Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Ciencias marinas
Print version ISSN 0185-3880
Cienc. mar vol.31 n.2 Ensenada Jun. 2005
Artículos
Assessment of sediment metal contamination in the Mar Menor coastal lagoon (SE Spain): Metal distribution, toxicity, bioaccumulation and benthic community structure
Valoración de la contaminación por metales en los sedimentos de la laguna costera del Mar Menor (SE de España): Distribución de metales, toxicidad, bioacumulación y estructura de las comunidades bentónicas
Lázaro Marín-Guirao1*, Augusto Cesar2, Arnaldo Marín1 and Rubén Vita1
1 Departamento de Ecología e Hidrología Facultad de Biología Universidad de Murcia 30100-Murcia, Spain. * E-mail: lamarin@um.es
2 Departamento de Ecotoxicología Universidade Santa Cecilia Santos, 11045-907, SP, Brazil.
Recibido en marzo de 2004;
aceptado en enero de 2005.
Abstract
The Mar Menor coastal lagoon is one of the largest of the Mediterranean Sea. Ancient mining activities in the mountains near its southern basin have resulted in metal contamination in the sediment. The metal bioavailability of these sediments was determined through laboratory toxicity bioassays using three Mediterranean sea urchin species and two amphipod species, and by means of field bioaccumulation measurements involving the seagrass Cymodocea nodosa. The effect of sediment metal contamination on benthic communities was assessed through benthic infaunal analyses, applying classical descriptive parameters and multivariate techniques. The sediments affected by the mining activities presented high levels of toxicity and metals were also accumulated in the seagrass tissues, pointing to metal bioavailability. Although the classical benthic indices were not clear indicators of disturbance, the multivariate techniques applied provided more consistent conclusions.
Key words: metals, toxicity, bioaccumulation, amphipods, sea urchins, seagrass, coastal lagoon, Mediterranean Sea.
Resumen
El Mar Menor es una de las mayores lagunas costeras en el Mediterráneo. Actividades mineras desarrolladas históricamente en las montañas situadas en su orilla sur han causado la contaminación por metales de sus sedimentos. La biodisponibilidad de los metales en estos sedimentos fue determinada por medio de bioensayos de toxicidad, empleando tres especies de erizos marinos y dos especies de anfípodos, y por medio de medidas de bioacumulación en la fanerógama marina Cymodocea nodosa. El efecto de la contaminación de los sedimentos por metales en las comunidades bentónicas fue valorado a través de análisis de la infauna bentónica, aplicando parámetros descriptivos clásicos y técnicas multivariantes. Los sedimentos afectados por las actividades mineras presentaron altos niveles de toxicidad y estos metales fueron incluso acumulados en los tejidos de la fanerógama marina, indicando la biodisponibilidad de los mismos. Aunque los índices bentónicos clásicos no resultaron ser claros indicadores de la perturbación, las técnicas multivariantes aplicadas ofrecieron conclusiones más consistentes.
Palabras clave: metales, toxicidad, bioacumulación, anfípodos, erizos marinos, fanerógama marina, laguna costera, Mar Mediterráneo.
Introduction
Coastal lagoons and estuaries are physicochemically unique because of their strong gradients in salinity, temperature, pH, dissolved oxygen, redox potential, sediment chemistry and species composition. Moreover, they can fundamentally be considered as receivers of sediments that act as a trap for materials. Sediments serve as a filter for many contaminants between land and sea, and not only accumulate metals but also act as a source of contaminants to marine biota (Ingersoll, 1995). However, specific toxicity and other environmental assessment methods for the sediments of lagoons or estuaries are few and relatively new, since many marine and fresh-water techniques are not generally applicable. Lagoons tend to have a low number of species and low species diversity compared with fresh or marine waters, and for this reason traditional univariate analyses of populations can be difficult to interpret in such naturally stressed environments. Hence, ecological stress, from any source, is best measured using mul-tivariate methods and analyses (Chapman and Wang, 2001).
The study area is located in Mar Menor, SE Spain, one of the biggest coastal lagoons in Europe and the Mediterranean Sea. The lagoon is relatively shallow, with a mean depth of 3.5 m and a maximum depth of just over 6 m. La Manga, a sandy bar (22 km long) crossed by five channels that regulate the water circulation with the Mediterranean Sea, encloses the lagoon. Owing to the low fresh-water input and the high level of water evaporation, the salinity values of the lagoon range between 42 and 47. Common shallow-water species of the Mediterranean Sea inhabit the lagoon. The semi-arid climate of SE Spain means that watercourses remain dry for a 5-10 year period, and fresh water does not reach the lagoon unless sporadic and torrential rainfall occurs. For several centuries two watercourses, the Beal and Ponce wadis, have brought to the lagoon drainage and sedimentation wastes associated with mining activities and abandoned mined lands. In fact, the mining activity in the mountains enclosing the southern part of the lagoon was amongst the most substantial in Spain in the last two centuries. All mining activities ceased in 1991, but during flood episodes the metals of mine tailings are released into the Mar Menor lagoon. Mine tailings may release and leach metals for several hundreds of years after the mining activity has ceased (Gundersen et al., 2001). Previous studies carried out in the area showed Zn, Pb and Cd to be the main metals remaining from the mining activity and released into the environment (Simoneau, 1973; De Leon et al., 1982).
