Introduction
The yellowtail kingfish (YTK), Seriola lalandi, is a carnivorous marine species with high energy requirements (Pirozzi and Booth 2009). Due to its rapid growth (Moran et al. 2009) it is cultured worldwide, mainly in net cages in the sea (Fernandes and Tanner 2008), and there is increasing interest in farming them in recirculating aquatic systems (Abbink et al. 2011). Synthetic seawater has also been used for YTK culture (Orellana et al. 2013). The YTK commercial pellets are based on a high energy content ranging from 52% crude protein (CP) and 20% crude fat (CF) to 55% CP and 16% CF (Orellana et al. 2013). As with most diets for cultured fish species, efforts are being made to replace fish-derived ingredients such as fish oil and fish meal with poultry oil, canola oil, and soybean meal (Bowyer et al. 2012, 2013), among others. The need to reduce fish oil and fish meal is well known, and it has been a central point of research in fish nutrition for more than a decade (Tacon and Metian 2009, Hardy 2010).
Most research on the substitution of fish oil by vegetable oils has focused on the eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) requirements (Turchini et al. 2009, Nasopoulou and Zabetakis 2012), but not enough attention has been paid to cholesterol content. Fish oil is rich in cholesterol, with concentrations ranging from 5210 mg kg-1 in menhaden oil to 7660 g kg-1 in herring oil, whereas in vegetable oils phytosterols are present (Abidi 2001, Moreau et al. 2002), and cholesterol is not (Turchini et al. 2009). Thus, when fish oil is replaced by vegetable oil, meeting the essential fatty acid requirements is a concern, but supplying a minimum level of cholesterol is not, resulting in a cholesterol reduction in the aquafeeds without knowing the real effects of its deprivation.
Although fish can synthesize cholesterol (Deng et al. 2010) there is no concrete evidence of how much cholesterol or how efficiently the cholesterol can be synthesized nor of sterol metabolism in fish. Since cholesterol is being synthesized in fish, it is not considered an essential nutrient; however, fish like European sea bass (Dicentrarchus labrax) (Kaushik et al. 2004), gilthead sea bream (Sparus aurata) (Sitja-Bobadilla et al. 2005), and Atlantic cod (Gadus morhua) (Hansen et al. 2007) have shown signs of hypocho-lesterolemia when fish meal is reduced in their feeds and replaced by vegetable meals. Likewise, fish oil substitution by vegetable oil has induced the same effect in D. labrax (Richard et al. 2006), Atlantic salmon (Salmo salar) (Jordal et al. 2007), and YTK (Bowyer et al. 2012). On the other hand, when taurine is supplemented in aquafeeds for yellow-tail (Seriola quinqueradiata) the hypocholesterolemic effect is reduced (Maita et al. 2006).
In several marine fish species, taurine has been declared essential due to its low synthesis rate in the animals (Takagi et al. 2008), including YTK (Jirsa et al. 2014). In juvenile turbot (Scophthalmus maximus), the dietary addition of both taurine and cholesterol resulted in maximum growth as compared with those animals fed diets containing only taurine or cholesterol or neither of them. In this respect, Yun et al. (2012) proposed a synergic interaction between cholesterol and taurine. The lack of cholesterol has also been found to decrease the immunologic capacity of rainbow trout (Oncorhynchus mykiss) (Deng et al. 2013). Cholesterol and taurine supplementation also improved the resistance to bacterial infection of juvenile Seriola quinqueradiata artificially infected by Lactococcus garvieae (Maita et al. 2006).
Therefore, the aim of this study was to address the effect of four different cholesterol concentrations in aquafeeds on the growth performance of juvenile YTK, as well as the accumulation of cholesterol in the muscle and liver tissues.
