Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Ciencias marinas
versión impresa ISSN 0185-3880
Cienc. mar vol.31 no.1b Ensenada may. 2005
Artículos
A mussel watch in the Ria Formosa lagoon
Programa de vigilancia de mejillones en la Ría Formosa
M.E. Morgado and M.J. Bebianno*
* CIMA, Laboratório de Ecotoxicologia e Química Ambiental Universidade do Algarve, Campus de Gambelas 8000-810 Faro, Portugal. * E-mail: mbebian@ualg.pt
Recibido en junio de 2003;
aceptado en marzo de 2004.
Abstract
The present work analyzes the concentrations of various metals (Ag, Cd, Cr, Cu, Ni, Pb and Zn) in the edible part of the musselMytilus galloprovincialis from the Ria Formosa lagoon (Portugal). Mussels of a similar size (5-7 cm) were collected. The results indicate that the dispersion and accumulation of metals in mussels is influenced essentially by the sources of pollution and the bioavailability of the metal. The highest concentrations of Ag, Cu and Ni occurred in the mussels from sites 1, 6 and 7, which are mainly related to inputs from urban discharges. The highest concentrations of Cr were found in the mussels from sites 2 and 5, located near urban discharges and metallic structures. The highest concentrations of Pb were recorded in the mussels from site 9, associated with fuel from boats and diffuse inputs of this metal. The mussels at sites 3, 4 and 9 presented the highest concentrations of Zn and Cd, probably caused by physical and chemical processes that occur in the boundary between the lagoon and the ocean. The concentrations of Ag and Ni in the mussels of Ria Formosa are relatively low when compared to other geographical locations. The concentrations of Cd, Cr, Cu and Pb accumulated in the mussels reveal low contamination. As for the concentration of Zn, the values in the Ria Formosa mussels are higher in the eastern part of the lagoon than in the western part, revealing signs of contamination by this metal.
Key words: Mussel Watch, metals, Ria Formosa.
Resumen
Se analizaron las concentraciones de varios metales (Ag, Cd, Cr, Cu, Ni, Pb y Zn) en la parte comestible del mejillón Mytilus galloprovincialis de la Ría Formosa (Portugal), para lo cual se recolectaron ejemplares de tamaño similar (5-7 cm). Los resultados indican que la dispersión y acumulación de metales en los mejillones es influenciada esencialmente por las fuentes de contaminación y la biodisponibilidad del metal. Las concentraciones más elevadas de Ag, Cu y Ni se registraron en los mejillones de las estaciones 1, 6 y 7, relacionadas principalmente con el vertido de descargas urbanas. Las concentraciones mayores de Cr se observaron en los mejillones de las estaciones 2 y 5, localizadas cerca de descargas urbanas y estructuras metálicas. Las concentraciones más altas de Pb se presentaron en los mejillones de la estación 9, lo cual se asocia con el combustible de barcos y aportes difusos de este metal. Los mejillones de las estaciones 3, 4 y 9 tuvieron las concentraciones más elevadas de Zn y Cd, probablemente como consecuencia de los procesos físicos y químicos que se presentan en el límite entre la laguna y el océano. Las concentraciones de Ag y Ni en los mejillones de la Ría Formosa son relativamente bajos en comparación con otros sitios geográficos. Las concentraciones de Cd, Cr, Cu y Pb acumuladas en los mejillones muestran poca contaminación. En cuanto a la concentración de Zn, los valores para los mejillones de la Ría Formosa son mayores en la parte oriental de la laguna que en la parte occidental, lo que indica contaminación por este metal.
Palabras clave: programa de vigilancia de mejillones, metales, Ría Formosa.
Introduction
The concentration of trace metals in the environment can be measured through the water, sediments or biota. Biota integrates in space and time the metal concentration in the environment and this concentration is a measure of the bioavailability of the metal. This is because many organisms are able to concentrate metals in much higher concentrations than those present in the marine environment (Rainbow, 1996).
