves are continuously flooded (Nagelkerken et al. 2008). Therefore, spatial structure (in terms of location and landscape characteristics of mangrove sites) may be of primary importance while assessing the origin of carbon sources assimilated by fishes (Vaslet et al. 2012).
This study considered different fringing mangroves (i.e., offshore mangrove islet, coastal mangroves) in a Caribbean lagoon and investigated the primary food sources of transient juvenile fishes. We examined the influence of mangrove location and habitat structure on the feeding behavior of 12 juvenile transient fishes by analyzing spatial patterns of stable isotopes. Carbon and nitrogen isotopic compositions provide good insights into the main food sources assimilated by fishes the preceding weeks (Gearing 1991). As a result of different inorganic carbon sources and photosynthesis, carbon isotopic fingerprints of mangroves are on average more depleted in 13C than are those of seagrass beds (Hemminga and Mateo 1996, Nagelkerken and van der Velde 2004a).
Carbon isotopes can thus be used as proxies to investigate the habitat from which fish species derive their diet. Trophic levels of consumers in food webs are revealed by nitrogen values (Peterson and Fry 1987). Consequently, stable isotope analyses enable tracing mangrove- and seagrass-derived organic matter in coastal food webs and assessment of trophic linkages between these habitats.
The objectives of the present study were (1) to investigate the influence of habitat structure on the feeding behavior of juvenile transient fishes and (2) to examine potential variations in fish feeding behavior regarding their feeding strategy (i.e., trophic category) and life stage (i.e., juveniles, adults).
Materials and methods
Study location
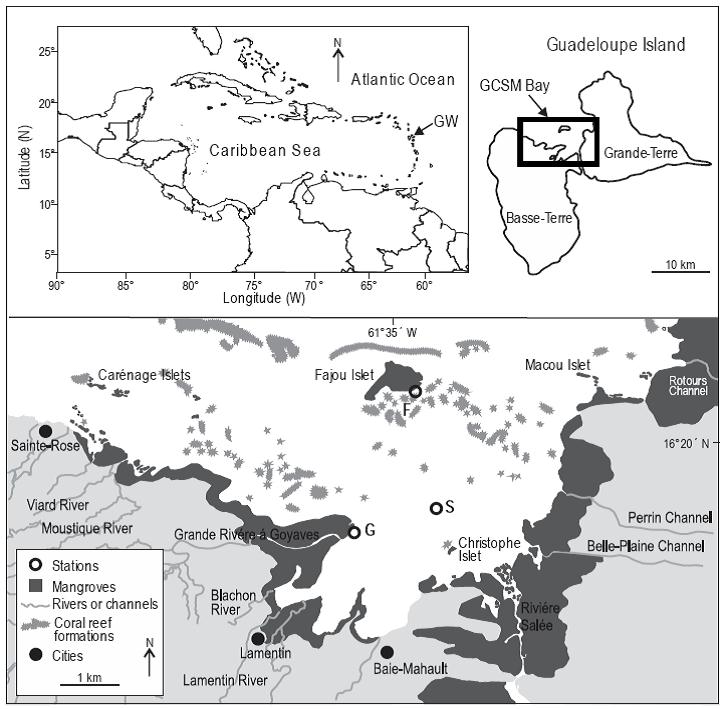
The study was carried out in Grand Cul-de-Sac Marin (GCSM), a bay on Guadeloupe Island (Lesser Antilles) (Fig. 1). A barrier reef encloses this bay and delimits a lagoon of 11,000 ha. The shoreline is fringed by Rhizophora mangle (Linnaeus 1753) mangroves with prop-roots always inundated. The GCSM lagoon presents large areas of seagrass beds, mainly composed of Thalassia testudinum Banks and Soland ex König, 1805 and Syringodium filiforme (Kütz 1860), covering about 8200 ha (Chauvaud et al. 2001). With the low tidal amplitude (0.5 to 0.6 m) occurring in the area (Assor 1988), mangroves are permanently flooded. Water depth varies from a few decimeters on shallows and coral keys to 30 m in reef passes. Salinity in the GCSM lagoon varies between 20.1 near river mouths to 36.5 around mangrove islets close to the barrier reef (Fajou and Carenage offshore islets; Vaslet et al. 2010). Three sites were studied between September and December 2007: Fajou offshore mangrove islet located near the barrier reef (site F), coastal fringing mangroves located close to the mouth of Grande Rivière à Goyaves (site G), and a seagrass bed in the middle of the GCSM lagoon (site S) (Fig. 1). The mangrove forest is more developed at site G than at site F, with a forest width of 1.2 km and 760 m, respectively (Vaslet et al. 2010).