Materials and methods
Dietary treatments
Four diets were formulated to contain a similar proximate composition (Table 1), consisting of 45% CP, provided by a mixture of soy protein concentrate and defatted fish meal. The CF concentration was 21%, and consisted of canola oil, olive oil, and cholesterol-free fish oil. Cholesterol (C8503, 92.5%, Sigma-Aldrich Corp. St. Louis, MO, USA) was added to the diets by its dissolution into the oil mixtures as follows: diet Ch0.0, no cholesterol was added; diet Ch0.05, 5.8023 g for each 10 kg of feed; diet Ch0.12, 12.5874 g; and diet Ch0.19, 19.3988 g. The identified sterols are shown in Figure 1. Starch and gelatin were cooked separately and added to the ingredients, and mechanically blended to produce a homogeneous mixture with 60% moisture content. The resulting mixture in the form of dough was then cold-pressed through a meat grinder into 4.0-mm diameter pellets, from which pieces were cut and then dried at 65 °C for 24 h. The feed was then stored at -30 °C until the day used for feeding.
Table 1 Ingredients and proximate composition on a dry weight basis of four experimental diets containing different concentrations of cholesterol. The four diets were fed to juvenile yellowtail kingfish (Seriola lalandi) for 60 days.
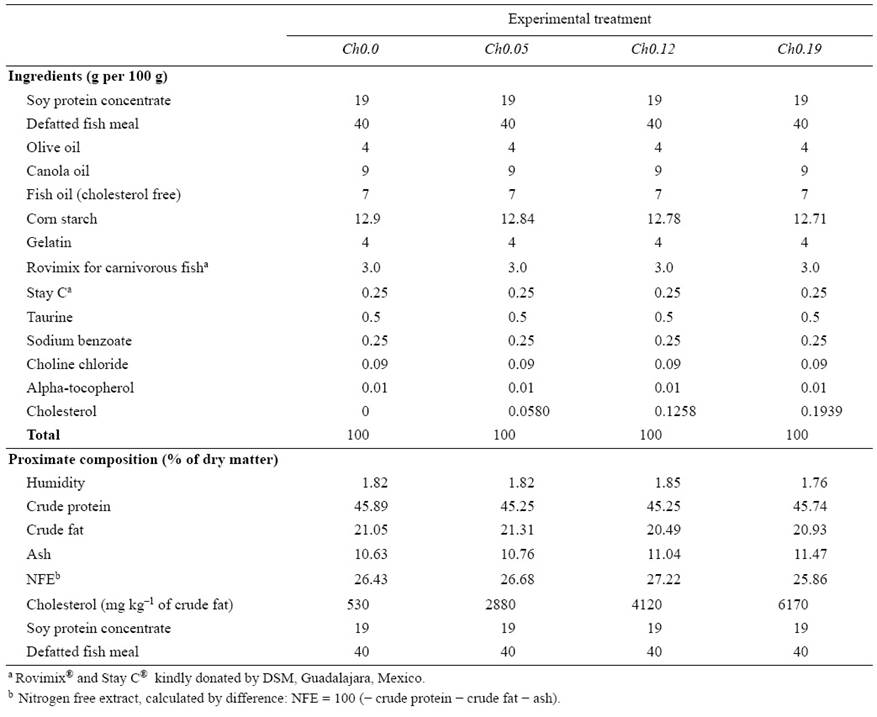

Figure 1 Sterols identified in the experimental diets formulated to contain four cholesterol levels (diets Ch0.0, Ch0.05, Ch0.12, and Ch0.19 containing 530, 2880, 4120, and 6170 mg kg-1 of crude fat, respectively). I.S. = internal standard (5a-cholestane).
The fish meal used in the diets was cold defatted using hexane as the carrier solvent. Batches of 5 kg of fish meal were soaked in 10 L of technical grade hexane (1:2 fish meal:hexane, w:v) for 24 h in 20-L buckets using a cheese cloth to retain the fish meal. After 24 h the fish meal was drained by hanging the cheese cloth and wrung by hand to release the excess of hexane. The fish meal was returned to the buckets and 5 L of hexane was added to the already washed batch and soaked for an additional 24 h to repeat the washing cycle. The fish meal batches were given a third wash with an additional 5 L of hexane, for a total of 20 L of hexane used for each 5 kg of fish meal. After the final wash cycle the fish meal was transferred to plastic trays and dried at ambient temperature using an extraction hood to assure the complete evaporation of the solvent residues.
