Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Ciencias marinas
versão impressa ISSN 0185-3880
Cienc. mar vol.39 no.4 Ensenada Dez. 2013
Difference in reproductive strategies of two scleractinian corals (branching vs massive) along the west coast of Mexico
Diferencia en las estrategias reproductivas de dos corales escleractinios (ramificado vs masivo) a lo largo de la costa occidental de México
Héctor Efraín Chávez-Romo1,2, David Arturo Paz-García3*, Francisco Correa-Sandoval2, Héctor Reyes-Bonilla4, Ramón Andrés López-Pérez5, Pedro Medina-Rosas6
1 Facultad de Ciencias Marinas, Universidad Autónoma de Baja California (UABC), Carretera Transpeninsular Ensenada-Tijuana No. 3917, Fraccionamiento Playitas, Ensenada 22860, Baja California, México.
2 Instituto de Investigaciones Oceanológicas, Universidad Autónoma de Baja California, Carretera Transpeninsular Ensenada-Tijuana No. 3917, Fraccionamiento Playitas, Ensenada 22860, Baja California, México.
3 Laboratorio de Genética para la Conservación, Centro de Investigaciones Biológicas del Noroeste (CIBNOR), Instituto Politécnico Nacional 195, Colonia Playa Palo de Santa Rita Sur, La Paz 23096, Baja California Sur, México. *Corresponding author. E-mail: dpaz@cibnor.mx.
4 Departamento de Biología Marina, Universidad Autónoma de Baja California Sur (UABCS), Carretera al Sur Km 5.5, La Paz 23280, Baja California Sur, México.
5 Instituto de Recursos, Universidad del Mar, Puerto Ángel 70902, Oaxaca, México.
6 Departamento de Ciencias, Centro Universitario de la Costa-Universidad de Guadalajara, Av. Universidad de Guadalajara No. 203, Delegación Ixtapa, Puerto Vallarta 48280, Jalisco, México.
Received January 2013,
received in revised form October 2013,
accepted October 2013.
ABSTRACT
This study addressed the relative contribution of sexual and asexual reproduction to the genetic composition of populations of two scleractinian corals, Pocillopora damicornis and Porites panamensis, off the west coast of mainland Mexico. Reproductive indexes showed that P. damicornis reproduced both sexually and asexually; P. panamensis reproduced sexually, but colonies with an asexual origin were also observed (10-30%). Asexual reproduction is usually attributed to fragmentation caused by hurricanes; however, no significant association between reproductive index values and frequency of hurricanes was observed for either species. Environmental conditions in the Gulf of California seem to be more favorable for sexual reproduction in both species than other parts of the west coast of Mexico. This study contributes baseline information of differences in sexual and asexual reproduction in massive and branching corals.
Key words: sexual reproduction, genotypic diversity, reproductive mode, genetic composition, Eastern Pacific.
RESUMEN
Se estudió la contribución relativa de la reproducción sexual y asexual a la composición genética de dos corales escleractinios, Pocillopora damicornis y Porites panamensis, de la costa occidental de México. Los índices reproductivos mostraron que P. damicornis presentó reproducción sexual y asexual; P. panamensis presentó reproducción sexual, pero también se observaron colonias que tuvieron un origen asexual (10-30%). La reproducción asexual es usualmente atribuida a la fragmentación por causa de huracanes; sin embargo, no hubo una asociación significativa entre los índices reproductivos de las dos especies y la frecuencia de huracanes. Las condiciones ambientales en el golfo de California parecen ser más favorables para la reproducción sexual en ambas especies que otras partes del Pacífico mexicano. Este estudio contribuye con información básica sobre las diferencias en reproducción sexual y asexual en las especies de coral masivo y ramificado.
Palabras clave: reproducción sexual, diversidad genotípica, modo reproductivo, composición genética, Pacífico oriental.
INTRODUCTION
Corals are long-lived organisms composed of thousands of individuals in a single colony that can potentially employ different strategies of sexual or asexual reproduction in their life history. Strategies of sexual and asexual reproduction of coral species are important for maintaining resilience of their populations; these strategies represent fundamental evolutionary processes resulting from environmental disturbances of natural or human causes (Harrison 2011). The contribution of sexual and asexual reproductive strategies can vary among populations of a single species across its range; in some species, the contribution can be approximately equal, while in others, one strategy can dominate in a specific area and time (Whitaker 2006, Starger et al. 2010).
During the life history of these long-lived organisms, sexual reproduction produces opportunities for generating new genetic combinations and opens possibilities for the dispersion of gametes and larvae within and between coral reefs or the colonization of new locations (van Oppen and Gates 2006). Asexual reproduction enables well-adapted local genotypes to increase the possibility of surviving disturbances and die-off events and expanding to nearby localities (Highsmith 1982, Baums et al. 2006). Asexual reproduction by fragmentation is advantageous because it provides a means of reaching sites where larvae are unable to settle (such as sandy areas at the periphery of a reef), it facilitates local dispersion and rapid occupation of space for the persistence of populations when conditions are unfavorable for sexual reproduction or larval recruitment, and it reduces the risk of genetic extinction (Highsmith 1982).
