Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Ciencias marinas
versión impresa ISSN 0185-3880
Cienc. mar vol.31 no.1b Ensenada may. 2005
Artículos
Bioavailability of heavy metals in the Guadalete River Estuary (SW Iberian Peninsula)
Biodisponibilidad de metales pesados en el Estuario del Río Guadalete (SO Península Ibérica)
Olivia Campana, Antonio Rodríguez, Julián Blasco*
* Instituto de Ciencias Marinas de Andalucía Campus Río S. Pedro 11510 Puerto Real, Cádiz, España * E-mail: julian.blasco@icman.csic.es
Recibido en junio de 2003;
aceptado en febrero de 2004.
Abstract
Sediments in the Guadalete River estuary were studied to determine the bioavailability of five common metals (Cu, Zn, Cd, Pb and Ni) in order to evaluate the toxicity of the sediment. Total sediment dry weight concentrations of heavy metals, simultaneously extracted metals (SEM) and acid volatile sulfides (AVS) were analyzed. Interstitial water criteria toxic units (IWCTU) were calculated and used as criteria to evaluate toxicity, making reference to the Sediment Quality Guidelines developed by the US Environmental Protection Agency. Diffusive fluxes through the sediment-water interface were also analyzed. The balance of deposition and return fluxes demonstrated the efficiency of estuarine sediment from Guadalete as sink for metal contaminants. The ratio between SEM and AVS was smaller than one, apparently indicating that the sediment was not toxic; however, the high concentrations of Cu and Pb in pore water make further investigations necessary to evaluate the real bioavailability and bioaccumulation of trace metals to show the obviousness of toxicity.
Key words: Sediment, bioavailability, metals, SEM, AVS.
Resumen
Se analizaron los sedimentos del Estuario del Río Guadalete con el fin de determinar la biodisponibilidad de los metales Cu, Zn, Cd, Pb y Ni, y evaluar la toxicidad de los sedimentos. Se analizó la concentración total de metales, los metales extraídos simultáneamente (SEM), y los sulfuros volátiles ácidos (AVS). Para la evaluación de la toxicidad se calcularon y emplearon los criterios de unidades tóxicas para el agua intersticial (IWCTU). Para ello se emplearon las referencias propuestas por las Guías de Calidad de Sedimentos desarrolladas por la Agencia de Protección Ambiental de Estados Unidos (USEPA). También se ha procedido al análisis de los flujos difusivos a través de la interfase agua-sedimento. Los balances de flujo de entrada y salida mostraron la eficiencia de los sedimentos del Estuario del Guadalete como sumidero de contaminación metálica. La proporción entre SEM y AVS fue menor que 1, e indicaba, en principio, que el sedimento no era tóxico. No obstante, las altas concentraciones de Cu y Pb en el agua intersticial ponen de manifiesto la necesidad de evaluar la biodisponibilidad real y la bioacumulación de los metales a fin de establecer de manera inequívoca la no toxicidad de los sedimentos.
Palabras clave: Sedimento, biodisponibilidad, metales, SEM, AVS.
Introduction
Estuaries are very important ecosystems because they undergo numerous chemical and biological processes and they are vital for the growth of several marine species. Nevertheless, too often, these systems are subjected to heavy anthropogenic impact due to agricultural, industrial and urban wastes, and bed sediments act as sink of organic and inorganic contaminants. The adsorbed metals on particulate matter can be desorbed and released into the water column and pore water. In this way, the metals will be uptaken and bioaccumulated by benthic and pelagic organisms. Hence, the measure of bioavailability and subsequent toxicity of the metals represents the first step to assess the risk of sediment contamination and eventually implement regulatory strategies to limit or prevent additional contamination. Aiming to protect the environment, Sediment Quality Guidelines (SQG) have been developed by the US Environmental Protection Agency in an effort to assess sediment toxicity with broad applicability. However, in view of the complexity of sediment environments, most studies still focus on establishing SQG for specific districts or situations, such as fresh-water and marine sediments (Liu et al., 1999).