Marine organisms used to quantify metal bioavailability are designated as bioindicators, sentinel organisms or biomonitors. They should comply with several criteria, such as being sedentary and representative of a wide area, having a long life span, being abundant, easy to identify and available for sampling all year round, providing sufficient tissue for analysis, being resistant to stress caused by handling and transportation, and tolerating wide variations of physical and chemical parameters. Many species selected as biomonitors do not comply with all of these criteria, but they show a high accumulation potential for the metals under study (Rainbow and Phillips, 1993; Rainbow, 1995; Boening, 1999). The use of species of the genus Mytilus as for this purposes is very frequent since they are cosmopolitan, tolerate eutrophic coastal waters and great variations of salinity and temperature, and are efficient accumulators of several trace metals (Kennish, 1992; Rainbow, 1995). In the past decades, intensive monitoring programs for assessing contamination levels in marine ecosystems have been made using mussels as sentinel organisms. Among these, the Mussel Watch developed by Goldberg et al. (1978) is considered of extreme importance (Lauenstein et al., 1990).
The monitoring of trace metals in the Ria Formosa lagoon is also of extreme importance since this system is highly productive and dynamic, and supports innumerable organisms, for which it has been designated a Natural Park, a special protection zone and an important bird area. Moreover, Ria Formosa has a long mariculture tradition, especially of Ruditapes decussatus that constitutes about 90% of the Portuguese production, and has important fishery industries (Bebianno, 1995; Mudge and Bebianno, 1997; Matos et al., 1999). Also an important tourism area, Ría Formosa is under the influence of several anthropogenic wastewater sources that have contributed to the deterioration of the system's water quality. The most significant inputs of contaminants are from point sources, like urban and industrial wastewaters, and from diffuse sources, like agricultural runoffs, road runoffs, ports, marinas and aquaculture (Bebianno, 1995; Mudge and Bebianno, 1997).
The aim of this study was to monitor, using the species Mytilus galloprovincialis as bioindicator, the level of seven trace metals, namely Ag, Cd, Cr, Cu, Pb, Ni and Zn, in several sampling sites in the Ria Formosa lagoon, and evaluate their spatial and temporal variation in comparison to previous studies.
Material and methods
Several specimens of M. galloprovincialis were collected in April 2001 at a total of nine sampling sites along the Ria Formosa lagoon (fig. 1), from site 1 (Ilha de Faro) to site 9 (Tavira). At every site, about 20 individuals ranging in size from 5 to 7 cm were collected. In the laboratory, specimens were depurated for a 48-h period in order to clear out the gut content. Each sample was labelled and frozen at -20°C until laboratory analysis. Six mussels from each sampling site were thawed. The length, width, height, sex and maturation stage were recorded for each organism. The byssus was removed from the organisms and the total edible part placed in a Teflon vessel. A high performance microwave oven was used to dry and digest the tissue samples. For the digestion, 4 mL of HNO3 69% Aristar and 1 mL of H2O2 30% Sigma were added.

Metal analysis
The metals were quantified with a Perkin-Elmer Analyst 800 atomic absorption spectrophotometer; Zn was determined by flame and Ag, Cd, Cr, Cu, Ni and Pb by graphite furnace.
Certified reference material was used to validate the results, namely TORT-1 (defatted lobster hepatopancreas, Homarus americanus) and LUTS-1 (non-defatted lobster hepatopan-creas, H. americanus) from the National Research Council of Canada. The recoveries of all metals, except for Pb and Zn, ranged from 80% to 120%. Concentrations of all elements are reported as mg g-1 on a dry weight basis.
Statistical analysis
The mean concentration, standard deviation and coefficient of variation were determined for each metal at the nine sampling sites. In order to verify the differences in metal concentration between sites, a Kruskal-Wallis non-parametric test of variance was applied. The null and alternative hypotheses were: H0 = the metal concentration is the same among the stations and H1 = the metal concentration is different among the stations. The level of significance established was 5%.
Statistical treatments also included a numeric classification method, cluster analysis (CA), and an ordination method, the principal component analysis (PCA). In order to reduce the effects of different scales of measurement in different characters, the data matrix was standardized (by subtraction and reduction). The correlation coefficient was selected to determine the similarities between sampling sites (mode Q) and metals (mode R). The method of agglomeration selected for the CA was the UPGMA (unweighted pair-group method using arithmetic averages) and in both analyses the data matrix consisted of the average concentrations of each metal at the nine sites. The software used for the CA and PCA was NTSYS-PC (numerical taxonomy and multivariate analysis system for IBM PC microcomputer), version 2.02.
