Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Ciencias marinas
versión impresa ISSN 0185-3880
Cienc. mar vol.39 no.4 Ensenada dic. 2013
Ontogenetic distribution of Callinectes ornatus (Decapoda, Portunoidea) in southeastern Brazil
Distribución ontogenética de Callinectes ornatus (Decapoda, Portunoidea) en el sureste de Brasil
Luciana Segura de Andrade1*, Vivian Fransozo2, Valter José Cobo3, Antônio Leão Castilho1, Giovana Bertini4, Adilson Fransozo1
1 Departamento de Zoologia, Instituto de Biociências, NEBECC (Crustacean Biology, Ecology and Culture Study Group), Universidade Estadual Paulista, Campus de Botucatu, Distrito de Rubião Junior, s/n, 18618000, Botucatu, SP, Brazil. *Corresponding author. E-mail: andradels@ibb.unesp.br.
2 Departamento de Ciências Naturais, Universidade Estadual do Sudoeste da Bahia, Estrada do Bem Querer, Km 04, 45031-900, Vitória da Conquista, BA, Brazil.
3 Laboratório de Zoologia, Departamento de Biologia, Universidade de Taubaté. Praça Marcelino Monteiro 63, 12030-010, Taubaté, São Paulo, Brazil.
4 Universidade Estadual Paulista, Campus Experimental de Registro, Rua Nelson Brihi Badur, 430, 11900-000, Registro, SP, Brazil.
Received February 2013,
Received in revised form October 2013,
accepted October 2013.
ABSTRACT
The spatial and seasonal distribution of different demographic groups of the swimming crab Callinectes ornatus was analyzed with respect to its population biology in three bays (Ubatumirim, Ubatuba, and Mar Virado) in southeastern Brazil. In each bay, monthly sampling was performed during two years along six transects, established at 5, 10, 15, and 20 m depth (parallel to the beach line), in a wave-sheltered area (7.5 m depth), and at an exposed site (10 m depth). Total abundance of crabs was similar among the bays. The presence of immature individuals, adult females, and breeding females was positively correlated with the bottom and surface temperatures, whereas adult males showed an opposite trend with respect to these factors. Immature and smaller individuals were most abundant along the sheltered and shallower transects. Males were more abundant along the shallower transects, and females at 15 and 20 m. This study revealed that different demographic groups occupy the habitat in different ways, according to local features. In general, southeastern Brazil offers a range of conditions that favor reproduction and consequently the maintenance and growth of the local population of C. ornatus, contributing to the relatively high abundance of the species in this coastal area.
Key words: coastal monitoring, differential habitat occupation, ontogeny, predatory fishing, swimming crabs.
RESUMEN
Se analizó la distribución espacial y temporal de distintos grupos demográficos del cangrejo nadador Callinectes ornatus en cuanto a su biologia poblacional en tres bahias (Ubatumirim, Ubatuba y Mar Virado) en el sureste de Brasil. En cada bahia se realizaron muestreos mensuales durante dos años en seis transectos, establecidos a 5, 10, 15 y 20 m de profundidad (paralelo a la linea de costa), en una zona protegida del oleaje (7.5 m de profundidad) y en un sitio expuesto al oleaje (10 m de profundidad). La abundancia total de cangrejos fue similar entre bahias. Se observó una correlación positiva entre la presencia de individuos inmaduros, hembras adultas y hembras ovigeras con la temperatura del fondo y superficial, mientras que los machos adultos mostraron una tendencia opuesta con respecto a estos factores. La mayor abundancia de individuos inmaduros y pequeños fue detectada en los transectos protegidos y menos profundos. Los machos fueron más abundantes en los transectos menos profundos, y las hembras a 15 y 20 m de profundidad. Este estudio reveló que los diferentes grupos demográficos ocupan el hábitat de diferentes maneras, de acuerdo con las caracteristicas locales. En general, el sureste de Brasil ofrece diferentes condiciones que favorecen la reproducción y consecuentemente la continuidad y el crecimiento de la población local de C. ornatus, contribuyendo a la relativamente alta abundancia de la especie en esta zona costera.
Palabras clave: monitoreo costero, ocupación preferida, ontogenia, pesca destructiva, cangrejos nadadores.