Branching corals are particularly susceptible to fragmentation from strong waves caused by hurricanes. Some corals appear to use fragmentation as part of their life histories after reaching a certain size (Highsmith 1982); however, fragmentation, as a reproductive strategy, is not exclusive to branched species. Long-lived massive corals, although less affected by storms, can be overturned or fragmented during these events (Lirman et al. 2001), and persist in local populations (Boulay et al. 2012). Genetic studies show that fragmentation contributes up to 25% of massive coral species (Miller and Ayre 2008). Thus, the evidence indicates that massive corals can propagate by asexual means, even though sexual strategies predominate (Miller and Ayre 2008, Boulay et al. 2012). To date, few massive coral species have been studied and no comparison has been made between species with different morphologies from the same sites.
Traditionally, the success of sexual reproduction has been addressed by following the presence or release of gametes and settlement of recruits (Harrison 2011, Schmidt-Roach et al. 2012), while the success of asexual reproduction by fragmentation has been addressed by counting fragments before and after disturbances and following the condition of naturally- or artificially-generated fragments (Smith and Hughes 1999). Genetic markers can be used to determine the genotypic diversity of a population, the number of distinct genets (genetically-different colonies originated by sexual means) or ramets (colonies generated by fragmantaion), giving the contribution of the reproductive strategy (sexual vs asexual reproduction) to the existing genetic structure of the populations (Aranceta-Garza et al. 2012, Pinzón et al. 2012).
After the 1997-1998 El Niño event, coral reefs along the west coast of mainland Mexico experienced massive die-offs, with losses of 60-90%; in the Gulf of California, die-offs were less than 18% (Reyes-Bonilla et al. 2002). Despite massive die-offs, coral reefs along the west coast of Mexico recovered by sexual reproduction, as suggested in histological (Chávez-Romo and Reyes-Bonilla 2007, Carpizo-Ituarte et al. 2011, Rodríguez-Troncoso et al. 2011) and recruitment studies (Medina-Rosas et al. 2005, López-Pérez et al. 2007). However, to understand the function of coral communities in reef recovery following disturbances, it is necessary to disentangle the importance of reproductive strategies in the genetic structure of the populations.
In this study, two scleractinian species, Pocillopora damicornis and Porites panamensis, were selected because they are abundant and widespread on the west coast of Mexico, display contrasting morphology, and have different reproductive strategies (Chávez-Romo and Reyes-Bonilla 2007, Carpizo-Ituarte et al. 2011). Pocillopora damicornis is a branching and hermaphroditic species that reproduces sexually in the Gulf of California and Gulf of Panama by broadcast spawning (Glynn et al. 1991, Chávez-Romo and Reyes-Bonilla 2007), and is typically susceptible to fragmentation (fig. 1a). In contrast, P. panamensis is a massive and gonochoric species, sexually active along the west coast of Mexico and Panama (Glynn et al. 1994, Carpizo-Ituarte et al. 2011, Rodríguez-Troncoso et al. 2011). Asexual reproduction in massive corals is rare, but abrasion of whole colonies and multiple fragmentations of P. panamensis have been observed in the Gulf of California (fig. 1b). From this unusual situation, we explored the relative contribution of sexual and asexual reproductive strategies of P. damicornis and P. panamensis to the existing genetic structure of populations along the west coast of Mexico.
MATERIALS AND METHODS
Locations and collection
Collections were made in the most important regions of coral development off the west coast of mainland Mexico (Gulf of California, Bahía de Banderas, and Bahías de Huatulco; fig. 2). In the Gulf of California, samples of P. damicornis were collected at El Portugués (POR) and in the southern part of Bahía de La Paz (BLP); samples of P. panamensis were collected at Bahía de los Ángeles (BLA), Bahía Concepción (BCO), and BLP. Samples of both species were collected at Punta Arena de la Ventana (PAV), close to the entrance of the Gulf of California, and further south, at Isla Redonda (IRD) in Bahía de Banderas. In Bahías de Huatulco, P. damicornis was collected at Dos Hermanas (DOH) and La Entrega (LET); P. panamensis was collected only at LET. All coral samples were frozen in liquid nitrogen, transported to the laboratory, and stored at -80 °C.
Allozyme electrophoresis
Extractions of coral tissue were conducted in Stoddart's modified buffer (Stoddart 1983, Weil 1992) with a sonic dismembrator (model 100, Fisher Scientific, Waltham, MA). Then, 2 mL of the blastate was centrifuged at 2600 x g for 10 min at 4 °C. The resulting supernatant was placed in vials and stored at -80 °C until analysis. The samples were analyzed after protein separation by polyacrylamide gel electrophoresis at 4 °C by a discontinuous gel system under native conditions (Manchenko 1994) using 8% acrylamide. The multi-genotype of each colony was determined in four enzyme systems (five polymorphic loci) in both species (Paz-García et al. 2008b, Chávez-Romo et al. 2009). These enzyme systems were leucine-glycyl-glycyl peptidase (LGG-1, E.C. 3.4.11.1), malic enzyme (ME-1, E.C. 1.1.1.40), glutamate dehydrogenase (GDH-1 and GDH-2, E.C. 1.4.1.3), and esterase (EST-1 and EST-2, E.C. 3.1.1.1). Two loci were observed in the EST and GDH enzyme systems in P. damicornis and P. panamensis, respectively. Alleles at each locus were labeled alphabetically, based on decreasing mobility from the origin.