Study design
Fishes were collected from mangroves using fish traps (see description in Vaslet et al. 2010) and from seagrass beds with a seine net, composed of a bag (3 m long, 2 m high) with two wings (23 m long, 2 m high) (Bouchon-Navaro et al. 1992). Twelve transient fish species of similar size ranges were collected at the three sites. The fish species Bairdiella ronchus (Cuvier 1830) was also sampled to examine food source contributions to the diet of this resident species that spends its entire life cycle in mangroves (Louis 1985).
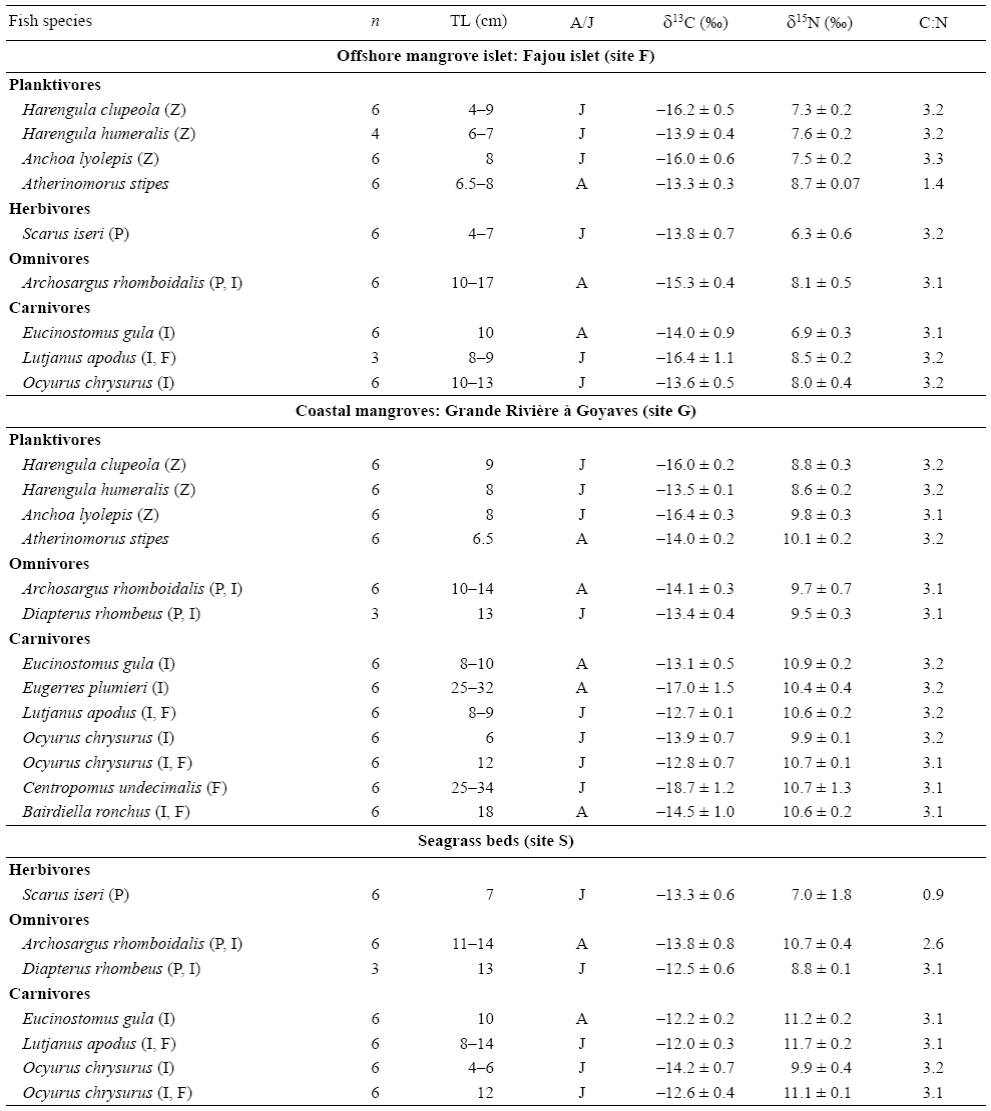
Fish species were assigned to four trophic guilds based on dietary analyses of fish gut contents (Table 1) and a review of literature on fish diets (Randall 1967, Austin and Austin 1971, Aguirre-León and Yáñez-Arancibia 1984, Bouchon-Navaro et al. 1992, Sierra et al. 1994, Nagelkerken et al. 2006, Chiaverini 2008): herbivores consuming algae, seagrass leaves, and their epiphytic algae; omnivores feeding on invertebrates and at least 10% of algae; planktivores consuming zooplankton; and carnivores ingesting benthic invertebrates and some fish species.