INTRODUCTION
Portunid crabs of the genus Callinectes are highly abundant along the coast of Brazil. The distribution of Callinectes ornatus Ordway 1863 is restricted to the western Atlantic, from North Carolina to Florida, in the Gulf of Mexico and the Caribbean, and along the coasts of Colombia, Venezuela, Guyana, and Brazil (Amapá to Rio Grande) (Melo 1996). This species occurs mainly in environments with sand or mud bottoms and less-saline waters, and can reach a depth of 75 m in some areas (Melo 1996). Maximum abundance of C. ornatus in the Ubatuba region (Brazil) was found at 5 to 15 m depth, and the abundance decreases significantly toward 25 m (Bertini and Fransozo 2004). It is unknown, however, if the abundances of male and female crabs differ in relation to depth.
Habitat selection by swimming crabs depends on the particular physiological requirements of each stage of their life cycle (Guillory et al. 2001). Blue crabs have planktonic, nektonic, and benthic stages, with offshore marine to near-shore estuarine phases (Guillory et al. 2001). In some species of the genus Callinectes, immature females that have not reached their pubertal molt seek out low-salinity zones of estuaries with high densities of mature males for mating (Guillory et al. 2001). Juvenile females molt and copulate in the brackish waters of the upper estuary (Johnson and Perry 1999). After successful mating, females migrate out of the estuaries for the purpose of larval dispersal.
The distribution patterns of blue crabs seem to be a result of habitat preferences combined with intra- and interspecific interactions (Buchanan and Stoner 1988). The different demographic groups within a species have different distribution patterns. For instance, ovigerous females of the genus Callinectes display a cryptic habit in order to protect their offspring, thus preferring waters with higher salinity (Pita et al. 1985, Mantelatto 2000). Females of C. ornatus can be found farther off the coast than females of the congener Callinectes danae, since the larvae of C. ornatus are less tolerant to variations in salinity and temperature (Negreiros-Fransozo et al. 1999). Keunecke et al. (2012), studying the reproductive strategies of two sympatric species of Callinectes in an estuarine system, suggested that ovigerous females of C. ornatus may occupy the ecotone areas nearer the open sea.
To investigate the hypothesis of differential occupation of space and depths by demographic groups of C. ornatus, their spatial and temporal distribution and sex ratio were studied, taking environmental factors into consideration. This information is important, not only to elucidate the biology of the species, but also to assist in environmental management, particularly in relation to the delimitation of marine protected areas.
MATERIALS AND METHODS
Field collections
Swimming crabs were collected monthly from January 1998 to December 1999 at Ubatumirim, Ubatuba, and Mar Virado bays on the southeastern Brazilian coast (23°35'00" S, 45°12'30" W; 23°22'30" S, 44°53'24" W). Six transects were established in each bay, at 5, 10, 15, and 20 m depth (parallel to the beach line), in an area protected from wave action (7.5 m depth), and at an exposed site (10 m depth), the latter two near rocky shores and perpendicular to the shoreline (fig. 1a). The characteristics of the exposed and sheltered areas were determined according to the position of the transect in relation to exposure to wave action from the ocean currents, which flow from north to south. The crabs were collected from a shrimp fishing boat equipped with doublerig nets with the following net specifications: 11 m in length, 4.5 m at the mouth, 25 mm body mesh diameter, and 15 mm codend mesh diameter. Each transect was trawled over a 30-min period, covering an area of 18,000 m2 at a speed of 2.1 mph.
Environmental variables
For each transect, the bottom and surface water temperatures were recorded using a thermometer attached to a Nansen bottle. An ecobathymeter coupled with a GPS was used to record depths at the sampling sites. Sediment samples were collected in each season with a Van Veen grab, sampling a bottom area of 0.06 m2 in order to measure the organic-matter content and grain size of the sediments. In the laboratory, the sediment was oven-dried at 72 °C for 72 h. The sediment organic-matter content (%) was obtained by ash-weighing: three aliquots of 10 g each per station were placed in porcelain crucibles, incinerated for 3 h at 500 °C, and the ashes were weighed (Mantelatto and Fransozo 1999). Sediment grain-size composition (phi) was analyzed according to Bertini and Fransozo (2004). The sediment remaining after analysis of the organic matter was re-dried and passed through a series of sieves with graduated mesh sizes, following the Wentworth (1922) scale.