Reproductive strategies
We followed different approaches to estimate the contribution of sexual and asexual reproduction to the genetic structure. First, samples that had identical alleles at all five loci were identified as clone mates belonging to the same genet. Then, we obtained the number of unique multi-genotypes detected at each location (Ng) for each species. The probability of identity (PID) was calculated to give a conservative estimate of the probability that two individuals sampled in the same general location share a multi-locus genotype by chance, not by descent (Waits et al. 2001). An overall unbiased PID was calculated after sequentially multiplying PID values for all loci, using the GIMLET software (Valière 2002); low overall PID values for P. damicornis (PID = 2.9 × 10-3) and P. panamensis (PID = 0.88 ×10-3) indicated that the loci used in this study were accurate to assign ramets to clones.
Second, we calculated three reproductive indexes: (a) genotype richness (Ng:N), where Ng was calculated as described above and N is the number of individuals analyzed at a location; (b) genotypic diversity (GO:Ge), where Go is the observed genotype diversity and Ge is the expected genotype diversity; and (c) genotypic evenness (GO:Ng). Values close to one in the Ng:N and GO:Ge ratios indicate that sexual reproduction is dominant at the location; values close to zero indicate locations that have colonies with high levels of asexual reproduction (Stoddart and Taylor 1988, Ayre et al. 1997). The Go:Ge ratio was calculated using the equations of Stoddart and Taylor (1988). Values close to one in the GO:Ng ratio indicate locations where each genet is represented by equal numbers of ramets; values close to zero indicate locations with one or few dominant clones (Coffroth and Lasker 1998).
Cloning and frequency of disturbance
To test whether there is a relationship between the level of cloning and frequency of disturbance, we tabulated the number of hurricanes that occurred at each location from 1958 through 2006. Standard buffer zones around each location were defined according to storm strength: 35 km for category 1 and 2 hurricanes (maximum sustained winds of 120-176 km/h), 60 km for category 3 hurricanes (177212 km/h), and 100 km for category 4 and 5 hurricanes (>213 km/h) (Stoddart et al. 1985, Done 1992, Gardner et al. 2005). Data on occurrence and strength of hurricanes were obtained from the NOAA Historical Hurricane Tracks website (http://csc.noaa.gov/hurricanes/). Using this data set, a storm was considered if it entered a strength-specific buffer zone. Linear regressions were performed, where the number of storms approaching each location was the independent variable and the Ng:N, Go:Ge, and Go:Ng ratios were the dependent variables.
RESULTS AND DISCUSSION
Reproductive strategies of Pocillopora damicornis
Two hundred P. damicornis colonies were sampled and most colonies showed unique genotypes (Ng = 136). The average percentage of unique multi-genotypes (Ng) was 66.66 (± 16.45, SD), and the values at each location ranged from 35% to 83% (fig. 3). The Ng:N average was 0.67 (±0.16), ranging from 0.35 to 0.83 (table 1); observed and expected genotypic diversity (Go:Ge) averaged 0.67 ± 0.16, ranging from 0.33 to 0.82. The percentage of unique genotypes and reproductive indexes (Ng:N and Go:Ge) indicate that P. damicornis uses sexual and asexual strategies. The Go:Ng ratio indicated that each genet is represented by almost equal numbers of ramets (0.72 ± 0.20), with values ranging from 0.31 to 0.84 (table 1). This result indicates that most of the locations are dominated by a few clones (i.e., high sexual reproduction indicated by Go:Ng values close to one), whereas in DOH there is high asexual reproduction (Coffroth and Lasker 1998).
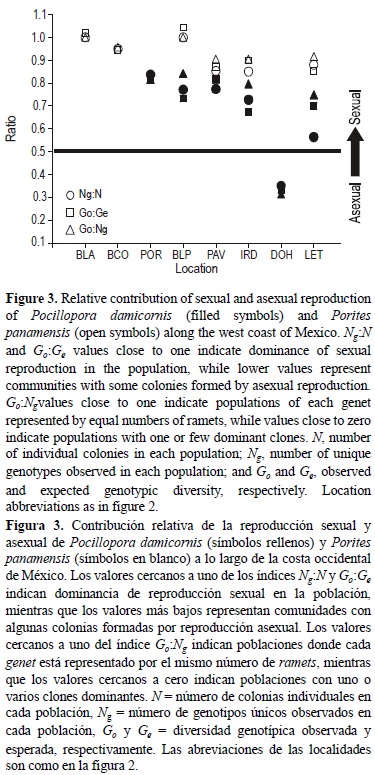
The highest numbers of clonal colonies (42-56%) were found in Bahías de Huatulco (DOH and LET), supporting earlier findings in the region. Previous histological studies based on surveys in Bahía de Banderas and Bahías de Huatulco over several years (2001-2004) did not detect mature gametes (only stages I-III), suggesting the predominance of asexual reproduction in these areas (Carpizo-Ituarte et al. 2011, Rodríguez-Troncoso et al. 2011). Interestingly, P. damicornis in Bahías de Huatulco had years of reproductive inactivity followed by periods of reproduction in subsequent years, but the causes for this have not yet been determined (Rodríguez-Troncoso et al. 2011).