Table 1: Fish gut contents based on average frequency of occurrence (F%O) and gravimetric (F%W) or volumetric (F%V) estimates of each food category. Data indicate frequencies and the sum of each line can be >100%. The three main prey identified for each fish species are presented in this table.
The following fish prey were collected in mangroves and seagrass beds: mangrove litter, seagrass leaves, epiphytes scraped from seagrass leaves, algae, annelids, gastropods, amphipods, crabs, and shrimps. Samples of seston were collected above the seagrass site and between 10 and 20 m from mangrove fringes by towing a plankton net (100 μm mesh size) at 0.5 m below the water surface during 5 min. Zooplankton (dominated by copepods) was sorted from dead suspended materials at the laboratory.
Stable isotope analyses and mixing models
Muscle tissues of fish, decapods, and molluscs, and other whole prey were oven dried at 50 °C and ground to powder. As carbonate can interfere with organic carbon values (Bosley and Wainright 1999), calcified samples (i.e., algae, crustaceans) were decalcified with 10% HCl. As acid treatments have been reported to affect δ15N values, subsamples dedicated to δ15N analyses were not acidified (Sweeting et al. 2006). Stable isotope analyses of carbon and nitrogen were performed with a mass spectrometer (Optima; Isoprime, UK) coupled to a CNS elemental analyser (NA1200, ThermoScientific, European Union) at MARE Center, Liege University (Belgium). Isotopic ratios of carbon (δ13C) and nitrogen (δ15N) were expressed as the relative per mil (%) difference between samples and standards (Vienna PDB for carbon, atmospheric N for nitrogen). Average reproductibili-ties based on replicate measurements were 0.19% for δ13C and 0.15% for δ15N. Variation in lipid content among organisms can affect the δ13C values and consequently the ecological interpretations (Post et al. 2007). Thus, the mathematical normalization outlined by Post et al. (2007) for aquatic organisms was used to standardize for lipid content: δ13Cnormalized = δ13Cuntreated - 3.32 + 0.99 × C:N. Normalization of δ13C values was based on C:N values (i.e., C:N ratios > 3.5 for lipid-rich tissue) and they were calculated for gastropods, amphipods, annelids, and Majidae crabs (Table 2).