Laboratory procedure
Individuals of C. ornatus were identified (Melo 1996) and separated by bay, transect, and collection month. All crabs were sexed by examining the abdominal morphology. The adherence of the abdomen to the thoracic sternites was checked to identify immature individuals (Mantelatto and Fransozo 1999) and to assess the size at sexual maturity. For females, the presence of eggs on the pleopods was also observed, and those that carried embryonated eggs attached to their pleopods were considered breeding females. Thus, the crabs were separated into four demographic categories: adult males, non-breeding adult females, breeding females, and immature individuals. The absolute frequency of these categories was calculated based on the total number of individuals recorded along each transect in each month. Each crab was measured for carapace length and width without the lateral teeth, using a caliper (0.01 mm).
Statistical analyses
Abundance data were log-transformed, and a one-way analysis of variance (ANOVA) was carried out to assess differences in the total abundance of individuals among bays (α = 0.05) (Zar 1996). To investigate the temporal variation in the abundance of each demographic group, a correspondence analysis was conducted, followed by a multi-response permutation procedure (MRPP) test. In the correspondence analysis, the percentage of explanation is obtained by dividing the eigenvalue by the total inertia. A principal components analysis (PCA) was used to identify the relationship between environmental variables (bottom temperature, salinity, organic matter, and phi) and the 18 transects and between the four demographic groups and the transects in each bay. The axes (determined by the broken-stick cut-off criterion, following McCune and Grace 2002) of the "environment vs transects" PCA were used as the environmental matrix in a canonical correspondence analysis (CCA), in which the biotic matrix was represented by the abundance of different demographic groups by transect.
Coordination points were calculated using linear combination scores (fitted values from the weighted averaging regression of the species scores; see McCune and Grace 2002), and the relative contributions of variables on each axis were determined by the intraset correlation (McCune and Grace 2002). The statistical significances of the eigenvalues and the species vs environment correlations were evaluated by randomization (Monte Carlo tests), using 1000 randomized runs for each analysis. In each randomization, sample units in the environmental matrix were shuffled. This destroys the relationship between the species and environmental matrices, while preserving the species matrix and the correlation structure of the environmental matrix. All multi-variate analyses were carried out using the software PC-ORD 6.0, conforming to all assumptions of normality.
The mean carapace width (mm) was tested for normality and homoscedasticity through the Shapiro-Wilks and Levene tests, and was compared between males and females using a Mann-Whitney test (the significance level adopted was 5%). To assess the structure of the population according to bays and transects, a factorial ANOVA (bays vs transects vs within-group carapace width) was performed, and Tukey's multiple comparison tests were carried out to analyze for statistical differences between means at the 5% significance level. The male to female sex ratio, by season and transects, was compared by the binomial test (Wilson and Hardy 2002).
RESULTS
Distribution, abundance, and environmental variables
A total of 20,521 specimens, including 6464 adult males, 5036 adult females (871 breeding), and 9021 immature individuals, were analyzed. Although the total abundance of crabs among the bays was similar (ANOVA; F = 0.32; P = 0.73), the ordination analysis of demographic groups by transects indicated an ontogenetic shift toward deeper waters (fig. 1b). This shift was most evident in the case of females, whereas juveniles were most commonly found in shallow water near rocky shores (fig. 1b).
The overall abundance of individuals oscillated over time (fig. 2). Immature individuals and females showed a peak in abundance during the warmer months, except in Ubatumirim, where the peak abundance of immature individuals occurred in midwinter of 1998. Adult males were more abundant during the colder months (fig. 2). This fluctuation was detected by reciprocal averaging, which indicated a positive correlation between the abundance of adult and breeding females with warmer months, and between adult males and months with lower temperatures. Immature individuals and breeding females were negatively associated with low-temperature months (table 1). These results were confirmed by MRPP (average within-group) (table 1).

During the study period, the widest variations in bottom temperature occurred in 1998, when significant differences were observed among transects, as detected by PCA. Similar differences in salinity occurred in 1999, varying among transects and also among bays. In general, silt and clay proportions (C fraction), phi values, and organic-matter content (%) increased from north to south in the different bays. Within each bay, the lowest proportions of silt and clay were observed at 20 m depth, with an increase toward shallower transects (fig. 3a).