Results
Figure 2 shows the mean concentrations (± standard deviation) of Ag, Cd, Cr, Cu, Ni, Pb and Zn in the edible part of M. galloprovincialis at the nine sites.
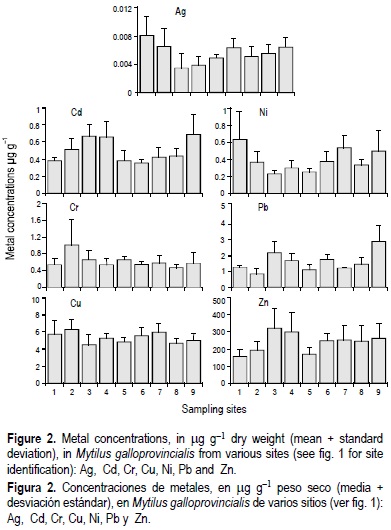
The concentrations of Ag ranged from 0.0036 ± 0.0021 mg g-1 at site 3 (Deserta) to 0.0082 ± 0.0021 mg g-1 at site 1 (Ilha de Faro) (fig. 2). The differences in Ag among the sites were statistically significant (P < 0.05). The Ag concentrations were generally lower in the areas near inlets, namely sites 3 (Deserta) and 4 (Farol), with values of 0.0036 ± 0.0021 and 0.004 ± 0.0012 mg g-1, respectively.
The concentrations of Cd ranged from 0.36 ± 0.04 to 0.69 ± 0.23 mg g-1 dry weight, the highest concentrations occurring at stations 3 (Deserta), 4 (Farol) and 9 (Tavira). These concentrations were significantly different from the other sampling sites (P < 0.05). The lowest concentrations were found at sites 1, 5 and 6.
The concentrations of Cr ranged from 0.46 ± 0.08 to 1.01 ± 0.61 mg g-1. The highest Cr concentrations were found at site 2 (Cais Comercial de Faro), which was significantly different from all the other sites (P < 0.05). Between the other sites there was no significant spatial variability of Cr concentrations.
In comparison to the other metal concentrations determined in the edible part of mussels from the Ria Formosa lagoon, Cu levels did not show any significant spatial variability (4.57 ± 1.21 to 6.35 ± 1.22 mg g-1). However, the highest concentrations were recorded at sites 1 (5.82 ± 1.52), 2 (6.35 ± 1.22), 6 (5.62 ± 0.87) and 7 (6.00 ± 0.96).
The concentrations of Ni in the whole soft tissues ranged from 0.22 ± 0.04 to 0.61 ± 0.32 mg g-1. The highest Ni concentrations occurred at sites 1 (0.61 ± 0.32), 7 (0.52 ± 0.14) and 9 (0.47 ± 0.24), and were significantly different from the other sites (P < 0.05).
The concentrations of Pb ranged from 0.84 ± 0.34 to 2.89 ± 0.96 mg g-1. The highest Pb concentrations occurred at station 9 (2.89 ± 0.96), which was significantly different from the other parts of the lagoon. The lowest concentrations were found at sites 1 (1.24 ± 0.16), 2 (0.84 ± 0.34), 5 (1.10 ± 0.36) and 7 (1.19 ± 0.08).
In contrast to Cu, Zn concentrations in the mussels showed greater variability, ranging from 156 ± 41 to 321 ± 120 mg g-1.
Similarly to Cd, the highest concentrations of Zn were also found at sites 3 (321 ± 120) and 4 (301 ± 109).
Data were treated together and CA was performed. The CA in mode Q is presented in figure 3a. As can be seen, the sampling sites were divided into two main groups. The first one includes sites 1, 2, 5, 6 and 7, whereas the second one includes sites 3, 4, 8 and 9. The correlation matrix between the sites (table 1) shows that, in the first group, the most similar sites are those on the main Faro-Olhao channel, namely stations 2 and 5 (r = 0.779), and secondly sites 1, 6 and 7. In the other group it is possible to distinguish two subgroups, one including the sites near inlets (3 and 4) and the other the sites in the eastern zone of Ria Formosa (8 and 9). The most similar sites in these subgroups are stations 3 and 4, with a correlation coefficient of r = 0.822 (P < 0.05).