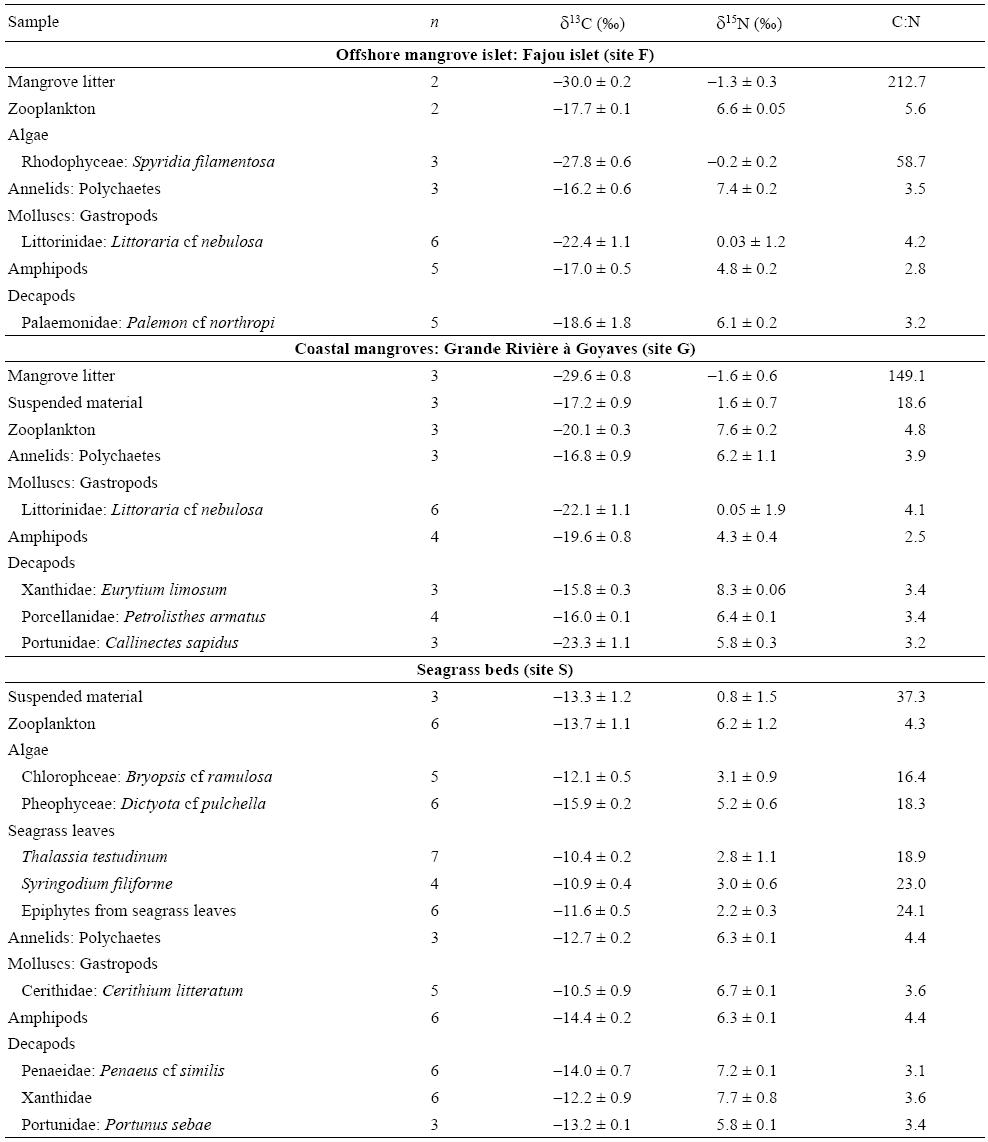
Table 2: Mean δ13C and δ15N values (± standard deviation) and C:N ratios of prey items sampled in mangroves and seagrass beds (n, number of samples). See figure 1 for location of sampling sites.
The SIAR (stable isotope analysis in R) mixing model was used to determine the relative contribution of prey from mangroves and seagrass beds to the fish diets (Parnell et al. 2010). This model has the advantage of taking into account uncertainties associated with isotopic values of consumers and prey and trophic enrichment (Parnell et al. 2010). The SIAR mixing model allows considering the relative contribution of groups of prey a posteriori. In this model, fish isotopic values were corrected for trophic enrichment: ΔC = 1.0 ± 0.3%% and ΔN = 2.2 ± 0.3%% for planktivores, herbivores, and omnivores, and ΔC = 0.5 ± 0.1% and ΔN = 3.4 ± 0.2% for carnivores (Vander Zanden and Rasmussen 2001, McCutchan et al. 2003).
Statistical analyses
Statistical differences in δ13C and δ15N values were tested using nonparametric tests (Mann-Whitney and Kruskal-Wallis tests) to overcome assumptions for normality and homogeneity of variance (Legendre and Legendre 1998). Spearman rank correlation coefficients between δ15N and total length were computed using data from fish species in order to observe whether ontogenetic changes in diet could be detected. A significance level of P < 0.05 was used in all analyses. Statistical analyses were performed using XL-STAT and the SIAR package was run in R2.15.0 (R Development Core Team 2012).
Results
The prey collected from magroves were more depleted in 13C (δ13Cmean = -20.4 ± 1.2%) than those collected from seagrass beds (δ13Cmean = -12.7 ± 0.5%) (Mann-Whitney test, P < 0.001) (Table 2; figs. 2, 3). Mangrove invertebrates presented δ13C values varying between -25.1% (Littorinidae gastropods) and -15.5% (amphipods) (Table 2; figs. 2, 3). On average, the mangrove invertebrates that were the most depleted in 13C were Portunidae crabs and Littorinidae gastropods (mean δ13C values of -23.3% and -22.3%, respectively; Table 2).