In the present study we have tested sediment toxicity using three species of sea urchin, Arbacia lixula (Linné), Paracentrotus lividus (Lamarck) and Sphaerechinus granularis (Lamarck), and two species of amphipod, the burrowing amphipod Microdeutopus gryllotalpa (A. Costa) and Siphonoecetes sabatieri (Rouvile), employed for the first time in toxicity testing. The bioavailability of metals was also determined by studying the bioaccumulation of metals in different fractions (leaves, rhizomes, roots) and in leaf-biofilm of the seagrass Cymodocea nodosa (Ucria) Aschers. To determine the effect of mineral wastes on the benthic communities of Mar Menor, classical descriptive parameters were used together with multivariate analyses that distinguish anthropogenic stresses from natural events.
The aim of the present study was to evaluate the environmental quality of the soft-bottoms from the southern basin of the Mar Menor lagoon influenced by historical mining activities. The concentration of metals in sediments and metal bioavailability were assessed through different approaches: (i) toxicity testing employing amphipods and sea urchin, (ii) accumulation measurements in the seagrass Cymodocea nodosa, (iii) physicochemical analyses measuring acid volatile sulfides and simultaneously extracted metals, and (iv) the study of benthic communities.
Materials and methods
Sampling stations and study area
Sampling stations were selected in the southern basin of the lagoon, with similar salinity and confinement ranges to minimize possible differences in structure and composition between populations due to the natural gradient characteristics of coastal lagoons. The location of the sampling sites and stations is shown in figure 1. Station UR (Los Urrutias) receives diffuse stormwater inputs and is influenced by the temporary Miranda stream located several hundreds of meters to the north. Station BW (Beal wadi) is located at the outlet of the temporary Beal stream historically employed to discharge mining wastes, and because of the existence of mine tailings in its head-area, great quantities of metals are introduced into the lagoon when torrential rains occur. Station PW (Ponce wadi), situated in the mouth of the temporary Ponce stream, shorter than Beal, channels the rainwater from mined areas where old mining wastes exist. Station PH (Playa Honda) is located near an urban area and a salt mine, midway between both wadis and station CI, selected as reference in the north side of El Ciervo Island, situated in the southern basin of the coastal lagoon and far from the influence of both temporary streams. A second station on the south side of El Ciervo Island (CII) was selected as a second reference station for bioaccumulation and infaunal measurements. For toxicity assays with amphipods we selected an additional reference station in the Encañizadas channel where the amphipod M. gryllotalpa was abundant. The Encañizadas channel, which permits the communication of the lagoon waters with the Mediterranean Sea, presents very different characteristics compared with the other sites selected in the southern basin, so its faunal distribution was not studied.

Collection of sediment, plants and organisms
Sediment samples were collected with a hand-grab, taking the top 5 cm of the surface, and stored in 0.5-L polyethylene jars. Prior to sample collection and between uses, all the containers used for the collection and storage of sediments were thoroughly cleaned with acid (10% HNO3). All samples were transported refrigerated to the laboratory to be stored in the dark at 4°C, and subsampled for chemical quantification and toxicological characterization. Before testing, the sediment samples were homogenized and sieved, discharging large pieces of debris and potential predators. For the benthic infaunal samples (n = 4), an area of 0.09 m2 was sampled, collecting the superficial 5 cm of the sediment with a hand-grab. Organisms were removed by wet-sieving at the study site with a 0.5-mm mesh, and preserved with 10% buffered formalin. Adults of the sea urchin species (A. lixula, P. lividus and S. granularis) were obtained by scuba diving in an unpolluted area of the Mediterranean Sea far from the lagoon (El Fraile Island, Aguilas). In the laboratory, the sea urchins were kept in glass aquaria with flowing natural seawater until used, and supplied with macroalga from their collection site as food. The amphipods (M. gryllotalpa and S. sabatieri) were collected from Las Encañizadas and El Ciervo island, respectively, two areas located within the coastal lagoon and selected as reference for the amphipod toxicity testing. Organisms were transported in polyethylene buckets containing collection site sediment and water, at a constant temperature. Before testing, experimental organisms were acclimated to test conditions.