The CA in mode R is represented in figure 3b. In this dendogram metals are divided into two main groups (the correlation matrix between the seven metals is shown in table 2). The first group includes Ag, Ni, Cu and Cr and the second Cd, Zn and Pb. There is a significant correlation between Ag and Ni (r = 0.737) and between Cd and Zn (r = 0.695) bioaccumu-lated in the mussels' whole soft tissue. The concentrations of Pb are also related to Cd and Zn (r = 0.692) and those of Cu are to Ag and Ni (r = 0.548). There is no significant relationship between Cr and Ag, nor between Ni and Cu (r = 0.120) (P > 0.05).
The application of PCA presented in figure 4 complements the results obtained by CA, and with the biplot it is possible to observe sites (mode Q) and metals (mode R) simultaneously. The first axis (PC1) accounts for 46% of the total variation, while the second (PC2) for only about 25%. PC1 and PC2 represent about 71% of the total variability. As in the CA, the division of the sites (mode Q) is probably related to their location in the Ria Formosa. Thus, three main areas can be distinguished in the lagoon: the area near one of the inlets (sites 3 and 4), the western area (sites 1, 2, 5, 6 and 7) and the eastern area (sites 8 and 9).

From figure 4 it is possible to observe that the metals occur near the sites in which their concentrations are higher. Hence, Ni and Ag are closer to site 1; Pb to site 9; Zn and Cd to sites 3, 4 and 9; Cr to sites 2 and 5; and Cu to sites 1, 2 and 7.
Discussion
Metal concentrations in whole soft tissues of mussels revealed that in spite of the considerable water renovation that occurs between tides, specific metal differences are observed among sites. These differences detected for certain metals highlight the presence of important pollution sources.
A metal associated with human activities is Ag, of which the natural sources are practically absent in coastal areas and estuaries (Muñoz-Barbosa et al., 2000). Contamination by Ag can be due to various sources, the most likely being its use in photography, X-rays, batteries and electric equipment (Clark, 2001). The highest Ag concentrations observed in the mussels from Ria Formosa occurred at sites 1, 2, 6 and 9. Sites1, 2 and 6 are located in an interior zone (with less efficient water renovation) and, therefore, more susceptible to the deposition of contaminated suspended particles. Site 9 is located in a channel where the renovation of water is higher, but it is near an urbanized area directly influenced by domestic and industrial wastewater discharges. Furthermore, it is located near the impact of the Gilao River, which can transport other contaminated materials from the inner part of the land.
The concentration of Ag in the edible part of the mussels is very low in comparison to other coastal areas (table 3).
The concentrations of Cd in the Ria Formosa lagoon are higher at sites 3, 4 and 9. The concentrations observed at site 9 could be related to urban discharges and agricultural runoffs. The transport of fertilizers and pesticides from agriculture can constitute an important non-point source of Cd to the marine environment (Laws, 1993). On the other hand, site 9 is located in the area of influence of the Gilao River, where the changes in salinity in surrounding areas can enhance the bioavailability of Cd (Wright, 1995). At sites 3 and 4 there is a strong oceanic influence (Andrade, 1990). In this case, the sources of Cd could be related to the resuspension of sediments or to the transport of Cd from the ocean by physical processes, like advection and upwelling (Munoz-Barbosa et al., 2000), which are known to occur occasionally in that area; these processes have also been related to Cd levels in the water column (results not published).
In comparison to other studies it is possible to say that Cd concentrations in the Ria Formosa lagoon are low (table 3). However, considering the Cd value of 0.5 mg g-1 in the edible part of the mussel as an indicator of contamination by this metal (Burt and Scrimshaw, 1993), we can observe that Cd levels are slightly higher at a few sites (3, 4 and 9).
The Cr concentrations detected in the edible part of the mussels did not show any significant variation along the Ria Formosa, except for site 2, where the concentrations were significantly higher. This could be related to industrial discharges, which according to Walsh and O'Halloran (1998) constitute an important source of Cr in the environment. Another significant source could be the oxidation of the metallic structures and ships found at that site, possibly releasing Cr into the water (Irwin et al., 1997).