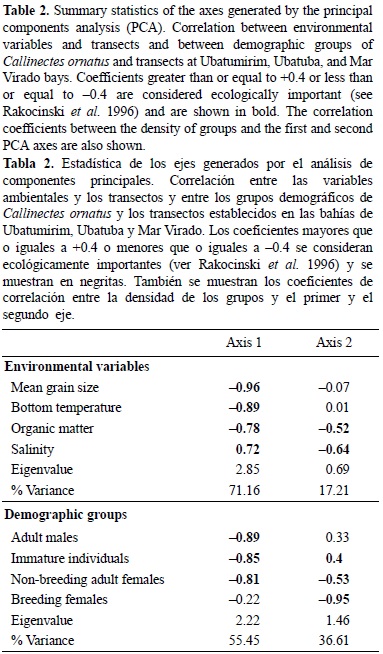
The PCA indicated that the environmental variables (bottom temperature, salinity, organic matter, and phi), as well as the demographic groups (adult males, non-breeding adult females, breeding females, and immature individuals), were strongly correlated among transects (table 2; figs. 1b, 3b). In the ordination plots, variables are represented as arrows, and their length indicates the relative importance of each variable. The angle between arrows represents the degree of correlation between the corresponding environmental variables, whereas the location of species in relation to the arrow indicates the characteristics of the locations and preferences of the species. This result demonstrates that the transects can be characterized by their environmental variables, as well as the demographic groups. The CCA revealed two axes of influence of environmental factors on the distribution of C. ornatus, with a total variance (inertia) of 12.47% (table 3). The correlation among the demographic groups and the two canonical axes was statistically significant (Monte-Carlo permutation test, P < 0.001), indicating correspondence between the demographic groups and the predictor variables (table 3). These canonical axes explained 17.1% of the variation in the distribution of the demographic groups of C. ornatus. Breeding females made a high contribution to this variation, since it was the group that better correlated with the axis. The occurrence of immature individuals showed a positive relationship with mean grain size (phi). The strength and direction of the relationships between the predictor variables and demographic groups are shown in the CCA scores and biplots (table 4).

Population structure and sex ratio
The differential distribution between genders was confirmed by a binomial test, which indicated higher proportions of females at 15 m (mean of bays = 1M:1.4F; P < 0.01) and 20 m depth (mean of bays = 1M:2.3F; P < 0.01). Along the other transects, the sex ratio favored males or showed equal proportions. Females were more abundant than males (1M:1.1F, P < 0.01) in autumn 1999, whereas in summer 1999 this proportion was the same (1M:1F, P = 0.3). During the other seasons, males predominated (P < 0.05).
Generally, the size structure of the population was stable. The mean (± standard deviation) length of the C. ornatus carapace was 61.24 ± 1.2 mm in adult males, 51.30 ± 1.2 mm in breeding females, 50.97 ± 0.50 mm in adult females, and 35.50 ± 1.63 mm in immature individuals. There were no fluctuations in carapace length in relation to depth or the wave exposure site for all individuals. The mean carapace width (CW) for the entire population was 47.4 ± 13.7 mm. All individuals were grouped in 14 size classes of 6 mm each. The size-frequency distribution was normal; males were significantly larger than females (Mann-Whitney; Z = 79.3; P < 0.05). The size of specimens ranged from 5.1 mm CW (an immature female caught in Ubatuba) to 84.0 mm CW (an adult male caught in Mar Virado). Males predominated in the smallest size classes, followed by a balance between the numbers of males and females, next a predominance of females, and again a predominance of males in the 50 mm CW and larger size classes. The largest female, captured in Ubatuba, measured 67.6 mm CW. When comparing the CW of each age group among regions and transects, it became evident that there were significant differences in the size of immature individuals and adult males among transects. The smallest adult males were found along the transect at 20 m depth in Ubatumirim (59.04 ± 4.27 mm) and Ubatuba (59.14 ± 5.36 mm) (factorial ANOVA: F = 5.20; MS = 3.4; P < 0.01); yet the smallest immature individuals were found along the sheltered transects in Ubatumirim (34.84 ± 9.46 mm) and Ubatuba (32.13 ± 7.28 mm) (factorial ANOVA: F = 2.74; MS = 19.98; P < 0.01). Breeding females and adult females did not show significant differences in CW among bays and transects.