Genetic studies of Pocillopora corals have shown evidence of asexual reproduction by fragmentation or by asexual larvae in Japan (Adjeround and Tsuchiya 1999), Taiwan (Yeoh and Dai 2010), the west coast of Australia (Stoddart 1983), and the Gulf of California (Aranceta-Garza et al. 2012, Pinzón et al. 2012), indicating the tendency of this group to form clonal communities. The contribution of asexual reproduction to the coral communities is usually attributed to fragmentation caused by storms and hurricanes, low frequency of sexual reproduction, and disturbance (Highsmith 1982, Hunter 1993).
From 1958 through 2006, in the Gulf of California 44 hurricanes impacted the surrounding waters of these coral communities; 21 were category 1 and 2, 12 were category 3, and 11 were category 4 and 5 hurricanes. Contrary to expectations, no significant association was found between the P. damicornis reproductive index values and frequency of hurricanes along the west coast of Mexico (table 2). Few studies have explored the association of hurricanes and reproductive indexes in corals from genetic studies (Baums et al. 2006, Aranceta-Garza et al. 2012) or reproduction effects in disturbed habitats (Hunter 1993). Similar to the findings in this study, Baums et al. (2006) found higher clonal structure of the branching coral Acropora palmata in the Caribbean and no correlation with the incidence of hurricanes.
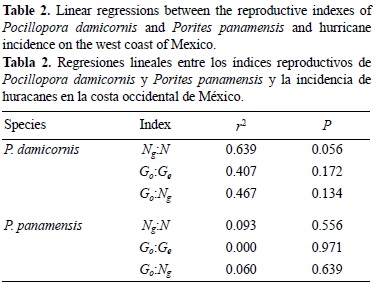
Aranceta-Garza et al. (2012) found a significant relationship between hurricane frequency and reproductive indexes of Pocillopora verrucosa in the Gulf of California (i.e., asexual reproduction by fragmentation). The differences in the results of their study and this study may be attributed to the following factors. First, P. verrucosa is more abundant than P. damicornis; in fact, some coral reef communities are composed almost entirely of P. verrucosa colonies (Aranceta-Garza et al. 2012, Paz-García et al. 2012a, Pinzón et al. 2012). Therefore, this species is more susceptible to fragmentation than P. damicornis. Second, during storms, large pieces of reef framework can be broken, coral colonies can be overturned or transported, and branches can be fragmented into smaller sizes (Lirman et al. 2001). Survival of fragments after disturbances is related to their size (Smith and Hughes 1999); thus bigger fragments have a better chance of survival. As P. verrucosa has wider and longer branches than P. damicornis, it may be more successful at asexual reproduction by fragmentation and hence present an association with storms.
The highest number of colonies with unique genotypes (72-83%) and the highest reproductive indexes in this study were found in the Gulf of California and Bahía de Banderas, suggesting that sexual reproduction plays a major role in these areas. These results are also supported by previous studies, which indicate higher recruitment levels of Pocillopora sp. in the Gulf of California (0.36 ind m-2 yr-1, table 3), compared to the tropical part of the west coast of Mexico (López-Pérez et al. 2007, Cabral-Tena 2012; table 3). Moreover, it has been suggested that there was an exchange of propagules between coral communities after the 1997-1998 El Niño in the southern Gulf of California and Bahía de Banderas (Paz-García et al. 2012b). These findings indicate more favorable environmental conditions for sexual reproduction for P. damicornis and other coral species in the Gulf of California, which has an extended warm season and relatively more stable oceanographic conditions than Bahías de Huatulco (Chávez-Romo and Reyes-Bonilla 2007, Cabral-Tena 2012, Paz-García et al. 2012b). Thus, coral communities from the Gulf of California represent an important resource for generating genetic diversity by sexual reproduction and exporting propagules for the recovery of damaged coral communities.
Pocillopora corals have been extensively studied, and the relative contribution of reproductive strategies has been inferred in different regions using genetic markers (Stoddart 1983, Adjeroud and Tsuchiya 1999, Miller and Ayre 2004, Sherman et al. 2006). In Japan, Taiwan, Australia, southwestern Mexico and the southern Gulf of California, asexual reproduction by fragmentation or the production of asexual larvae are the dominant reproductive strategy (Ward 1992, Adjeroud and Tsuchiya 1999, López-Pérez et al. 2007, Carpizo-Ituarte et al. 2011). In Indonesia, eastern Australia, and elsewhere in the Gulf of California, sexual reproduction is dominant (Sherman et al. 2006, Starger et al. 2010, Pinzón et al. 2012).