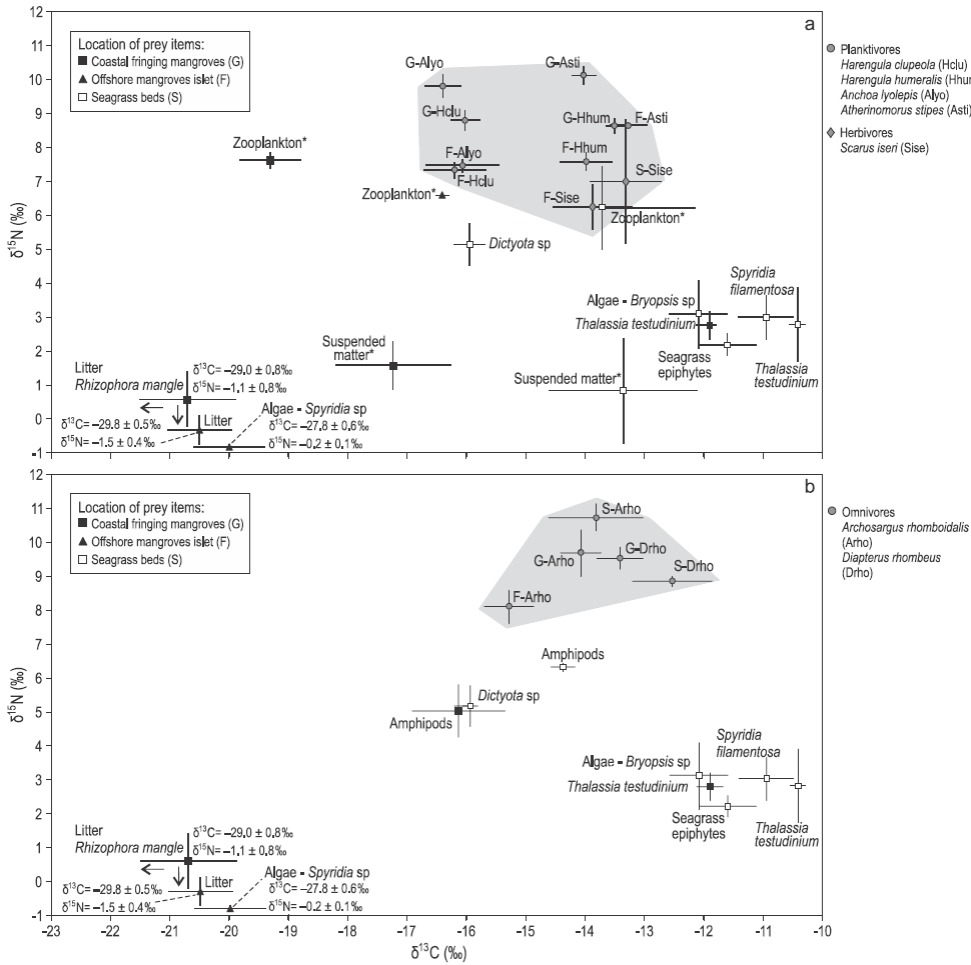
Figure 2: Mean δ13C and δ15N values (± standard deviation) of (a) planktivorous and herbivorous fishes and (b) omnivorous fishes (grey areas) and their prey sampled in mangroves and seagrass beds. The asterisks indicate potential prey of planktivorous fishes.
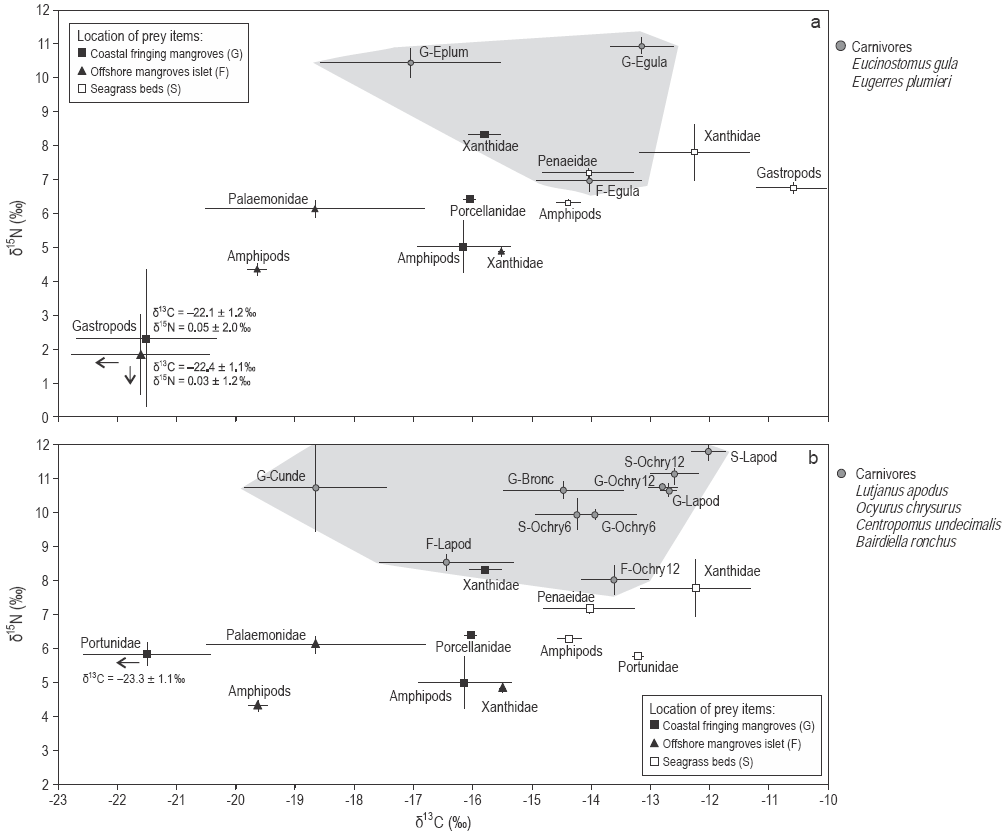
Figure 3: Mean δ13C and δ15N values (± standard deviation) of the carnivorous fishes (a) Eugerres plumieri and Eucinostomus gula and (b) Lutjanus apodus, Ocyurus chrysurus, Centropomus undecimalis, and Bairdiella ronchus (grey areas) and their prey items sampled in mangroves and seagrass beds.
Mean δ15N values were lowest for primary producers, such as mangrove litter, algae, seagrasses, and epiphytic algae (-1.5% to 5.2%); intermediate (4.5% to 6.3%) for amphipods; and higher (5.8% to 8.3%) for other invertebrates, such as zooplankton, annelids, molluscs, and decapods (Table 2). Littorinidae gastropods from mangroves were characterized by much lower mean δ15N values (0.04%) compared to other invertebrates (Table 2). Elemental ratios (C:N, w:w), which are indicative of prey nutritional values in terms of digestibility, presented lower values in animal food sources (C:Nmean = 3.9 ± 0.1) than in plant items (C:Nmean = 43.3 ± 16.3), testifying the high nutritive quality of the former (Mann-Whitney test, P < 0.001) (Table 2).