The cholesterol from fish oil was removed taking advantage of its affinity for phospholipids. Granular soy lecithin was used as the source of phospholipids. Lecithin paste was prepared by mixing granular soy lecithin and distilled water (1:1 w:v). Batches of 900 g of fish oil were mixed with 90 g of lecithin paste in a food processor (3/4 HP motor). The resulting mixture was poured into Falcon tubes and centri-fuged at 4 rcf for 10 min, favoring the precipitation of the cholesterol-enriched lecithin paste. The fish oil was poured back into the food processor and the process was repeated for two more extraction cycles always using fresh lecithin paste.
The cholesterol-enriched lecithin paste was discarded after each centrifugation.
Experimental design
A total of 84 juvenile YTK (125.0 ± 0.2 g) obtained from a commercial farm (Baja Seas, SA de CV, Ensenada, BC, Mexico) were randomly distributed in twelve 500-L tanks (seven fish per tank) connected to a biofilter under a recirculation system with 5% water renewal every day. Water was maintained at 21.0 ± 1.0 °C by heating the water in a reservoir after filtration through the biofilter. The fish were hand-fed until apparent satiation four times a day (8:00, 11:00, 14:00 and 17:00 h) during 60 days. All treatments were performed in triplicate. At the end of the experimental period, fish were fasted for 24 h prior to sacrifice by immersion in ice-cold water (hypothermia) in 200-L plastic tanks in accordance with our institution's health and safety policy. Liver and muscle tissue were obtained from five random fish per tank. Samples were individually frozen at -30 °C until further analysis. The following indices were calculated to evaluate growth performance:
A quadratic regression model was applied to growth data to estimate a preliminary cholesterol requirement for the basal diet presented. The requirement was estimated as Imax x 95%.
where R is the final body weight (g) and I is the cholesterol concentration (mg kg-1 of CF).
Proximate analysis
The proximate composition of diets was measured (in triplicate) and expressed on a dry matter basis according to standard procedures (AOAC 1990). The moisture content of each diet was calculated from samples (2 g) dried to constant weight at 60 °C. Total nitrogen content was determined by the micro-Kjeldahl method, and percent CP was then calculated as % N x 6.25. Total fat concentration was determined by Soxhlet extraction with petroleum ether as a solvent and the CF was calculated gravimetrically. Ash content was determined by heating samples to 550 °C for 6 h. Nitrogen-free extract was calculated by difference:
Lipid extraction
Sterols were extracted from liver and muscle samples according to the AOAC (1990) methods. Tissue samples were dried at 100 °C for 24 h and ground with a hand mortar. Fat sterols were extracted following a modified Bligh and Dyer (1959) method, substituting chloroform with dichlo-romethane. An aliquot of 5a-cholestane dissolved in methanol was added to the samples as an internal standard prior to lipid extraction. Total lipid extracts were saponified with 1mL o 1.0 N potassium hydroxide (90% ethanol, 10% distilled water) at 70 °C for 30 min in capped glass vials. After cooling to ambient temperature, 1 mL of distilled water was added and the non-saponifiable fraction was extracted with 400 µL of hexane, a procedure that was repeated three times. Hexane was evaporated under nitrogen atmosphere and the dried samples were silylated with HTP (hexamethyldisila-zane:trimethylchlorosilane:pyridine, 2:1:5, v:v:v); 700 of HTP were added to each sample, and incubated at ambient temperature for 15 min in a nitrogen atmosphere. Residual pyridine was evaporated under nitrogen flow and the samples were diluted in hexane for gas chromatography-flame ioniza-tion detection (GC-FID) analysis.