Additionally, histological studies have provided evidence that the reproductive strategy of P. damicornis varies (Glynn et al. 1991, Ward 1992, Chávez-Romo and Reyes-Bonilla 2007). Across its range, this species can spawn gametes for external fertilization or broadcast larvae (Stoddart 1983, Ayre and Miller 2004; see Harrison 2011 for a review). Shifts in modes of reproduction, from brooding larvae to free-spawning gametes, seem to occur in peripheral populations of Pocillopora off the coast of South Africa and the southwestern coast of Australia, and in the Red Sea and the Eastern Pacific (Glynn et al. 1991, Ward 1992, Chávez-Romo and Reyes-Bonilla 2007, Harrison 2011). However, different reproductive traits in P. damicornis across its distribution may be associated with cryptic species diversity (Schmidt-Roach et al. 2012). Recent studies show that morphological plasticity is higher than previously thought (Flot et al. 2008, Souter 2010, Pinzón and LaJeunesse 2011). This will require detailed and integrated taxonomic studies (morphological and host-symbiont genetics) to unambiguously recognize Pocillopora species. In the studied locations, Pocillopora corals comprise one genetic group (Pocillopora type 1, sensu Pinzón and LaJeunesse 2011).
Reproductive strategies of Porites panamensis
We sampled 139 colonies of P. panamensis; most of the colonies had unique genotypes (Ng = 127). The unique multi-genotypes ranged from 85% to 100% at each location (on average, 92.21 ± 6.4; fig. 3). The Ng:N average was 0.92 ± 0.06, ranging from 0.85 to 1.00 among locations (table 4). Observed and expected genotypic diversity (Go:Ge ratio) averaged 0.93 ± 0.07, ranging from 0.85 to 1.04. The Go:Ng ratio revealed that, on average, each genet is represented by an equal number of ramets (0.94 ± 0.04), ranging from 0.90 to 1.00 among locations (table 4); this result indicates high sexual reproduction, with Go:Ng values close to one (Coffroth and Lasker 1998). The percentage of unique genotypes and reproductive indexes showed that the massive coral P. panamensis mainly reproduced sexually along the west coast of Mexico, but also had low asexual reproduction at the southern locations. Recent studies of massive coral species show that fragmentation is higher than previously thought (Miller and Ayre 2008, Boulay et al. 2012). In this study, 24-30% of P. panamensis colonies at PAV, IRD, and LET originated by asexual reproduction. Similar percentages of asexual reproduction (8-25%) have been estimated for other four massive species: Porites lobata (Boulay et al. 2012), Montastraea annularis (Severance and Karl 2006), Montas-traea faveolata (Severance and Karl 2006), and Platygyra daedalea (Miller and Ayre 2008).
In the Gulf of California, detached fragments and whole P. panamensis colonies were observed (fig. 1). No significant association was found between the reproductive index values and frequency of hurricanes along the west coast of Mexico for P. panamensis colonies (table 2). Different processes may contribute to fragmentation in this species. For example, bioerosion by polychaetes, bivalves, and excavating sponges could make the coral skeleton more susceptible to fragmentation (Carriquiry and Reyes-Bonilla 1997, Reyes-Bonilla 2003), or differences in regeneration and partial mortality may cause fission of large colonies (Paz-García and Reyes-Bonilla 2006).
Histological studies of P. panamensis indicate that successful sexual reproduction is feasible along the west coast of Mexico (table 3). Release of larvae occurs most of the year in BLP (Gulf of California), but usually during summer (two-four months) in Bahía de Banderas and Bahías de Huatulco (Carpizo-Ituarte et al. 2011, Rodríguez-Troncoso et al. 2011, Paz-García et al. 2008a; see table 3). Other differences along the west coast of Mexico are that the Gulf of California had the highest Ng:N and Go:Ge ratios and number of oocytes per polyp (Mora-Pérez 2005, Carpizo-Ituarte et al. 2011, Rodríguez-Troncoso et al. 2011), suggesting that even with relatively large changes in environmental conditions over an annual cycle, this coral is well adapted for survival and reproduces under harsh conditions.
High recruitment ratios of Porites in the Gulf of California and Bahías de Huatulco (0.63-78.71 ind m-2 yr-1, see table 3) suggest recovery of coral reefs in other areas of the west coast of Mexico because their reproduction ratios are high (López-Pérez et al. 2007, Cabral-Tena 2012, Paz-García et al. 2012b). Differences in reproductive strategies of coral communities in the Gulf of California could have evolutionary significance because there is high reproduction potential when there are multiple larval releases. This may be a survival strategy under fluctuating environmental conditions. Long-term studies and more attention to massive species are necessary to understand the role of asexual reproduction and population dynamics among coral reefs.
In summary, this study provides genetic evidence of the importance of sexual and asexual reproduction in the reef corals P. damicornis and P. panamensis at diverse sites along the west coast of Mexico. These results support previous histological and recruitment studies in this region. Our findings indicate that locations in the Gulf of California have high levels of sexual reproduction, recruitment, and genotypic diversity of corals that could be important for recovery in other locations (Saavedra-Sotelo et al. 2011, Paz-García et al. 2012b). Further integrated research of coral communities would improve our understanding of population structure and reproductive response to global climate change in these ecosystems.