Toxicity
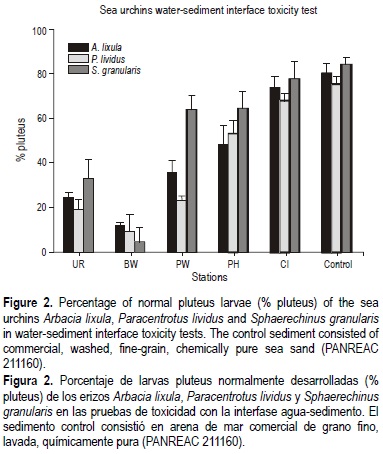
Short-term chronic toxicity tests were performed with gametes of the sea urchins, according to the US Environmental Protection Agency (1995). The sea urchin A. lixula was stimulated to release gametes by touching the shell with steel electrodes connected to a 35-V transformer (about 10 s each time), whereas P. lividus and S. granularis were spawned by injection of 5 mL 0.5 M KCl through the peristomal membrane into the coelomic cavity. Four replicates were used per treatment and approximately 400 fertilized eggs were added to each test chamber. Experiments were conducted in a constant temperature chamber (ASL-Snijders) at 20 ± 0.5°C, with a 16-h/8-h light/dark photoperiod. Tests were finished when control embryos reached fully developed four-arm plu-teus larval stage, each test tube being fixed with buffered formalin. The exposure period varied from 28 h for P. lividus to 38 h for A. lixula and S. granularis. Using a microscope, the first 100 embryos encountered in each tube were counted for normal or abnormal development. Reference toxicant tests were conducted with each batch of organisms using sodium dodecyl sulfide (C12H25NaSO4). At the beginning and the end of every test, temperature, salinity, dissolved oxygen, pH and ammonia were measured, to ensure the acceptability of the tests. Sediment-water interface toxicity tests involved whole-sediment exposures in 15-mL polystyrene tubes, introducing 2 mL of whole-sediment sample using a syringe with the head cut-off and 8 mL of control water (added with care to minimize resuspension), following the procedures of Cesar et al. (2004). Sediments were allowed to settle overnight and a membrane (0.45 |am) was placed on the water-sediment interface. Control sediment consisted of commercially, washed, fine-grain, chemically pure sea sand (PANREAC 211160).
Static 10-day exposures with the estuarine amphipods, M. gryllotalpa and S. sabatieri, were conducted according to methods detailed in the testing manual of the US Environmental Protection Agency (1994). The test chambers consisted of 1-L polyethylene jars. Five replicate test chambers were used per treatment, with each replicate receiving 10 healthy, randomly selected amphipods. After the exposure period at 20 ± 0.5°C, the test organisms were sieved from the sediment, and the survivors were transferred into beakers with clean control sediment to count the number of animals that were not able to rebury. The measured endpoints were mortality and sublethal effects, including the inability to rebury and immobilization. Temperature, salinity, dissolved oxygen, pH and ammonia were measured at the beginning and at the end of the test. During the development of the whole-sediment toxicity test, 48-h water-only reference toxicity tests using sodium dodecyl sulfide were performed to assess the state of health of the field-collected organisms.
Seagrass metal accumulation
In order to evaluate whether the metal contents in sediments were bioavailable to rooted aquatic plants in the field, we analyzed metal concentrations in the seagrass C. nodosa. Sediment and macrophyte samples were collected by snorkel diving. Entire plants and the top 5 cm of sediments were collected at stations BW, PW, CI and CII, where the phanerogam grows to form seagrass beds. The plant material was placed in clean polythene bags and transported to the laboratory on ice. In the laboratory, phanerogam leaves were scraped with a glass slide to separate the particulate material, extracellular polymeric substances and the epibionts that form the biofilm on the leaf surface. In the rest of the plant tissues, the adhering particulate material and sediments were removed rubbing off the tissues with a soft toothbrush, washing them with ultrapure nitric acid (approximately 2%) and then rinsing with distilled water. Plants were divided into the following fractions: roots, rhizomes and leaves. Each sample was dried at 60°C until constant weight. Prior to digestion, samples were powdered in a porcelain mortar and homogenized. Digestions were carried out in triplicate on the homogenized total-sediment and plant fraction. About 0.2 g of sample was digested, adding 1 mL of a nitric, perchloric and sulphuric acid mixture (8:8:1). Samples were heat-digested by a stepwise increase in temperature to 380°C until total evaporation, and then rediluted with deionized water in an ultrasonic bath, acidified with suprapure hydrochloric acid. The concentrations of Zn, Pb, and Cd were measured polarographically by anodic stripping voltammetry (Metrohm 646 VA Processor) with hanging mercury drop. Quality assurance procedures included the use of the certified reference material NIST 1577b (bovine liver). Mean recovery for selected metals was higher than 90%.
Physicochemical analyses
Measurements of pore-water salinity were made using a multiparametric recorder (WTW, MultiLine P4) extracting the interstitial water by sediment centrifugation. Sediment particle size distribution was determined by mechanical dry sieving (Buchanan, 1984). Samples of oven-dried sediments were sieved through a stacked set of graded sieves within the range 2000-62 µm. The percentage of organic matter was determined as the percentage of weight lost upon ignition of dry sediment in a muffle furnace (6 h at 450°C). The dried sediments were finely ground and carefully sieved through a stainless steel mesh. Total organic carbon content was determined with an elemental analyzer (Carlo Erba Instruments, EA1108) following sample preparation with 1 N HCl to decompose carbonates (Verdardo et al., 1990). Sediment samples for the acid volatile sulfide (AVS) and simultaneously extracted metals (SEM) were frozen without airhead space, to minimize sulfide oxidation. Sediment samples (n = 4) were analyzed for AVS by a cold-acid purge-and-trap technique described in detail by Allen et al. (1993). The sediment solid-phase sulfide was converted to hydrogen sulfide by adding hydrochloric acid. The hydrogen sulfide was purged with nitrogen and trapped in a NaOH solution and determined with an ion selective silver/sulfide electrode (ThermoOrion, model 9616). The sediment-water-hydrochloric acid slurry remaining in the reaction flask was immediately filtered, and Zn, Cd, Pb and Cu concentrations were measured by anodic stripping voltammetry (Metrohm 646 VA Processor) with hanging mercury drop. This SEM fraction is the most appropriate for evaluating metal/AVS interactions in sediments (Allen et al., 1993).