DISCUSSION
The migration of breeding females to areas farther offshore seems to be a behavior to increase dispersal and maximize larval survival; however, for C. ornatus, this behavior was only inferred from catch records. The C. danae and Callinectes sapidus juveniles are euryhaline and the life stages move between seawater, estuaries and fresh water, which does not occur with C. ornatus. The data from this study indicated a differential use of habitat according to the requirements of the different developmental stages. Immature individules and adult males were more common in shallow areas and near rocky shores. Adult males were also abundant in areas favorable for mating (shallower depths). In contrast, breeding females migrated to deeper areas.
The mean size of sediment particles in a certain area reflects the local hydrodynamics and the geological history, which determine the sediment stratification. Silt particles only accumulate in more sheltered conditions, whereas sand particles reflect a more dynamic and high-energy environment (Pires 1992). This may explain the high abundance of immature crabs in Ubatuba Bay, where the high concentration of organic matter and quantity of silt and clay led to more available food for immature individuals, especially in the sheltered area and near the rocky shores (Mascaré et al. 2007). Santos et al. (1995) observed a higher abundance of immature individuals of Portunus spinimanus in shallow water (4 m deep) in Fortaleza Bay (near the study area), where crabs as small as 10 mm CW were caught. The larger numbers of immature individuals at shallower depths in protected areas may be related to the availability of shelter and food, in addition to high productivity, which leads to high growth rates (Hines et al. 1987). In this study, the exposed transect of Ubatuba also had an abundance of immature crabs, which can be explained by the presence of several large rocks near the transect. Immature individules of some portunid species preferentially use brackish habitats to molt, possibly because of the osmotic advantages and also the lower predation rates in these locations (Hines et al. 1987). A majority of immature individuals of Persephona mediterranea occupied Ubatuba Bay, which suggested that this bay provides especially favorable conditions for them (Bertini et al. 2001). Furthermore, it has been suggested that there are ontogenic differences in the feeding strategies of Callinectes rathbunae, which may also favor the presence of immature crabs near the coast (Mascaré et al. 2007). In a study on the bathymetric distribution of crabs, Bertini and Fransozo (2004) suggested that the population of C. ornatus predominates at depths of 2 and 5 m, where there is a low diversity of species. This distribution restricted to lower depths can be explained by an abundance of immature individuals, since small crabs survive better where there are fewer predators and better protection (Buchanan and Stoner 1988).
The greater abundance of breeding females along the transect at 20 m depth in Mar Virado Bay seems to follow the same pattern observed in Ubatuba Bay. This site has a high proportion of silt and clay and a higher percentage of organic matter compared to transects at the same depth in the other bays. The confluence of tropical waters from the Brazil Current with subantarctic waters from the Falklands Current occurs between latitudes 30° and 46° S. This subtropical convergence in the southwestern Atlantic (Pires 1992) forms a water mass called the South Atlantic Central Water (SACW). During certain times of the year, this water mass, depending on the intensity and influence of currents and winds (Castro-Filho et al. 1987), can influence coastal regions, causing horizontal and vertical changes, as well as seasonal mixing of tropical and subantarctic waters (Odebrecht and Castello 2001). Changes in bottom and surface temperatures and salinity are related to the hydrodynamics of the water masses in the Ubatuba region (see Pires 1992). These changes can lead to a higher abundance of breeding females in this location, since large-sized larvae can float more easily in high-salinity waters and are more easily dispersed by ocean currents (Pita et al. 1985, Mantelatto 2000). Johnson and Perry (1999) noted that seasonal circulation patterns driven by average wind stress allowed blue-crab larvae to be dispersed offshore and return to nearshore areas during the appropriate period in their development for settlement as megalopae.