ACKNOWLEDGMENTS
This study was funded by the Project AWARE Foundation (to DAPG) and SEMARNAT project 2002-c01-0605 (to Luis Calderon of CICESE). HECR (#198888) and DAPG (#160065) are recipients of fellowship grants from the National Council for Science and Technology (CONACYT, Mexico). We thank the work group at the Parque Nacional Islas Marietas, and Diana Sánchez, Luis Lombardo, and Karina Xolaltenco (UABC) for their laboratory assistance. Salwa El Khattabi (UABCS) helped with the Spanish translation of the paper. Ira Fogel (CIBNOR) provided editorial services.
REFERENCES
Adjeround M, Tsuchiya M. 1999. Genetic variation and clonal structure in the scleractinian coral Pocillopora damicornis in the Ryukyu Archipelago, southern Japan. Mar. Biol. 134: 753-769. http://dx.doi.org/10.1007/s002270050592 [ Links ]
Aranceta-Garza F, Balart EF, Reyes-Bonilla H, Cruz-Hernández P. 2012. Effect of tropical storms on sexual and asexual reproduction in coral Pocillopora verrucosa subpopulations in the Gulf of California. Coral Reefs 31: 1157-1167. http://dx.doi.org/10.1007/s00338-012-0941-9 [ Links ]
Ayre DJ, Miller KJ. 2004. Where do clonal coral larvae go? Adult genotypic diversity conflicts with reproductive effort in the brooding coral Pocillopora damicornis. Mar. Ecol. Prog. Ser. 277: 95-105. http://dx.doi.org/10.3354/meps277095 [ Links ]
Ayre DJ, Hughes TP, Standish RJ. 1997. Genetic differentiation, reproductive mode, and gene flow in the brooding coral Pocillopora damicornis along the Great Barrier Reef, Australia. Mar. Ecol. Prog. Ser. 159: 175-187. http://dx.doi.org/10.3354/meps159175 [ Links ]
Baums IB, Miller MW, Hellberg ME. 2006. Geographic variation in clonal structure in a reef-building Caribbean coral, Acropora palmata. Ecol. Monogr. 76: 503-519. [ Links ]
Boulay JN, Cortes J, Nivia-Ruiz J, Baums IB. 2012. High genotypic diversity of the reef-building coral Porites lobata (Scleractinia: Poritidae) in Isla del Coco National Park, Costa Rica. Rev. Biol. Trop. 60: 279-292. [ Links ]
Cabral-Tena RA. 2012. Reclutamiento coralino en un arrecife restaurado en La Paz, BCS. In: Pérez-Ramírez M, Lluch-Cota SE (eds.), Biodiversidad y Vulnerabilidad de Ecosistemas Costeros en Baja California Sur. Centro de Investigaciones Biológicas del Noroeste, La Paz, BCS, México, pp. 331-345. [ Links ]
Carpizo-Ituarte E, Vizcaíno-Ochoa V, Chi-Barragán G, Tapia-Vázquez O, Cupul-Magaña AL, Medina-Rosas P. 2011. Evidence of sexual reproduction in the hermatypic corals Pocillopora damicornis, Porites panamensis, and Pavona gigantea in Banderas Bay, Mexican Pacific. Cienc. Mar. 37: 97-112. http://dx.doi.org/10.7773/cm.v37i1.1773 [ Links ]
Carriquiry JD, Reyes-Bonilla H. 1997. Estructura de la comunidad y distribución geográfica de los arrecifes coralinos de Nayarit, Pacífico de México. Cienc. Mar. 23: 227-248. [ Links ]
Chávez-Romo HE, Reyes-Bonilla H. 2007. Sexual reproduction of the coral Pocillopora damicornis in the southern Gulf of California, Mexico. Cienc. Mar. 33: 495-501. [ Links ]
Chávez-Romo HE, Correa-Sandoval F, Paz-García DA, Reyes-Bonilla H, López-Pérez RA, Medina-Rosas P, Hernández-Cortés MP. 2009. Genetic structure of a scleractinian coral, Pocillopora damicornis, in the Mexican Pacific. Proc. 11th Int. Coral Reef Symp., pp. 429-433. [ Links ]
Coffroth MA, Lasker HR. 1998. Population structure of a clonal gorgonian coral: The interplay between clonal reproduction and disturbance. Evolution 52: 379-393. [ Links ]
Done TJ. 1992. Effects of tropical cyclone waves on ecological and geomorphological structures on the Great Barrier Reef. Cont. Shelf Res. 12: 859-872. [ Links ]
Flot J-F, Magalo H, Cruaud C, Couloux A, Tillier S. 2008. Patterns of genetic structure among Hawaiian corals of the genus Pocillopora yield clusters of individuals that are compatible with morphology. C. R. Biol. 331: 239-247. [ Links ]
Gardner TA, Cote IM, Gill JA, Grant A, Watkinson AR. 2005. Hurricanes and Caribbean coral reefs: Impacts, recovery patterns, and role in long-term decline. Ecology 86: 174-184. http://dx.doi.org/10.1890/04-0141 [ Links ]