Benthic community analyses
Macrobenthic invertebrates were sorted from the sediment with the help of a binocular magnifying glass and classified into major constituent taxa. Taxonomic identifications were then performed to the lowest possible level. The structure of the benthic community was analyzed in terms of species composition and abundance, diversity and evenness. The following descriptive community parameters were calculated for each sample (n = 4) and then summarized for each station: total abundance, species richness (Margelef's d), Shannon-Wiener diversity (log2, H'), evenness (Pielou's J) and Simpson's dominance index (S). The numerical contribution of major taxonomic groups (Polychaeta, Mollusca, Crustacea) was calculated as the proportion of abundance of a taxon to total abundance for each sample and station; mean proportions were also determined and expressed as percentages.
Data analyses
Data from the toxicity tests, expressed as percentage of amphipod survival and normally-developed sea urchin larvae, were analyzed using the Toxstat® V3.3 statistical program (Gulley et al., 1991). Sea urchin results were arc sine root transformed prior to statistical analysis. Data were analyzed for normality and homogeneity of variances with Shapiro-Wilk's test and Hartley's test, respectively. Once data passed these tests, they were subjected to Dunnet's test (ANOVA, P < 0.05). The EC50-48 h values for the amphipod sensitivity tests were calculated with the statistical program Trimmed Spearman-Karmber, applying Abbott's correction (Hamilton et al., 1977). The IC50 values for the sea urchin reference toxicant test were estimated with the ICp method (US Environmental Protection Agency, 1993). Data from the seagrass bioaccumulation were log transformed and later subjected to Dunnet's test (ANOVA, P < 0.05) to check differences in metal accumulation between stations.
The survival in toxicity bioassays and field abundance of S. sabatieri were correlated by the Spearman correlation analysis. The descriptive analyses applied to the infaunal data and the multivariate analyses were performed using the PRIMER (Plymouth Routines in Multivariate Ecological Research, v5) software package (Clarke and Gorley, 2001; Clarke and Warwick, 2001). In order to determine faunal similarities between stations, further comparisons were made using a multivariate ordination technique: non-metric multidimensional scaling (MDS) based on the pooled data with the Bray-Curtis similarity index (Clarke, 1993).
Bray-Curtis similarity matrices were derived using fourth root transformation to absolute abundance data. MDS was used to derive a two-dimensional ordination of sites. Associations between environmental variables and patterns of multivariate community composition were explored using the BIOENV procedure (Somerfield et al., 1994), which exhaustively searches for the combination of environmental variables that produces the similarity matrix most highly correlated to the similarity matrix of sites based on the biota. Environmental similarity matrices were calculated using normalized Euclidian distance and correlations were calculated using the harmonic Spearman rank correlation coefficient.
Multivariate relationships between the sediment physico-chemical parameters were evaluated using correlation-based principal components analysis (PCA). Associations were evaluated between the eight sediment physicochemical variables (fine fraction, organic matter, total organic carbon content, and Zn, Pb, Cu, Cd and AVS concentrations). A component loading cut-off of 0.40 was used to select variables for inclusion in factors, based on suggestions by Tabachnick and Fidell (1996).
Results
Toxicity
Sea urchins
The embryo-larval bioassays showed consistent and similar patterns of toxicity between the three equinoderm species employed. The results, summarized in figure 2, show that, except for S. granularis at station CI , all stations presented significant differences (Dunnet's test, P < 0.05) from the reference for all three species of sea urchins. The results indicated toxicity moving from the sediments to the water column, with consequent adverse effects on sea urchin embryo development. Generally, S. granularis was the species that presented the highest percentage of normally-developed pluteus larvae at every station. The 50% inhibiting concentrations (IC50) for A. lixula, P. lividus and S. granularis in the reference toxicity tests with sodium dodecyl sulfide were 1.63 mg L-1 (±SD 0.23), 1.71 mg L-1 (±SD 0.28) and 1.87 mg L-1 (±SD 0.01), respectively, similar concentrations to those obtained by Cesar et al. (2002).