Mean δ13C values for the 12 transient fish species collected in mangroves and seagrass beds ranged from -18.7 ± 1.2% for the snook Centropomus undecimalis (site G) to -12.0 ± 0.3% for the snapper Lutjanus apodus (site S) (Table 3). On average, fish species collected in seagrass beds (site S) were more enriched in 13C (δ13Cmean = -13.0 ± 0.3%) than species from the mangrove sites (sites F and G, -14.9 ± 0.4% and -14.7 ± 0.5%, respectively) (Table 3). The δ15N values were lower for herbivores; intermediate for plankti-vores, omnivores, and carnivores foraging exclusively on invertebrates; and higher for carnivores consuming both invertebrates and fishes (Table 3).
Table 3: Mean δ13C and δ15N values (± standard deviation) and C:N ratios of fish species sampled in mangroves and seagrass beds (see Fig. 1 for location). Abbreviations: n, number of samples; TL, total length; A/J, adult or juvenile specimens. Fish diets are indicated after the name of the species: P, plant material; Z, zooplankton; I, invertebrates; F, fish.
As δ13C values significantly differed between food sources from mangroves and seagrass beds, the relative importance of these prey items in the fish diets could be assessed. Three out of 12 fish species had depleted δ13C values that are closer to those of mangrove prey: the plankti-vores Anchoa lyolepis (sites F, G) and Harengula clupeola (sites F, G), and the carnivore C. undecimalis (site G). Specimens of L. apodus from all sites had C signatures closer to those of prey from seagrass beds (Fig. 3). The values of both δ13C and δ15N for Ocyurus chrysurus increased between small (6 cm) and large specimens (12 cm; Spearman rank correlation, P < 0.0001) (Table 3; Fig. 3b), suggesting different diets for this fish species depending on specimen sizes (Fig. 3).