The Cr levels in the Ria Formosa mussels are lower (table 3) than those reported for other geographical areas, except in comparison to those of Mytilus edulis in Canada (Lobel et al., 1990). The Ria Formosa specimens reveal Cr concentrations higher than 0.5 mg g-1, which according to Burt and Scrimshaw (1993) is an indicator of Cr contamination.
The Cu concentrations in the Ria Formosa mussels, like those of Cr, show a certain homogeneity between sites. Considering that the most significant Cu sources are the urban discharges, release from metallic structures, antifouling paints and fertilizers (Clark, 2001), one could assume that the highest concentrations occurred at sites near urbanized areas, like stations 2, 6 and 9. This reduced variation of Cu concentrations can be related to the fact that mussels easily eliminate this metal; therefore, Cu levels in this lagoon could be underestimated (Langston and Spence, 1995; Brown and Depledge, 1998).
The Cu levels in the Ria Formosa mussels are lower than those observed by several authors in different coastal areas (table 3), but they are higher than 1.5 mg g-1, which indicates contamination by this metal (Burt and Scrimshaw, 1993).
The Ni concentrations in the Ria Formosa mussels were highest at sites 1, 7 and 9. This metal enters the marine environment essentially through urban and industrial discharges (Irwin et al., 1997). The levels at site 1 could be related to domestic inputs, the major source of contamination at this site (Newton, 1995). At sites 7 and 9 the contamination is probably due to industrial and domestic inputs, which occur near both sites. Even though site 7 is far from the main source of contamination, the water circulation in Ria Formosa could explain the transport of Ni from those areas.
The concentration of Ni in the Ria Formosa mussels is lower than those in mussels from coastal areas of Europe and North America (table 3).
The highest Pb levels occur at sites 3 and 9. According to Cortesao et al. (1986) and Bebianno (1995), the highest concentrations of Pb in the Ria Formosa are related to the input by urban discharges and the use of Pb in boat fuel. The Pb concentrations observed at site 9 are probably due to urban discharges and boat fuel, since there is a lot of maritime traffic in this area. Additional sources could be road runoffs (Sadiq, 1992) and atmospheric deposition (Laws, 1993). Similarly, Cortesao et al. (1986) also observed high levels of Pb at site 3 (Deserta), which is probably related to the intense maritime traffic in that area.
In comparison to other studies it is possible to say that Pb concentrations in the Ria Formosa mussels are lower than those found in the United Kingdom and Italy (table 3), but higher than those for M. edulis in Canada (Lobel et al., 1990) and for M. californianus on the western coast of the USA (Muñoz-Barbosa et al., 2000). Taking into account the level of 1 mg g-1 as an indicator of Pb contamination (Burt and Scrimshaw, 1993), one can say that the concentrations of Pb in the Ria Formosa mussels are higher than that level.
The concentrations of Zn show a similar distribution to that observed for Cd, which is in agreement with Coimbra and Carraga's study (1990). Because Zn and Cd belong to the same group of metals (IIB), they possess a similar geochemical behaviour and so their distribution is usually very similar. The sources of Zn in this lagoon include industrial discharges, release from metallic structures and rubber materials (Hunter et al., 1995), probably occurring near sites 3, 4 and 9.
The Zn concentrations in the Ria Formosa mussels are lower than those observed by Bryan et al. (1985) in the United Kingdom, but higher than those observed by Lauenstein et al. (1990) on the eastern coast of the USA, by Lobel et al. (1990) in Canada and by Widdows et al. (1997) in Italy (table 3). The Ria Formosa mussels present Zn levels ten times higher than 30 mg g-1, which indicates Zn contamination (Burt and Scrimshaw, 1993).
With the application of CA and PCA in mode Q (by sites), there is a clear distinction between the sites located in the western area, eastern area and inlet area. In fact, one can divide Ria Formosa based on the water circulation. The water inflow splits the lagoon system into two main sectors: western and eastern; the latter is confined to the east of site 7 and is only connected during spring tide. This way the sources of contamination are restricted to a particular sector, separating sites 1, 2, 5, 6 and 7 from sites 8 and 9.