Amphipods
Whole-sediment bioassays with both species of amphipods showed similar toxicity patterns to those obtained with equino-derm embryo-larval bioassays. All stations, except for El Ciervo Island (CI), presented significant differences (Dunnet's test, P < 0.05) from the reference for both species of amphi-pods. For M. gryllotalpa, the mean percentage of amphipod mortality during the 10-day amphipod toxicity test ranged from 2-8% in the reference and CI stations to 46-54% at stations PW and BW (fig. 3). For S. sabatieri, the maximum percentage of amphipod mortality (88%) was observed at station BW. The effect concentration (EC50) mean value for the 48-h water-only reference toxicant tests with sodium dodecyl sulfide was 2.98 mg L-1 (±SD 0.26) for M. gryllotalpa, similar to that found by other authors (Cesar et al., 2000, 2002) and to that obtained for S. sabatieri, which presented a mean EC50 value of 3.10 mg L-1 (±SD 0.09).

Seagrass metal accumulation
Cymodocea nodosa plants collected from the Mar Menor lagoon contained Zn, Pb and Cd, indicating their bioavailabil-ity to rooted aquatic plants. The metal concentrations in roots, rhizomes, leaves and leaf-biofilm are represented together with total sediment metal concentrations (fig. 4). There were significant differences (Dunnet's test, P < 0.05) in each plant fraction metal concentration between sampling stations. Stations BW and PW located at the outlets of both desert streams presented the highest metal contents in sediments and in the different plant fractions analyzed, the former being the station that showed the highest concentrations. Both reference stations (CI and CII) presented similar metal concentrations in the different plant fractions analyzed. Except for station BW, where the Pb concentration was higher in roots than in the surrounding sediment, the metal concentrations determined in the sediments were higher than in the plant fractions (leaves, rhizomes and roots) for the three metals determined. The highest metal concentrations in plants from polluted stations were found in the leaf biofilm (3913 ± 326 µg Zn/g d.w. and 1568 ± 191 mg Pb/g d.w. for station BW, and 12489 ± 2592 ng Cd/g d.w. for station PW), possibly due to its ability to form complexes between metals and the extracellular polymeric substances that form the biofilm.

Different patterns were observed in plant metal concentrations between both wadis stations (BW and PW) for all three metals studied, the highest plant metal content being found in the roots from station BW, but in the leaves from station PW. The metal content in the rhizomes was lower than in the leaves and roots for every station and metal studied.
Physicochemical analyses
The granulometric analysis indicated that sediments are mainly composed of very fine sands (table 1). Station BW located at the mouth of Beal wadi presented the highest content of fine-grained sediments, the percentage of silt-clay (10.5%) being similar to that of station CII (8.4%). The organic matter analysis ranged from 2.23% to 8.17% of dry sediment for stations PH and BW, respectively, the higher organic carbon content corresponding to the latter station.
Station BW presented the highest sediment metal values for the metals analyzed simultaneously to sulfides, whereas the highest AVS concentration was observed at station PW. The SEM-AVS relation was positive at PH, UR, PW and BW, being highest in the last station. Negative values were found at CI, CII and the reference station.
The application of factor analysis to the sediment variables identified three new variables or principal factors, which explained 90.7% of the variance in the original data set. The first new variable, PC1, was predominant and accounted for 47.5% of the variance. This factor combined the chemical concentrations of Zn, Pb, organic matter and silt-clay content. The second new variable, PC2, accounted for 29.4% of the variance and combined the concentration of Cu and Cd simultaneously extracted to sulfides. The third new variable, PC3, accounted for 13.8% of the variance and was a combination of the AVS sediment concentration and the total organic carbon content.
The two-dimensional PCA plot is represented in figure 5, with the stations ordered along the first axis, according to Zn and Pb concentrations and organic matter and fine sediment content. All the samples from BW had PC1 scores greater than 3.5, suggesting that they are severely contaminated by Zn and Pb; they also presented a high content of fine-grained sediment and organic matter. Stations CI and CII presented PC1 and PC2 values close to 0, indicating low or moderate levels of metals; however, stations BW, PW and UR, with PC2 scores of between 1 and 2, had moderate contamination levels of Cu and Cd. The results of the PCA analysis are in accordance with those obtained in the MDS ordination.

Benthic community analyses
The results of the descriptive analyses are shown in table 2, together with the numerical contribution of the major taxo-nomic groups. Polychaetes dominated at the stations affected by the discharge of the desert streams (BW and PW), while crustaceans predominated at those stations furthest from the wadis (CI, CII and PH). The amphipod S. sabatieri presented the highest abundance. Other less abundant organisms were the Cirratulidae polychaetes, the bivalves Cerastoderma edule and Mytilaster minimus, and the isopod Sphaeroma serratum.
Biological communities of the Mar Menor lagoon are composed of a low number of marine species and, typically, all the stations exhibited low diversity. Stations CI and CII presented the highest number of species, although station CI showed the lowest diversity and evenness values and the highest dominance due to the high abundance of the amphipod Siphonoecetes sabatieri.
The two-dimensional MDS ordination (fig. 6), based on invertebrate community composition, produced three groups of sites (Bray Curtis similarity between groups of approximately 45%). One group contained the stations CI, CII and PH, within which replicates were consistently grouped, except replicate CII3 plotted outside the group. Another group included the four replicates of station UR, and a third group consisted of the two flood-way stations (BW and PW), whose replicates were mixed and dispersed to the right side of the plot, presenting the highest variability among replicates.