Adult males seem to display environmental preferences similar to those of immature individuals, possibly as a reproductive strategy to increase mating success by inhabiting areas where females undergo pubertal molt (changing from juveniles to adults). Santos (2000) suggested that when environmental conditions influence the reproduction of a population, the combination of prevailing environmental factors (physical, chemical, and biotic) should be considered, in addition to interactions such as competition, predation, and social structure. Callinectes ornatus occurs mainly in more saline, transparent, and deeper waters, and on fine- and medium-sand bottoms, that is, areas with greater marine influence (Carvalho and Couto 2011). The portunids Callinectes similis and C. sapidus migrate to more saline and stable waters to spawn and incubate their eggs (Aguilar et al. 2005). Migration of C. sapidus females to high-salinity waters tends to improve their reproductive success, because eggs and larvae will be subject to less fluctuation of physical and chemical parameters and also to lower predation pressure (Hines et al. 1987). Juvenile and adult males of C. danae may concentrate in low-salinity areas in bays, whereas females prefer more saline waters (Sforza et al. 2010). This partial sexual segregation is associated with the reproductive cycle of swimming crabs, and was mentioned for the first time by Norse (1977) in his study on the zoogeographical distributions of members of the genus Callinectes. Subsequently, other researchers also cited this differential occupation of habitat, mainly during breeding periods, such as Pita et al. (1985) and Hines et al. (1987) for C. danae and C. sapidus, respectively.
This differential habitat occupation by crabs might change according to temporal variations, given that, as previously reported, the different demographic groups prefer certain environmental conditions. Bottom and surface temperatures, as well as salinity, are the factors that oscillate the most during the year, due to the influence of SACW. These factors were significantly correlated, particularly with adult females, breeding females, and immature individuals, which are most abundant during months with higher temperatures and salinity (see Johnson and Perry 1999). These months are more favorable for reproduction, and during this period females migrate to deeper regions that are more suitable for larval hatching, while larvae migrate to shallower habitats to develop.
Not only immature individuals but also the smallest adult individuals of C. ornatus were found in protected areas. This is probably related to the presence of large amounts of organic detritus and the high organic-matter content in the sediment (see Mascaré et al. 2007). As suggested by Heck et al. (2001) for C. sapidus, juveniles may be more abundant in sheltered areas because of the reduced risk of predation and less competition for mates from smaller adult males. However, the smaller males may not succeed in fertilizing females, as the females may already have their seminal receptacles partly or completely filled with the spermato-phore of another male (see Johnson 1980).
The normality of the size-class distribution in the C. ornatus population analyzed here indicates an equilibrium between birth and mortality rates, and also a balance between immigration and emigration rates, that is, a stable population structure (Negreiros-Fransozo et al. 1999). Brachyuran populations in tropical and subtropical regions generally show these features, as reported by Santos et al. (1995) for Portunus spinimanus in Fortaleza Bay, by Chacur and Negreiros-Fransozo (2001) for C. danae in Ubatuba Bay, and by Cobo (2005) for Mithraculus forceps at Couves Island (Ubatumirim). Males were distributed in all size classes, whereas females were concentrated in the intermediate classes. A similar pattern was found by Ripoli et al. (2007) for Portunus spinimanus in the state of Espirito Santo, southeastern Brazil. Differential growth between sexes, also shown by C. ornatus, may explain the higher abundance of females in the intermediate size classes (Santos et al. 1995), because in certain life stages females expend more energy on reproduction than on growth. Males were on average larger than females, which is the usual case for portunid crabs (Branco and Masunari 1992). This attribute is an important adaptation for mating, as larger males can better manipulate females and a larger size maximizes their potential for combat with other males (Mantelatto and Fransozo 1999).
This study revealed that the different demographic groups occupy the habitat in different ways, according to local features. Despite being part of the shrimp fishery bycatch, C. ornatus is the most common portunid in the region. In general, the region offers a range of different conditions that favor breeding and consequently the maintenance and growth of the local population of C. ornatus.
ACKNOWLEDGMENTS
The authors thank the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) for providing financial support (#97/12106-8; #97/12108-6; #97/12107-0). They are also greatful to Maria Lucia Negreiros-Fransozo for her constructive comments on early drafts of the manuscript, to NEBECC co-workers for their help during the fieldwork, and to Janet Reid for her valuable help with the English language. All sampling in this study was conducted in compliance with applicable Brazilian state and federal laws.