Gas chromatography analysis
Gas chromatography was performed with an Agilent Technologies 6850 Network GC System (flame ionization detector) and an Agilent Technologies HP-1 capillary column (100% dimethylpolysiloxane, 30 m, 0.320 mm internal diameter, 0.25 um film thickness). The oven temperature conditions were as follows: initial 150 °C, held 3 min; initial 150 °C, held 3 min; raised to 200 °C at a rate of 25 °C min-1; to 280 °C at 5 °C min-1; and to 295 °C at 1 °C min-1. Hydrogen was used as carrier gas at an initial pressure of 20 psi, held 2 min, and 40 psi for the rest of the run. The injector was set at 280 °C and the flame ionization detector was set at a temperature of 330 °C, with a flow of 45 mL min-1 for hydrogen and 450 mL min-1 for air; 20 mL min-1 for column + makeup gas. The injection volume was 1 uL and the samples were manually injected in splitless mode. To identify the sterol content in tissues, five random samples were injected under similar conditions into a gas chromatograph mass spectrometer (GC-MS, Agilent Technologies 7890A GC System) with a DB-5 capillary column (5% diphenyl, 95% dimethylpolysiloxane, 30 m, 0.250 mm internal diameter, 0.25 um film thickness) using the selective ion monitoring (SIM) method. The ions monitored were the ones reported by Ahmida et al. (2006).
Statistical analyses
Statistical analyses were performed using the software package SigmaPlot (v12.3). Weight gain (WG), final body weight, thermal growth coefficient (TGC) and sterol concentration in tissues were subjected to a one-way analysis of variance. When needed, a Tukey test was applied to address the differences among groups.
Results
After 60 days of the feeding trial, no significant differences were found among groups regarding fish survival rate, although only groups Ch0.05 and Ch0.12 achieved 100% survival (Table 2). The maximum growth was obtained with the Ch0.05 treatment. The WG of fish fed Ch0.05 was the highest with 186.2 ± 8.8 g (mean ± SD), and it was significantly (P < 0.05) different from Ch0.0 (145.3 ± 16.7 g) and Ch0.19 (142.1 ± 16.5 g), but not from Ch0.12 (162.8 ± 6.9 g). Final body weight followed the same pattern, with Ch0.05 presenting the highest mean value (311.4 ± 9.4 g), which was significantly (P < 0.05) different from Ch0.0 (270.3 ± 15.5 g) and Ch0.19 (267.0 ± 16.4 g), but not from Ch0.12 (287.6 ± 6.4 g).
Table 2 Biological indices, survival rate, and cholesterol concentration in liver and muscle tissues of juvenile yellowtail kingfish (Seriola lalandi) fed four different cholesterol concentrations for 60 days (mean ± standard deviation). Values within the same row with different superscripts indicate significant differences (P < 0.05).

The TGC was significantly (P < 0.05) higher for Ch0.05, with a value of 1.41 ± 0.05 (mean ± SD), which was different from Ch0.0 and Ch0.19, but not from Ch0.12. No statistical differences were found among the other treatments, with TGC values of 1.16 ± 0.11 for Ch0.0, 1.27 ± 0.05 for Ch0.12, and 1.14 ± 0.11 for Ch0.19. The Imax value calculated with the quadratic regression for the cholesterol concentration (Fig. 2) was 3179 mg kg-1 of CF. Considering 95% of such value, the estimated requirement should be 3020 mg kg-1 of CF.

Figure 2 Quadratic regression adjustment between cholesterol concentration in the dietary treatments and final body weight of Serióla lalandi after 60 days of feeding experimentation.
Total cholesterol in the liver and muscle samples showed no significant variations among treatments (Table 2). Nevertheless, the cholesterol content in muscle tissue was noticeably lower in Ch0.19. The cholesterol precursors, lathosterol and desmosterol, were not detected in the liver samples (Fig. 3), or in the muscle tissue of any of the tissues analyzed by GC-MS. The phytosterols campesterol and p-sitosterol were detected by the GS-MS SIM method in the liver samples from all treatments even when the peaks were not clearly visible at plain sight (Fig. 3); such sterols, even when detected and identified, could not be quantified due to the lack of analytical standards. In the muscle samples, campesterol and P-sitosterol were not detected by the SIM method (Fig. 4).

Figure 3 Chromatogram obtained from liver tissue (sample from treatment Ch0.19). I.S. = internal standard (5a-cholestane).