The BIOENV analyses produced optimal correlations with the biotic matrix involving eight variables. Variables related to the metal concentrations in sediments best explained community composition, with a maximum correlation of 0.371 for Pb and Cu, followed by the three variables Zn, Pb and Cd with a correlation of 0.364.
Discussion
Our results indicated that sediments from the southern basin of the Mar Menor coastal lagoon are influenced by historical mining activities developed in the adjacent mountains and show high levels of metals. Sediments influenced by the discharge of mining residuals through both wadis (BW and PW) presented metal bioavailability to benthic invertebrates (toxicity bioassays) and to marine phanerogams (bioaccumulation). The highest sediment metal concentration and toxicity levels were found in samples taken near the mouth of Beal wadi (BW), where mining wastes were discharged directly during mine exploitation. At present, due to the arid climate of the area, when torrential rains occur the mining wastes remaining in the mountains are introduced into the lagoon through the Beal (BW) and Ponce (PW) wadis. Small and not well-defined streams also occur that produce diffuse contamination around station UR.
The sediments of the Mar Menor lagoon have higher Zn, Pb, Cu and Cd concentrations than other coastal areas (e.g., Ambatsian et al., 1997; Byrne and O'Halloran, 2000). These concentrations are in agreement with those observed in previous studies in the area during the last three decades and have not undergone any noticeable change during this period (Simoneau, 1973; De Leon et al., 1982; Rodríguez et al., 2001).
The whole-sediment toxicity tests performed with the amphipods M. gryllotalpa and S. sabatieri, and the sediment-water interface toxicity tests developed with the sea urchins, are in accordance and identified as toxic those sediments influenced by the discharge of both wadis. The toxicity results were well correlated with the SEM-AVS negative values, indicating the absence of toxicity, as demonstrated Hansen et al. (1996) in anoxic sediments, where the availability of divalent metals to organisms living in nearby oxic surface sediments or tubes has been related to AVS.
The SEM-AVS values may also be related to metal bio-accumulation in the seagrass Cymodocea nodosa. The great differences in SEM-AVS values between both wadi stations (BW and PW) might be responsible for the different pattern observed in the accumulation of metals between leaves and roots. Station BW presented very high SEM-AVS values, indicating that most of the metals were not bound to sulfides and were bioavailable, the roots being the plant fraction that presented the highest concentrations. On the other hand, stations PW, CI and CII showed higher metal concentrations in the leaf fractions and SEM-AVS values close to zero, indicating that most metals were associated with AVS, forming insoluble and biologically unavailable metal sulfides. Although the SEM-AVS ratio influences the availability of metals to living organisms, its role may be lower in the case of rooted aquatic plants because they transport oxygen to underground tissues, oxidizing the sulfides and reducing their effect on metal availability (Marbá and Duarte, 2001). The metal concentrations recorded in our study for C. nodosa may be considered to exceed the background level for non-polluted areas at stations BW and PW (Moore and Ramamoorthy, 1984) and are much higher than those found in other studies mentioned in the bibliography for C. nodosa (Malea and Haritonidis, 1994; Prange and Dennison, 2000; Sanchiz et al., 2000). Many metal ions, such as Cd2+, Cu2+, Cr3+, Pb2+, etc., are efficiently chelated by the extracellular polymeric substances secreted when bacteria and microalgae are associated with surfaces (e.g., sediment particles or plant surfaces), forming a "microbial biofilm" (Decho, 2000). The fact that sediments presented similar Zn and Pb concentrations to the leaf-biofilm at the wadi stations can be due to metal deposition over the bio-film from the stormwater runoff that introduces great quantities of metals associated with fine sediments through these two wadis.
The classical descriptive parameters employed to study the benthic community structure (Margalef richness, Shannon-Wiener diversity, Pielou evenness and Simpson dominance) were not clear indicators of disturbance in estuarine areas (DelValls and Chapman, 1998; DelValls et al., 1998; Drake et al. , 1999). These parameters were also poor indicators of the "state of health" of the Mar Menor lagoon, where the areas contaminated by metals (BW, PW and UR) showed higher diversity values than those stations (CI and PH) with low or moderate metal contamination. In our case, this inconsistency was mainly due to the high abundance of just one species (S. sabatieri) in non-impacted sites (high dominance values). Metal contamination probably has a deleterious effect on the amphipod S. sabatieri, decreasing its dominance and thus increasing the diversity values of the contaminated stations. The effects of metal contamination on the benthic community might be similar to the effect of top predators that control the abundance of lower trophic levels, sustaining communities of apparent high diversity. The abundance of S. sabatieri was correlated with the survival percentages obtained with this species in sediment toxicity assays (Spearman correlation r = 0.62, P < 0.01). This is the first time that the amphipod S. sabatieri has been used to evaluate sediment toxicity and seems to be suitable for sediment toxicity determination in the lagoon, not only because of the consistency of the results but also because of its ecological significance due to its broad distribution in Mar Menor.