The western sector is divided into two subgroups (figs. 3, 4): one comprised by sites 1, 6 and 7, and the other by sites 2 and 5. This division is not related to the proximity of the sites, but to the influence of the respective water inlet, which in a way restricts the water that flows into a particular site, and to the type of discharges that occur nearby, which would confer similar metal concentrations.
The inlet area that includes sites 3 and 4 is more influenced by the sea, showing different metal concentrations than those observed in the other areas of the lagoon. At these sites water renovation occurs more frequently and so the impact of the metal discharges from the interior of the lagoon is inferior. Sites 3 and 4 are associated with the highest concentrations of Cd and Zn, which can also come from the coastal zone. Another factor can be due to the regeneration of Cd and Zn in the water column, in a cycle similar to that of nutrients (Muñoz-Barbosa et al., 2000).
From the present work it is possible to conclude that metal concentrations in the Ria Formosa mussels decrease according to the order: Zn > Cu > Pb > Cr > Cd > Ni > Ag. Also, three main areas can be distinguished in the Ria Formosa lagoon, related to the similarities in metal concentrations: a western zone that includes sites 1, 2, 5, 6 and 7; an eastern zone that includes sites 8 and 9; and an inlet zone that comprises sites 3 and 4. Comparatively, the metal concentrations exhibited in the edible part of the Ria Formosa mussels are inferior to those observed in mussels from coastal areas in Europe and USA. Therefore, metal levels in mussels can be used, together with the suspension-feeder R. decussatus, as bioindicators of metal contamination in this ecosystem.
References
Andrade, P. (1990). O Ambiente de Barreira da Ria Formosa, Algarve, Portugal. Dissertação de Doutoramento em Geologia do Ambiente. Departamento de Geologia da Universidade de Lisboa. [ Links ]
Bebianno, M.J. (1995). Effects of pollutants in the Ria Formosa Lagoon, Portugal. Sci. Total Environ., 171: 107-115. [ Links ]
Bebianno, M.J. and Machado, L.M. (1997). Concentrations of metals and metallothioneins in Mytilus galloprovincialis along the south coast of Portugal. Mar. Pollut. Bull., 34(8): 666-671. [ Links ]
Boening, D.W. (1999). An evaluation of bivalves as biomonitors of heavy metal pollution in marine waters. Environ. Monit. Assess., 55: 459-470. [ Links ]
Brown, M.T. and Depledge, M.H. (1998). Determinants of trace metal concentrations in marine organisms. In: W.J. Langston and M.J. Bebianno (eds.), Metal Metabolism in Aquatic Environments. Chapman and Hall, London, pp. 185-217. [ Links ]
Bryan, G.W., Langston, W.J., Humerstone, L.G. and Burt, G.R. (1985). A guide to the assessment of heavy metal contamination in estuaries using biological indicators. Occas. Publ. Mar. Biol. Assoc. UK, 1: 1-73. [ Links ]
Burt, J.S. and Scrimshaw, C.E. (1993). A comparative survey of heavy metals in the mussel Mytilus edulis from Cockburn Sound and surrounding waters. Environmental Protection Authority, Perth, Western Australia, Tech. Ser. No. 56, 27 pp. [ Links ]
Clark, R.B. (2001). Marine Pollution. 5th ed. Oxford University Press, New York, 237 pp. [ Links ]
Coimbra, J. and Carraga, S. (1990). Accumulation of Fe, Zn, Cu, and Cd during the different stages of the reproductive cycle in Mytilus edulis. Comp. Biochem. Physiol., 95C(2): 265-270. [ Links ]
Cortesao, C., Mendes, R. and Vale, C. (1986). Metais pesados em bivalves e sedimentos na Ria Formosa, Algarve. Bol. Inst. Nac. Invest. Pescas, No. 14: 3-28. [ Links ]