REFERENCES
Aguilar R, Hines AH, Wolcott TG, Wolcott DL, Kramer MA, Lipcius RN. 2005. The timing and route of movement and migration of post-copulatory female blue crabs, Callinectes sapidus Rathbun, from the upper Chesapeake Bay. J. Exp. Mar. Biol. Ecol. 319: 117-128. http://dx.doi.org/10.10167j.jembe.2004.08.030 [ Links ]
Bertini G, Fransozo A, Costa RC. 2001. Ecological distribution of three species of Persephona (Brachyura, Leucosiidae) in the Ubatuba region, São Paulo, Brazil. Nauplius 9: 31-11. [ Links ]
Bertini G, Fransozo A. 2004. Bathymetric distribution of brachyuran crab (Crustacea, Decapoda) communities on coastal soft bottoms off southeastern Brazil. Mar. Ecol. Prog. Ser 279: 193-200. [ Links ]
Branco JO, Masunari S. 1992. Crescimento de Callinectes danae Smith (Decapoda, Portunidae) da Lagoa da Conceição, Florianópolis, Santa Catarina, Brasil. Rev. Bras. Zool. 9: 53-66. [ Links ]
Buchanan BA, Stoner AW. 1988. Distributional patterns of blue crabs (Callinectes sp.) in a tropical estuarine lagoon. Estuaries 11: 231-239. [ Links ]
Carvalho FL, Couto ECG. 2011. Environmental variables influencing the Callinectes (Crustacea: Brachyura: Portunidae) species distribution in a tropical estuary—Cachoeira River (Bahia, Brazil). J. Mar. Biol. Assoc. UK 91: 793-800. http://dx.doi.org/10.1017/S0025315410001700 [ Links ]
Castro-Filho BM, Miranda LB, Miyao SY. 1987. Condições hidrográficas na Plataforma Continental ao largo de Ubatuba: Variações sazonais e em média escala. Bol. Inst. Oceanogr. 35: 135-151. [ Links ]
Chacur MM, Negreiros-Fransozo ML. 2001. Spatial and seasonal distributions of Callinectes danae (Decapoda, Portunidae) in Ubatuba Bay, São Paulo, Brazil. J. Crust. Biol. 21: 414-425. http://dx.doi.org/10.1651/0278-0372(2001)021[0414:SASDOC]2.0.CO;2 [ Links ]
Cobo VJ. 2005. Population biology of the spider crab, Mithraculus forceps (A. Milne-Edwards 1875) (Majidae, Mithracinae) on the southeastern Brazilian coast. Crustaceana 78: 1079-1087. [ Links ]
Guillory V, Perry H, Steele P, Wagner T, Keithly W, Pellegrin B, Petterson J, Floyd T, Buckson B, Hartman L, Holder E, Moss C. 2001. The Blue Crab Fishery of the Gulf of Mexico, United States: A Regional Management Plan. Gulf States Marine Fisheries Commission, Ocean Springs, MS, 301 pp. [ Links ]
Heck Jr KL, Coen LD, Morgan SG. 2001. Pre- and post-settlement factors as determinants of juvenile blue crab Callinectes sapidus abundance: Results from the north-central Gulf of Mexico. Mar. Ecol. Prog. Ser. 222: 163-176. [ Links ]
Hines AH, Lipicus RN, Haddon AM. 1987. Population dynamics and habitat partitioning by size, sex and molt stage of blue crabs Callinectes sapidus in a subestuary of central Chesapeake Bay. Mar. Ecol. Prog. Ser. 36: 55-64. [ Links ]
Johnson DR, Perry HM. 1999. Blue crab larval dispersion and retention in the Mississippi Bight. Bull. Mar. Sci. 65: 129-149. [ Links ]
Johnson PT. 1980. Histology of the blue crab Callinectes sapidus: A model for the Decapoda. Praeger Scientific Publishing, New York, 440 pp. [ Links ]
Keunecke KA, D'Incao F, Verani JR, Vianna M. 2012. Reproductive strategies of two sympatric swimming crabs Callinectes danae and Callinectes ornatus (Crustacea: Portunidae) in an estuarine system, south-eastern Brazil. J. Mar. Biol. Assoc. UK 92: 343-347. http://dx.doi.org/10.1017/S0025315411000397 [ Links ]