The multivariate techniques applied in this study provided more consistent conclusions than those obtained with descriptive analyses and seem to be a useful tool to evaluate the environmental quality of the Mar Menor lagoon. The MDS ordination technique grouped the stations into three different groups that can be classified as non-impacted (PH, CI and CII), moderately impacted (UR) and severely impacted (BW and PW). The wadis stations (BW and PW) showed higher presence of Polychaeta and lower of Crustacea. At both stations, the abundance of Cirratulidae (Polychaeta), which are tolerant of a wide range of environmental conditions and inhabit sandy and silty sediments, was high (Fauvel, 1975). Some species of the Cirratulidae and Nephtyidae Polychaeta families are common in estuaries, including those draining metalliferous mining regions (Geoffrey and Gibbs, 1987). The high percentage of Crustacea, the most pollution-sensitive taxon, at stations PH, CI and CII pointed to low or moderate levels of disturbance. Metal concentrations in sediments were identified as the main parameters that determined the benthic community structure in the southern basin of Mar Menor, although other contaminants not analyzed in this study may also influence the community assemblages.
In this study we have evidenced the high load of metals in sediments of the southern basin of the Mar Menor lagoon influenced by mining wastes. The availability of metals to benthic organisms has been demonstrated through toxicity tests with amphipods and sea urchins and bioaccumulation measurements in the seagrass C. nodosa. This metal bioavailability seems to be closely related to the molar relation SEM-AVS and seems to influence the benthic faunal assemblages of those contaminated sediments.
Acknowledgements
The second author acknowledges the post-doc scholarship from the Ministerio de Educagao e Cultura de Brasil (CAPES/MEC-BR/BEX2558-03/3).
References
Allen, H.E., Gongmin, F. and Deng, B. (1993). Analysis of acid volatile sulfide (AVS) and simultaneously extracted metals (SEM) for estimation of potential toxicity in aquatic sediments. Environ. Toxicol. Chem., 12: 1441-1453. [ Links ]
Ambatsian, P., Fernex, F., Bernat, M., Parron, C. and Lecolle, J. (1997). High metal inputs to closed seas: The New Caledonian lagoon. J. Geochem. Explor., 59: 59-74. [ Links ]
Buchanan, J.B. (1984). Sediment analysis. In: N.A. Holme and A.D. Mcintyre (eds.), Methods for the Study of Marine Benthos. Blackwell Scientific Publ., pp. 41-65. [ Links ]
Byrne, P.A. and O'Halloran, J. (2000). Acute and sublethal toxicity of estuarine sediments to the Manila clam, Tapes semidecussatus. Environ. Toxicol., 15: 456-468. [ Links ]
Cesar, A., Marín, L., Vita, R., Gómez, M., Jiménez, B. y Marín, A. (2000). Test de toxicidad con sedimento marino en la costa Mediterránea empleando anfípodos: Gammarus aequicauda y Microdeutopus gryllotalpa. En: G. Espindola, R. Paschoal, O. Rocha, C. Bohrer y L. Neto (eds.), Ecotoxicologia: Perspectivas para o Século XXI. Sao Carlos, RiMa, pp. 17-27. [ Links ]
Cesar, A., Marín-Guirao, L., Vita, R. and Marín, A. (2002). Sensitivity of Mediterranean amphipods and sea urchins to reference toxicants. Cienc. Mar., 28: 407-417. [ Links ]
Cesar, A., Marín, A., Marín-Guirao, L. and Vita, R. (2004). Amphipod and sea urchin tests to assess the toxicity of Mediterranean sediments: The case of Portman Bay. Scientia Mar., 68(Suppl. 1): 205-213. [ Links ]
Chapman, P.M. and Wang F. (2001). Assessing sediment contamination in estuaries. Environ. Toxicol. Chem., 20: 3-22. [ Links ]
Clarke, K.R. (1993). Non-parametric multivariate analyses of changes in community structure. Aust. J. Ecol., 18: 117-143. [ Links ]
Clarke, K.R. and Gorley, R.N. (2001). PRIMER v5: User Manual/Tutorial. Plymouth, UK. [ Links ]
Clarke, K.R. and Warwick, R.M. (2001). Change in marine communities: An approach to statistical analysis and interpretation. 2nd ed. Plymouth Marine Laboratory, UK. [ Links ]
Decho, AW. (2000). Microbial biofilms in intertidal systems: An overview. Cont. Shelf Res., 20: 1257-1273. [ Links ]
De Leon, A.R., Guerrero, J. and Faraco, F. (1982). Evolution of the pollution of the coastal lagoon of Mar Menor. VI Journées Étud. Pollutions, Cannes, CIESM. [ Links ]