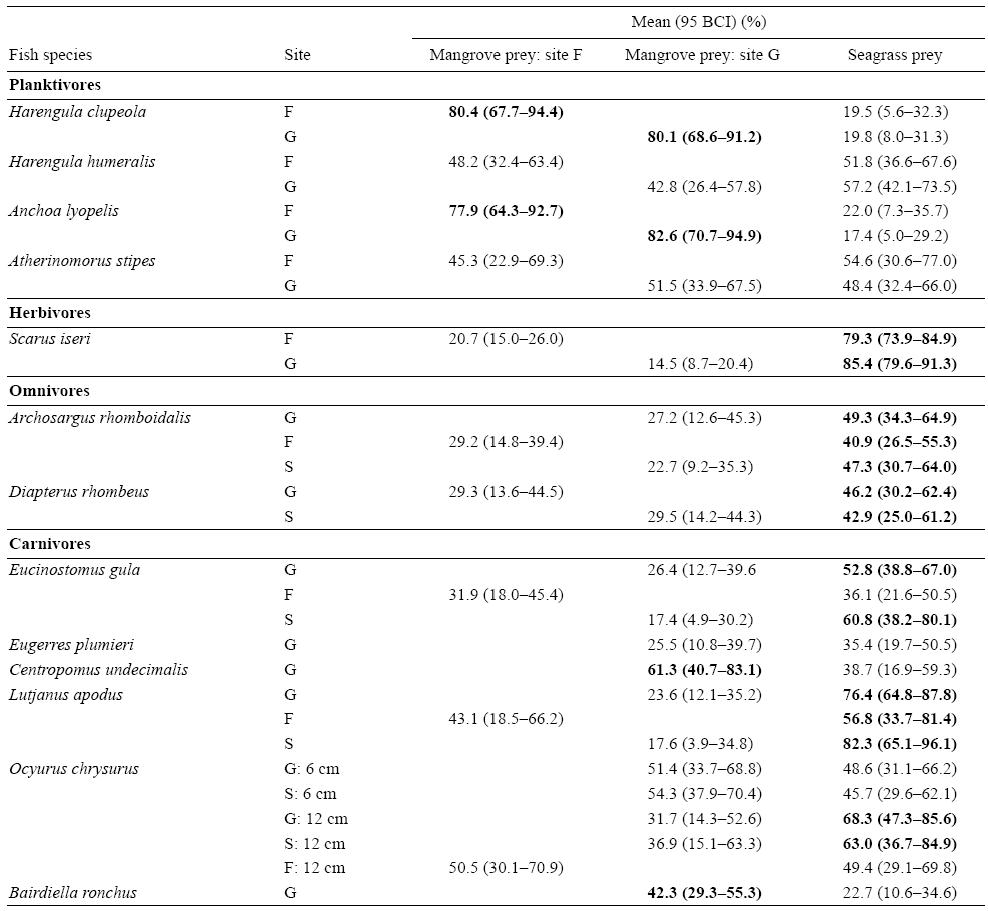
The SIAR mixing models showed that the two plankti-vores A. lyolepis and H. clupeola presented higher mean contributions (95% Bayesian confidence intervals [BCI]) of mangrove food sources in their diets (78-83%) than the other two planktivorous species, Harengula humeralis and Atherinomorus stipes, which relied equally on mangrove and seagrass bed food sources (Table 4). The carnivores C. undecimalis and the mangrove resident fish species B. ronchus presented mangrove prey mean contributions (95% BCI) of 61.3% (40.7-83.1%) and 42.3% (29.3-55.3%), respectively (Table 4). In contrast, the herbivore Scarus iseri, the omnivores Archosargus rhomboidalis and Diapterus rhombeus, and the carnivores Eucinostomus gula, L. apodus, and the large-sized juveniles of O. chrysurus relied more on seagrass prey than on mangrove prey, regardless of the sampling site (Table 4). However, the low contribution percentages obtained for the omnivores and the resident carnivore B. ronchus indicated that some additional prey were missing in the model. A slight increase in the proportion of seagrass prey items in the diet of O. chrysurus was observed from small specimens (95% BCI of seagrass prey varying between 29.6% and 62.1%) to larger ones (95% BCI of seagrass prey varying between 29.1% and 85.6%) (Table 4).
Table 4: Mean contributions (95% Bayesian confidence intervals [BCI]) of food sources from mangroves and seagrass beds in the fish diets. Bold numbers indicate the highest contributions of food sources. Sites: offshore mangrove islet (Fajou, F), coastal mangroves (Grande Rivière à Goyaves, G), and seagrass beds (S).