Glynn PW, Gassman NJ, Eakin CM, Cortés J, Smith DB, Guzmán HM. 1991. Reef coral reproduction in the eastern Pacific: Costa Rica, Panama, and Galapagos Islands (Ecuador). I. Pocilloporidae. Mar. Biol. 109: 355-368. http://dx.doi.org/10.1007/BF01313501 [ Links ]
Glynn PW, Colley SB, Eakin CM, Smith DB, Cortés J, Gassman NJ, Guzmán HM, Del Rosario JB, Feingold JS. 1994. Reef coral reproduction in the eastern Pacific: Costa Rica, Panama, and Galapagos Islands (Ecuador). II. Poritidae. Mar. Biol. 118: 191-208. http://dx.doi.org/10.1007/BF00349785 [ Links ]
Harrison PL. 2011. Sexual reproduction of scleractinian corals. In: Dubinsky Z, Stambler N (eds.), Coral Reefs: An Ecosystem in Transition. Springer, New York, pp. 59-85. [ Links ]
Highsmith R. 1982. Reproduction by fragmentation in corals. Mar. Ecol. Prog. Ser. 7: 207-226. [ Links ]
Hunter CL. 1993. Clonal diversity and population structure of scleractinian coral populations under different disturbance histories. Evolution 47: 1213-1228. [ Links ]
Lirman D, Glynn PW, Baker AC, Leyte-Morales GE. 2001. Combined effects of three sequential storms on the Huatulco coral reef tract, Mexico. Bull. Mar. Sci. 69: 267-278. [ Links ]
López-Pérez RA, Mora-Pérez MG, Leyte-Morales GE. 2007. Coral (Anthozoa: Scleractinia) recruitment at Bahías de Huatulco, western Mexico: Implications for coral community structure and dynamics. Pac. Sci. 61: 355-369. http://dx.doi.org/10.1353/psc.2007.0032 [ Links ]
Manchenko GP. 1994. Handbook of Detection of Enzymes on Electrophoretic Gels. CRC Press, Boca Raton, FL. 554 pp. [ Links ]
Medina-Rosas P, Carriquiry JD, Cupul-Magaña AL. 2005. Recruitment of Porites (Scleractinia) on artificial substrate in reefs affected by the 1997-98 El Niño in Banderas Bay, Mexican Pacific. Cienc. Mar. 31: 103-109. http://dx.doi.org/10.7773/cm.v31i11.75 [ Links ]
Miller KJ, Ayre DJ. 2004. The role of sexual and asexual reproduction in structuring high latitude populations of the reef coral Pocillopora damicornis. Heredity 92: 557-568. http://dx.doi.org/10.1038/sj.hdy.6800459 [ Links ]
Miller KJ, Ayre DJ. 2008. Population structure is not a simple function of reproductive mode and larval type: Insights from tropical corals. J. Anim. Ecol. 77: 713-724. http://dx.doi.org/10.1111/j.1365-2656.2008.01387.x [ Links ]
Mora-Pérez MG. 2005. Biología reproductiva del coral Porites panamensis Verrill 1866 (Anthozoa: Scleractinia), en Bahía de La Paz, Baja California Sur, México. MSc thesis, Centro Interdisciplinario de Ciencias Marinas, La Paz, BCS, México. 81 pp. [ Links ]
Paz-García DA, Reyes-Bonilla H. 2006. Temporal variation in the regeneration rate of artificial lesions in two morphotypes of Porites panamensis. Cienc. Mar. 32: 187-194. [ Links ]
Paz-García DA, Reyes-Bonilla H, Hernández-Cortés MP. 2008a. Genetic variation in two morphotypes of Porites panamensis from the Gulf of California, Mexico. Proc. 11th Int. Coral Reef Symp., pp. 444-448. [ Links ]
Paz-García DA, Correa-Sandoval F, Chávez-Romo HE, Reyes-Bonilla H, López-Pérez A, Medina-Rosas P, Hernández-Cortés MP. 2008b. Genetic structure of the massive coral Porites panamensis (Anthozoa: Scleractinia) from the Mexican Pacific. Proc. 11th Int. Coral Reef Symp., pp. 449-453. [ Links ]
Paz-García DA, Balart EF, García-de-León, FJ. 2012a. Cold water bleaching of Pocillopora in the Gulf of California. Proc. 12th Int. Coral Reef Symp. 9A-10: 1-5. [ Links ]
Paz-García DA, Chávez-Romo HE, Correa-Sandoval F, Reyes-Bonilla H, López-Pérez A, Medina-Rosas P, Hernández-Cortés MP. 2012b. Genetic connectivity patterns of corals Pocillopora damicornis and Porites panamensis (Anthozoa: Scleractinia) along the west coast of Mexico. Pacific Sci. 66: 43-61. http://dx.doi.org/10.2984/66.L3 [ Links ]
Pinzón JH, LaJeunesse TC. 2011. Species delimitation of common reef corals in the genus Pocillopora using nucleotide sequence phylogenies, population genetics and symbiosis ecology. Mol. Ecol. 20: 311-325. http://dx.doi.org/10.1111/j.1365-294X.2010.04939.x [ Links ]
Pinzón JH, Reyes-Bonilla H, Baums IB, LaJeunesse TC. 2012. Contrasting clonal structure among Pocillopora (Scleractinia) communities at two environmentally distinct sites in the Gulf of California. Coral Reefs. 31: 765-777. http://dx.doi.org/10.1007/s00338-012-0887-y [ Links ]