DelValls, T.A. and Chapman, P.M. (1998). The use of multivariate analysis to link the sediment quality triad components to site-specific sediment quality values in the Gulf of Cádiz (Spain) and in San Francisco Bay (USA). Cienc. Mar., 24: 313-336. [ Links ]
DelValls, T.A., Conradi, M., García-Adiego, E., Forja, J.M. and Gómez-Parra, A. (1998). Analysis of macrobenthic community structure in relation to different environmental sources of contamination in two littoral ecosystems from the Gulf of Cádiz (SW Spain). Hydrobiologia, 385: 59-70. [ Links ]
Drake, P., Baldó, F., Sáez, V. and Arias, A. (1999). Macrobenthic community structure in estuarine pollution assessment on the Gulf of Cádiz (SW Spain): Is the phylum-level meta-analysis approach applicable? Mar. Pollut. Bull., 38: 1038-1047. [ Links ]
Fauvel, P. (1975). Fauna de France: Polychétes. Fédération Frangaise des Sociétés de Sciences Naturelles. Office Central de Faunistique. Kraus Reprint. Neudeln/ Liechtenstein. [ Links ]
Geoffrey, W.B. and Gibbs, P.E. (1987). Polychaetes as indicators of heavy-metal availability in marine deposits. In: J.M. Capuzzo and D.R. Kester (eds.), Oceanic Processes in Marine Pollution. Vol. 1. Biological Processes and Wastes in the Ocean. Krieger Publ. Co., Melbourne, Florida, pp. 37-19. [ Links ]
Gulley, D.D., Boelter, A.M. and Harold, L.B. (1991). TOXSTAT® Computer Program. Version 3.3. Univ. of Wyoming, Laramie. [ Links ]
Gundersen, P., Olsvik, P.A. and Steinnes, E. (2001). Variations in heavy metal concentration in two mining-polluted streams in central Norway. Environ. Toxicol. Chem., 20: 978-984. [ Links ]
Hamilton, M.A., Russo, R.C. and Thurston, R.V. (1977). Trimmed Spearman-Karber method for estimating median lethal concentrations in toxicological bioassays. Environ. Sci. Tech., 11: 714-719; correction 12: 417, 1978. [ Links ]
Hansen, D.J., Berry, W.J., Mahony, J.D., Boothman, W.S., Di Toro, D.M., Robson, D.L., Ankley, G.T., Ma, D., Yan, Q. and Pesch, C.E. (1996). Predicting the toxicity of metal-contaminated field sediments using interstitial concentration of metals and acid-volatile sulfide normalizations. Environ. Toxicol. Chem., 15: 2080-2094. [ Links ]
Ingersoll, C.G. (1995). Sediment test. In: G.M. Rand (ed.), Fundamentals of Aquatic Toxicology. Taylor and Francis, USA, pp. 231-255. [ Links ]
Malea, P. and Haritonidis, S. (1994). Local distribution and seasonal variation of Fe, Pb, Zn, Cu, Cd, Na, K, Ca and Mg concentrations in the seagrass Cymodocea nodosa (Ucria) Aschers in the Antikyra Gulf, Greece. Mar. Ecol., 16(1): 41-56. [ Links ]
Marbá, N. and Duarte, C.M. (2001). Growth and sediment space occupation by seagrass Cymodocea nodosa roots. Mar. Ecol. Prog. Ser., 224: 291-298. [ Links ]
Moore, J.W. and Ramamoorthy, S. (1984). Heavy Metals in Natural Waters. Springer, New York, Berlin, Heidelberg, Tokyo. [ Links ]
Prange, J.A. and Dennison, W.C. (2000). Physiological responses of five seagrass species to trace metals. Mar. Pollut. Bull., 41: 327-336. [ Links ]
Sanchiz, C., García-Carrascosa, A.M. and Pastor, A. (2000). Heavy metal contents in soft-bottom marine macrophytes and sediments along the Mediterranean coast of Spain. Mar. Ecol., 21(1): 1-16. [ Links ]
Simoneau, J. (1973). Mar Menor: Evolution sedimentologique et geochimique recente du remplissage. Thesis, Université Paul Sebatier de Tolouse, France. [ Links ]
Somerfield, P.J., Gee, J.M. and Warwick, R.M. (1994). Soft sediment meiofaunal community structure in relation to a long-term heavy metal gradient in the Fal estuary system. Mar. Ecol. Prog. Ser., 105: 79-88. [ Links ]
Tabachnick, B.G. and Fidell, L.S. (1996). Using Multivariate Statistics. Harper Collins, New York. [ Links ]
US Environmental Protection Agency (1993). A Linear Interpolation Method for Sublethal Toxicity: The Inhibition Concentration (ICp) Approach. Duluth, MN. [ Links ]
US Environmental Protection Agency (1994). Methods for assessing the toxicity of sediment-associated contaminants with estuarine and marine amphipods. EPA/ 600/ R-94/025. Narragansett, RI. [ Links ]
US Environmental Protection Agency. (1995). Short-term methods for estimating the chronic toxicity of effluents and receiving waters to west coast marine and estuarine organisms. EPA/ 600/ R-95-136. Cincinnati, Ohio. [ Links ]
Verdardo, D.J., Forelich, P.N. and McIntyre, A. (1990). Determination of organic carbon and nitrogen in marine sediments using the Carlo Erba NA-1500 analyzer. Deep-Sea Res., 37: 157-165. [ Links ]