Discussion
Prey from mangroves are more depleted in 13C compared to those from seagrass beds (Kieckbusch et al. 2004, Nagelkerken et al. 2006, Lugendo et al. 2007), which allows assessing their relative importance in fish diets. The variations in carbon isotope values is due to different photosyn-thetic pathways and the different isotopic compositions in inorganic carbon used by primary producers (i.e., mangroves and seagrass beds) (Hemminga and Mateo 1996, Raven et al. 2002, Bouillon et al. 2008). This depletion in 13C is also observed for some invertebrates (e.g., annelids, amphipods, crabs) suggesting that they forage on mangrove resources (Christensen et al. 2001, Bouillon et al. 2002, Nagelkerken and van der Velde 2004b). Mangrove Littorinidae are characterized by low nitrogen values (Christensen et al. 2001, this study). This pattern could be due to the consumption of emerged epiphytes particularly depleted in 15N (Christensen et al. 2001, Bouillon et al. 2004). These epiphytes incorporate depleted nitrogen sources, such as N2 or volatilized ammonia coming from mangrove material remineralization (Bouillon et al. 2004, Fogel et al. 2008).
Two groups of transient fishes were distinguished in GCSM according to their δ13C: most of the fishes had enriched carbon values close to those of seagrass food sources and only a few specimens of transient fishes had relatively depleted carbon values, suggesting that these specimens complemented their diets with mangrove prey items. Mangrove food resources were consumed by mangrove resident species, such as B. ronchus, or by species that tolerate freshwater inputs and turbid waters, such as C. undecimalis (Louis 1985, Aguirre-León and Díaz-Ruiz 2000). Nutrient inputs and the abundance of mangrove infauna can explain the importance of mangrove prey in the diet of some transient fishes (Rodelli et al. 1984, Lugendo et al. 2007, Thollot et al. 1999).
For most juvenile transient fishes, seagrass beds represented their main feeding habitat even if the fishes sheltered in mangroves. These results concur with findings from studies performed in the Caribbean region highlighting that juvenile parrotfishes (Scaridae) shelter in mangroves and forage on epiphytic algae growing on seagrass leaves (Moncreiff and Sullivan 2001, Cocheret de la Morinière et al. 2003a, Nagelkerken et al. 2006). This feeding behavior could be due to high nutritional value and high productivity of epiphytic algae enhancing the vegetal food available to herbivores in seagrass beds (Montgomery and Gerking 1980, Moncreiff and Sullivan 2001).
Some transient fishes had different feeding habits according to the location of the mangrove site (offshore mangrove islet or coastal fringing mangroves). This can be due to day-night migrations during which fish species (such as Lutjanidae) forage in mangroves during the day and in seagrass beds at night (Nagelkerken and van der Velde 2004b, Nagelkerken et al. 2006). However, this does not seem to be the case in GCSM as few nocturnal migrations of fishes occur between mangroves and seagrass beds (Kopp et al. 2007), which concurs with the low contribution of mangrove prey to the diet of L. apodus (this study). Coastal mangroves can be considered open systems where the availability and exchange of allochthonous sources from seagrass beds, rivers, coral reefs or the lagoon in general can reduce the consumption of autochthonous food resources by transient species (Thimdee et al. 2004, Bouillon et al. 2008, Nyunja et al. 2009). However, it would be interesting to consider other sampling sites in fringing mangroves far from the river mouth to confirm this hypothesis.
Fishes can experience a change in diet during ontogenetic migrations between mangroves and seagrass beds (Nagelkerken and van der Velde 2004b, Lugendo et al. 2006). That was observed in the present study for O. chrysurus, with a slight decrease in mangrove food sources in the diets of large juveniles. Small juveniles of O. chrysurus foraged on zooplankton while larger juveniles consumed larger prey such as crabs and fishes (Cocheret de la Morinière et al. 2003b). This ontogenetic shift in the diet of O. chrysurus is confirmed by an increasing gradient in δ15N values, reflecting a variation of the trophic level of this fish species during its life-cycle migration (Cocheret de la Morinière et al. 2003b, present study), as commonly observed in previous studies (Frédérich et al. 2010).
This study revealed that juvenile transient fishes in mangroves mostly foraged in adjacent seagrass beds. Thus, mangroves act more as shelters than feeding grounds. These findings coincide with those of Thimdee et al. (2004), Lugendo et al. (2007) and Vaslet et al. (2012), who showed that mangrove fishes can adopt different feeding behaviors related to mangrove settings (i.e., shoreline mangrove, enclosed mangrove lagoons, and ponds) and prey availability. As mangroves and seagrass beds constitute important ecological fish habitats by acting as either shelters, nurseries or feeding areas, it is of primary importance to preserve these interlinked ecosystems to sustain coastal fisheries.











 texto en
texto en