Reyes-Bonilla H. 2003. Coral reefs of the Pacific coast of Mexico. In: Cortés J (ed.), Latin American Coral Reefs. Elsevier Science, Amsterdam, pp. 331-349. [ Links ]
Reyes-Bonilla H, Carriquiry JD, Leyte-Morales GE, Cupul-Magaña AL. 2002. Effects of the El Niño-Southern Oscillation and the anti-El Niño event (1997-1999) on coral reefs of the western coast of Mexico. Coral Reefs 21: 368-372. http://dx.doi.org/10.1007/s00338-002-0255-4 [ Links ]
Rodríguez-Troncoso AP, Carpizo-Ituarte E, Leyte-Morales GE, Chi-Barragán G, Tapia-Vázquez O. 2011. Sexual reproduction of three coral species from the Mexican South Pacific. Mar. Biol. 158: 2673-2683. http://dx.doi.org/10.1007/s00227-011-1765-9 [ Links ]
Saavedra-Sotelo NC, Calderón-Aguilera LE, Reyes-Bonilla RA, López-Pérez RA, Medina-Rosas P, Rocha-Olivares A. 2011. Limited genetic connectivity of Pavona gigantea in the Mexican Pacific. Coral Reefs 30: 677-686. http://dx.doi.org/10.1007/s00338-011-0742-6 [ Links ]
Schmidt-Roach S, Miller KJ, Woolsey E, Gerlach G, Baird AH. 2012. Broadcast spawning by Pocillopora species on the Great Barrier Reef. PLoS ONE 7(12): e50847. http://dx.doi.org/10.1371/journal.pone.0050847 [ Links ]
Severance EG, Karl SA. 2006. Contrasting population genetic structure of sympatric mass-spawning Caribbean corals. Mar. Biol. 150: 57-68. http://dx.doi.org/10.1007/s00227-006-0332-2 [ Links ]
Sherman CDH, Ayre DJ, Miller KJ. 2006. Asexual reproduction does not produce clonal populations of the brooding coral Pocillopora damicornis on the Great Barrier Reef, Australia. Coral Reefs 25: 7-18. http://dx.doi.org/10.1007/s00338-005-0053-x [ Links ]
Smith LD, Hughes TP. 1999. An experimental assessment of survival, re-attachment and fecundity of coral fragments. J. Exp. Mar. Biol. Ecol. 235: 147-164. http://dx.doi.org/10.1016/S0022-0981(98)00178-6 [ Links ]
Souter P. 2010. Hidden genetic diversity in a key model species of coral. Mar. Biol. 157: 875-885. http://dx.doi.org/10.1007/s00227-009-1370-3 [ Links ]
Starger CJ, Barber PH, Ambariyanto Baker AC. 2010. The recovery of coral genetic diversity in the Sunda Strait following the 1883 eruption of Krakatau. Coral Reefs 29: 547-565. http://dx.doi.org/10.1007/s00338-010-0609-2 [ Links ]
Stoddart JA. 1983. Asexual production of planulae in the coral Pocillopora damicornis. Mar. Biol. 76: 279-284. http://dx.doi.org/10.1007/BF00393029 [ Links ]
Stoddart JA, Taylor JF. 1988. Genotypic diversity: Estimation and prediction in samples. Genetics 118: 705-711 [ Links ]
Stoddart JA, Ayre DJ, Willis B, Heyward AJ. 1985. Self-recognition in sponges and corals. Evolution 39: 461-463. [ Links ]
Valière N. 2002. GIMLET: A computer program for analyzing genetic individual identification data. Mol. Ecol. Notes 2: 377-379. http://dx.doi.org/10.1046/j.1471-8286.2002.00228.x-i2 [ Links ]
Van Oppen MJH, Gates RD. 2006. Conservation genetics and the resilience of reef-building corals. Mol. Ecol. 15: 3863-3883. http://dx.doi.org/10.1111/j.1365-294X.2006.03026.x [ Links ]
Waits LP, Luikart G, Taberlet P. 2001. Estimating the probability of identity among genotypes in natural populations: Cautions and guidelines. Mol. Ecol. 10: 249-256. http://dx.doi.org/10.1046/j.1365-294X.2001.01185.x [ Links ]
Ward S. 1992. Evidence for broadcast spawning as well as brooding in the scleractinian coral Pocillopora damicornis. Mar. Biol. 112: 641-646. http://dx.doi.org/10.1007/BF00346182 [ Links ]
Weil E. 1992. Genetic and morphlogical variation in Caribbean and eastern Pacific Porites (Anthozoa, Scleractinia). Preliminary results. Proc. 7th Int. Coral Reef Symp. 2: 643-656. [ Links ]
Whitaker K. 2006. Genetic evidence for mixed modes of reproduction in the coral Pocillopora damicornis and its effect on population structure. Mar. Ecol. Prog. Ser. 306: 115-124. http://dx.doi.org/10.3354/meps306115 [ Links ]
Yeoh S, Dai C. 2010. The production of sexual and asexual larvae within single broods of the scleractinian coral, Pocillopora damicornis. Mar. Biol. 157: 351-359. http://dx.doi.org/10.1007/s00227-009-1322-y [ Links ]