Discussion
The growth rates obtained here and given as TGC values are similar in the case of treatments Ch0.0 (1.16 ± 0.11), Ch0.12 (1.27 ± 0.05), and Ch0.19 (1.14 ± 0.11) to the range of values (1.09 to 1.22) obtained by Orellana et al. (2013); however, the mean value for treatment Ch0.05 (1.41 ± 0.05) was significantly higher relative to the other treatments. Cholesterol supplementation in fish diets has resulted in different effects on growth performance. A growth-promoting effect of cholesterol has been reported when the feed is based on high plant-protein content. Twibell and Wilson (2004) observed a significantly higher WG when 1% cholesterol was supplemented to a diet for channel catfish (Ictalurus punctatus) formulated with soybean meal as the only protein source. In the same study, there was a significant improvement in feed intake, WG, and specific growth rate when cholesterol was supplemented to a soy protein isolate diet. An improvement in WG and feed intake was also observed when 1% cholesterol was added to a diet with high amounts of plant protein (soybean meal and wheat gluten meal) for Scophthalmus maximus (Yun et al. 2011).
When the aquafeed is based on fishery ingredients, the opposite effect has been reported. Deng et al. (2010) found a significant reduction in WG and specific growth rate in the Japanese flounder (Paralichthys olivaceus) when cholesterol was supplemented to a fish-derived diet, consisting of fish protein concentrate or fish meal with 1% additional cholesterol. In a feed trial with hybrid striped bass (Morone chrysops x Morone saxatilis), the addition of 1% cholesterol to a fish meal diet decreased the WG and feed efficiency ratio, but not significantly (Sealey et al. 2001). In a study using Salmo salar, however, the addition of 1% cholesterol to a fish meal diet did not show significant differences in growth performance when compared with a diet without supplemented cholesterol, but cholesterol increase in feces and accumulation in liver tissue were observed (Bjerkeng et al. 1999). Nevertheless, when defatted fish meal was used to feed Scophthalmus maximus, the supplementation of 1% cholesterol failed to significantly improve the feed intake, but an increase in WG was observed (Zhu et al. 2014).
In the present work, growth decreased when fish were fed the lowest (diet Ch0.0, 530 mg kg-1 of CF) and highest (diet Ch0.19, 6170 mg kg-1 of CF) cholesterol concentrations compared to the treatment Ch0.05 (2880 mg kg-1 of CF). This result was unexpected because the cholesterol concentration of diet Ch0.19 resembles the cholesterol content that would be present if the diet was formulated using fish meal as the sole protein source without removing the fat and fish oil. In YTK, one of the uses of cholesterol, among others, is in the synthesis of bile salts, and in these fish the conjugation of bile acids occurs only with taurine and not with glycine-conjugated bile salts as has been reported for other organisms (Russell 2003). Therefore, it can be assumed that in Seriola species, as reported for S. quinqueradiata (Khaoian et al. 2014), the only cholesterol-degradation pathways available are taurocholic acid and chenodeoxycholytaurine.
Several studies have pointed out that higher dietary cholesterol concentrations promote the gene CYP7A1 expression (Yun et al. 2011, 2012; Zhu et al. 2014) that synthesizes the protein cholesterol-7a-hydroxylase, a rate-limiting enzyme responsible for the bile acid synthesized from cholesterol (Matsumoto et al. 2005). In a feed trial with juvenile S. quinqueradiata fed fish meal and soybean meal diets supplemented with or without cholesterol and/or taurine (Khaoian et al. 2014), hepatic cholesterol accumulation was significantly higher in fish fed the diets lacking taurine than in those fed the taurine-supplemented diets. Reduced growth and bile salt synthesis rates were also found in fish fed diets without taurine supplementation. When taurine was supplemented, no cholesterol accumulation was detected and the bile salt synthesis rate and excretion improved.