Mantelatto FLM. 2000. Allocation of the portunid crab Callinectes ornatus (Decapoda: Brachyura) in the Ubatuba Bay, northern coast of São Paulo State, Brazil. In: Klein JCVV, Schram FR (orgs.), Crustacean Issues, The Biodiversity Crisis and Crustacea. AA Balkema, Rotterdam. Vol. 12, 1st ed., pp. 431-443. [ Links ]
Mantelatto FLM, Fransozo A. 1999. Characterization of the physical and chemical parameters of Ubatuba Bay, Northern coast of São Paulo State, Brazil. Rev. Bras. Biol. 59: 23-31. http://dx.doi.org/10.1590/S0034-71081999000100004 [ Links ]
Mascaró M, Castillo AM, Simoes N, Chiappa-Carrara X. 2007. Variations in the feeding habits of Callinectes rathbunae in Las Palmas lagoon (southern Gulf of Mexico) on three temporal scales. Crustaceana 80: 139-160. [ Links ]
McCune B, Grace JB. 2002. Analysis of Ecological Communities. MJM Software Design, Gleneden Beach, Oregon, 300 pp. [ Links ]
Melo GAS. 1996. Manual de Identificação dos Brachyura (Caranguejos e Siris) do Litoral Brasileiro. São Paulo, Pleiade/FAPESP, 604 pp. [ Links ]
Negreiros-Fransozo ML, Mantelatto FLM, Fransozo A. 1999. Population biology of Callinectes ornatus Ordway, 1863 (Decapoda, Portunidae) from Ubatuba (SP), Brazil. Sci. Mar. 63: 157-163. http://dx.doi.org/10.3989/scimar.1999.63n2157 [ Links ]
Norse EA. 1977. Aspects of the zoogeographic distribution of Callinectes (Brachyura: Portunidae). Bull. Mar. Sci. 27: 440-447. [ Links ]
Odebrecht C, Castello JP. 2001. The convergence ecosystem in the southwest Atlantic. In: Seeliger U, Kjerfve B (eds.), Ecological Studies: Coastal Marine Ecosystems of Latin America. Vol. 144. Springer Verlag, Berlin, pp. 147-166. [ Links ]
Pires AMS. 1992. Structure and dynamics of benthic megafauna on the continental shelf offshore of Ubatuba, southeastern Brazil. Mar. Ecol. Prog. Ser. 86: 63-76. [ Links ]
Pita JB, Rodrigues ES, Graça-Lopes R, Coelho JAP. 1985. Observações bioecológicas sobre o siri Callinectes danae Smith, 1869 (Crustacea, Portunidae) no complexo baía-estuário de Santos, Estado de São Paulo, Brasil. Bol. Inst. Pesca 12: 35-43. [ Links ]
Rakocinski CF, Lyczkowski-Shultz J, Richardson SL. 1996. Ichthyoplankton assemblage structure in Mississippi Sound as revealed by canonical correspondence analysis. Estuar. Coast. Shelf Sci. 43: 237-257. http://dx.doi.org/10.1006/ecss.1996.0067 [ Links ]
Ripoli LV, Fernandes JM, Rosa DM, Araujo CCV. 2007. Dinâmica populacional de Portunus spinimanus Latreille 1819 (Crustacea, Portunidae) em um trecho litorâneo da Ilha do Frade, Vitória, ES. Bol. Inst. Pesca 33: 205-212. [ Links ]
Santos S. 2000. Influência dos fatores ambientais na abundância de Portunus spinimanus Latreille 1819 (Crustacea, Brachyura, Portunidae) na Região de Ubatuba (SP). Ciênc. Nat. 22: 129-144. [ Links ]
Santos S, Negreiros-Fransozo ML, Fransozo A. 1995. Estructura poblacional de Portunus spinimanus Latreille 1819 (Decapoda, Brachyura, Portunidae) en la Enseada de la Fortaleza, Ubatuba (SP), Brasil. Rev. Invest. Mar. 16 : 37-42. [ Links ]
Sforza R, Nalesso RC, Joyeux JC. 2010. Distribution and population structure of Callinectes danae (Decapoda: Portunidae) in a tropical Brazilian estuary. J. Crust. Biol. 30: 597-606. http://dx.doi.org/10.1651/09-3223.1 [ Links ]
Wentworth CK. 1922. A scale of grade and class terms for clastic sediments. J. Geol. 30: 377-392. [ Links ]
Wilson K, Hardy ICW. 2002. Statistical analysis of sex ratios: an introduction. In: Hardy ICW (ed.), Sex Ratios: Concepts and Research Methods. Cambridge, University Press, 1st ed., pp. 48-92. [ Links ]
Zar JH. 1996. Biostatistical Analysis. 3rd ed. Prentice-Hall, New Jersey, 918 pp. [ Links ]