Goldberg, E.D., Bowen, V.T., Farrington, J.W., Harvey, G., Martin, J.H., Parker, P.L., Risebrough, R.W., Robertson, W., Schneider, E. and Gamble, E. (1978). The Mussel Watch. Environ. Conserv., 5(2): 101-125. [ Links ]
Gutiérrez-Galindo, E.A., Villaescusa-Celaya, J.A. and Arreola-Chimal, A. (1999). Bioaccumulation of metals in mussels from four sites of the coastal region of Baja California. Cienc. Mar., 25(4): 557-578. [ Links ]
Hunter, C.L., Stephenson, M.D., Tjeerdema, R.S., Crosby, D.G., Ichiwaka, G.S., Goetzl, J.D., Paulson, K.S., Crane, D.B., Martin, M. and Newman, J.W. (1995). Contaminants in oysters in Kaneohe Bay, Hawaii. Mar. Pollut. Bull., 30(10): 646-654. [ Links ]
Irwin, R.J., VanMouwerik, M., Stevens, L., Seese, M.D. and Basham, W. (1997). Environmental Contaminants Encyclopedia. National Park Service, Water Resources Division, Fort Collins, Colorado. [ Links ]
Kennish, M.J. (1992). Ecology of Estuaries: Anthropogenic Effects. Marine Science Series, USA, 494 pp. [ Links ]
Langston, W.J. and Spence, S.K. (1995). Biological factors involved in metal concentrations observed in aquatic organisms. In: A. Téssier and D.R. Turner (eds.), Metal Speciation and Bioavailability in Aquatic Systems. Vol. 3. IUPAC, England, pp. 407-478. [ Links ]
Lauenstein, G.G., Robertson, A. and O'Connor, T.P. (1990). Comparison of trace metal data in mussels and oysters from a mussel watch programme of the 1970s with those from a 1980s programme. Mar. Pollut. Bull., 21(9): 440-447. [ Links ]
Laws, E.A. (1993). Aquatic Pollution. 2nd ed. John Wiley, USA, 611 pp. [ Links ]
Lobel, P.B., Belkhode, S.P., Jackson, S.E. and Longerich, H.P. (1990). Recent taxonomic discoveries concerning the mussel Mytilus: Implications for biomonitoring. Arch. Environ. Contam. Toxicol., 19: 508-512. [ Links ]
Matos, N.F., Partidário, M.R. and Oliveira, R. (1999). Estudo ambiental do "Projecto de requalificagao do Sistema Lagunar da Ria Formosa". 6th National Conference of Environmental Quality, Vol. 3, Lisbon, pp. 479-191. [ Links ]
Mudge, S.M. and Bebianno, M.J. (1997). Sewage contamination following an accidental spillage in the Ria Formosa, Portugal. Mar. Pollut. Bull., 34(3): 163-170. [ Links ]
Muñoz-Barbosa, A., Gutiérrez-Galindo, E.A. and Flores-Muñoz, G. (2000). Mytilus californianus as an indicator of heavy metals on the northwest coast of Baja California, Mexico. Mar. Environ. Res., 49: 123-144. [ Links ]
Newton, A. (1995). The water quality of the Ria Formosa Lagoon, Portugal. Ph.D. Thesis, University of Wales, 226 pp. [ Links ]
Rainbow, P.S. (1995). Biomonitoring of heavy metal availability in the marine environment. Mar. Pollut. Bull., 31(4-12): 183-192. [ Links ]
Rainbow, P.S. (1996). Heavy metals in aquatic invertebrates. In: W.N. Beyer, G.H. Heinz and A.W. Redmon-Norwood (eds.). Environmental Contaminants in Wildlife. Lewis Publ., Boca Raton, Florida, pp. 405-425. [ Links ]
Rainbow, P.S. and Phillips, D.J. (1993). Cosmopolitan biomonitors of trace metals. Mar. Pollut. Bull., 26(11): 593-601. [ Links ]
Sadiq, M. (1992). Toxic Metal Chemistry in Marine Environments. Marcel Dekker, New York, 390 pp. [ Links ]
Walsh, A.R. and O'Halloran, J. (1998). Accumulation of chromium by a population of mussels (Mytilus edulis (L.)) exposed to leather tannery effluent. Environ. Toxicol. Chem., 17(7): 1429-1438. [ Links ]
Widdows, J., Nasci, C. and Fossato, V.U. (1997). Effects of pollution on the scope for growth of mussels (Mytilus galloprovincialis) from the Venice Lagoon, Italy. Mar. Environ. Res., 43(1/2): 69-79. [ Links ]
Wright, D.A. (1995). Trace metal and major ion interactions in aquatic animals. Mar. Pollut. Bull., 31(1-3): 8-18. [ Links ]