Comparing these findings with the results of the present study, it is likely that the higher cholesterol concentration in diet Ch0.19 stimulated the synthesis of bile salts in juvenile YTK. This process would consume both cholesterol and taurine with an inevitable reduction in both nutrients. It has already been pointed out that taurine is an essential nutrient for YTK to sustain optimal growth (Jirsa et al. 2014). Though the synthesis of bile acids and nutrient digestibility were not evaluated, restrictions in taurine availability due to bile acid synthesis may have affected the growth of fish fed diet Ch0.19. This hypothesis can be supported by the negative correlation between the cholesterol concentration in the diet and muscle tissues observed in the present work, although no significant differences were observed. This could be due to the reverse cholesterol transport of conjugated high density lipoproteins (HDL) (Chen et al. 2003), which mobilizes the cholesterol from peripheral tissues into the liver. HDL-cholesterol would then be reabsorbed by the liver and secreted in bile as free cholesterol and bile salts by the ATP-binding cassette transporters G5 and G8 (ABCG5 and ABCG8) and the bile salt export pump (van der Wulp et al. 2013). The liver cholesterol concentration would thus not vary and the cholesterol content in muscle would decrease depending on how much cholesterol is required to synthesize the bile salts in the liver.
An important amount of energy expenditure is required for cholesterol biosynthesis (Haines 2001, Norambuena et al. 2013). In a recent study with O. mykiss (Norambuena et al. 2013), a diet supplemented with 0.1% cholesterol failed to improve the growth performance of fish compared to a similar diet without the supplement. However, despite the growth results reported, differential energy expenditure was attributed to the cholesterol biosynthesis by observing that fish fed the lower cholesterol concentration had a higher fatty acid p-oxidation rate. Since the muscle and the whole-body cholesterol concentrations did not differ between the experimental groups, it was suggested that cholesterol biosynthesis was compensating for the dietary deficiency (Norambuena et al. 2013).
In our study, the fish fed diet Ch0.0 presented a liver cholesterol concentration similar to that of fish fed the other experimental diets, whereas the cholesterol concentrations in muscle tissue were slightly, though not significantly, higher. Considering the conclusions reported by Norambuena et al. (2013), the lower growth of fish fed diet Ch0.0 could be attributed to the energy spent on the cholesterol biosynthesis necessary to compensate for the dietary deficiency in order to maintain the cholesterol content needed as observed in the rest of the treatments.
Zhu et al. (2014) concluded that even if juvenile Scophthalmus maximus were able to synthesize cholesterol, it was not enough to maximize growth, suggesting the need for supplementation. The same authors mention that excess cholesterol could be even harmful to fish health despite promoting higher weights. When cholesterol is provided at higher concentrations than needed, a higher concentration in feces is observed (Bjerkeng et al. 1999; Deng et al. 2010; Yun et al. 2011, 2012; Zhu et al. 2014), turning into a potential risk factor for health, like promoting atherosclerotic lesions (Deng et al. 2010). Here, cholesterol accumulation was not observed either in muscle or liver tissues, a result that suggests that the cholesterol concentrations used were not excessive for this fish species (up to 6170 mg kg-1 of CF). Cholesterol is absorbed in the proximal intestine due to the activity of the membrane transporter Niemann-Pick C1-Like 1 (NPC1L1) located in the apical zone of the enterocyte membrane (Altmann et al. 2004), a transporter that also works with phytosterols (Davis and Altmann 2009, Davis et al. 2004). Enterocytes also have the capacity to secrete cholesterol into the lumen, a process known as transintestinal cholesterol excretion. This occurs through the ATP-binding cassette transporters G5 and G8 (ABCG5 and ABCG8) (Yu et al. 2002a, 2002b), a process that has also been reported for phytosterols (Berge et al. 2000).
We conclude that the variations in the cholesterol content in partially-substituted diets for YTK have a significant effect on growth performance. When substituting fish meal and fish oil, it is important that proper cholesterol sources be found to blend together with vegetable oil as long as the other nutrients are present.
Acknowledgments
The authors wish to thank the National Council for Science and Technology (CONACYT, Mexico) for the scholarship granted to FMGO; José Vinicio Macias Zamora and Nancy Ramirez Alvarez for their support in the GC-MS analysis of samples and for providing all the necessary equipment; Juan Pablo Lazo Corvera for his help on YTK nutritional requirements; and Baja Seas, SA de CV, for providing the juvenile YTK.











 nueva página del texto (beta)
nueva página del texto (beta)