Although most studies have tried to evaluate metal bioavailability based primarily on metal concentration in interstitial water, it is critical to bear in mind all possible sources of metal bioavailability and identify the habits of the species studied, i.e., benthic or pelagic species, suspension feeders or detritivors, etc. For instance, it is inappropriate to consider interstitial water as the only route of exposure to contaminants in sediments when organisms that ingest substantial amounts of sediments are studied (Chapman, 1995). Moreover, it is insufficient to predict toxic effects on suspension feeders based on metal bioavailability in interstitial water.
The objective of this study is to determine the bio-availabilty of five common metals (Cu, Zn, Cd, Pb and Ni), considering different sources of metal bioavailability, and evaluate the toxicity of sediments in the Guadalete River estuary. Total sediment dry weight concentration for each metal was considered and compared with critical values found in the NOAA Screening Quick Reference Tables (SQUIRTs) (Buchman, 1999). With reference to SQG, according to two prevalent approaches, we calculated acid volatile sulfides (AVS) and interstitial water criteria toxic units (IWCTU), proposed by Ankley et al. (1996). Since particle-reactive metals released into estuarine or coastal marine waters can be scavenged by particles and removed to the sediment (deposition flux), or metals can move back up to the overlying water (return diffusion flux) due to digenetic reactions at the sediment-water interface, the balance of deposition and return fluxes is very important to determine the efficiency of estua-rine or marine coastal systems as sink for metal contaminants or source of contaminants for the overlying water column (Shine et al., 1998). In consequence, trace metal diffusive fluxes through the sediment-water interface were also calculated.
Material and methods
Study area
The Guadalete River flows into the Bay of Cádiz, located in the southwestern Iberian Peninsula (fig. 1). Since the 1960s, this river has been subjected to intense pollution due to discharges of urban, industrial, agricultural and livestock wastes from more than twenty urban centers of nearly 400,000 inhabitants. The recovery of the Guadalete River began in 1988 with a plan that included the construction of waste-depuration plants along the river to remove the contamination.
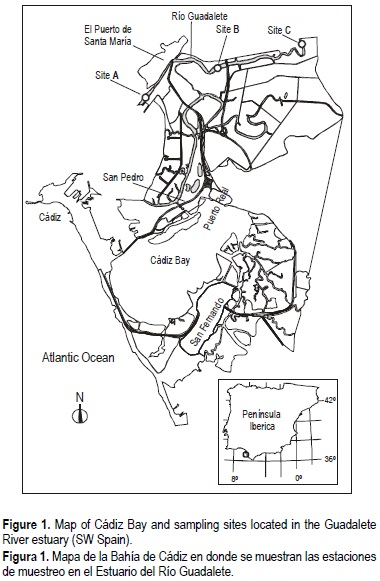
The sampling stations were selected in three different environments of the estuary, with a distance of approximately 1 km between each other. Site A is located near the town of El Puerto de Santa María, in front of the sailing harbor, in an area of commercial traffic, and its substrate is sandier than that of the other stations. Sites B and C were situated more inland, in areas surrounded by crops and salt-marshes. Severe mollusk harvesting occurs at site B. All sediments are anoxic and subject to a tidal regime.
Sample collection and handling
Sediments were collected in February 2001 and two replicates of sediment cores were taken from each station with a polycarbonate tube core (5 cm inner diameter, 50 cm in length). Immediately after collection, core tubes were kept in the upright position to avoid mixing, sealed in a plastic bag and frozen at -20°C within 3 h of collection, until analysis. In the laboratory, sediment cores were sub-sampled still frozen, in five 2-cm thick segments at different depths: 0-2, 4-6, 10-12 and 20-22 cm, and the last 2 cm of each core. Homogenizing was rapidly performed and sub-samples of sediments were frozen until AVS analysis or directly processed for total digestion and interstitial water determinations. No anaerobic condition was used for the handling or storage of samples, because according to Boothman and Helmstetter (1992), it is possible to process and then refrigerate or freeze sediments without a nitrogen atmosphere for up to 14 days with no loss of AVS.
Sediment analysis
Sediments were analyzed for AVS, simultaneously extracted metals (SEM), total metal concentration and porosity. The concentrations of AVS in the sediment were analyzed according to the method proposed by Simpson (2001). The SEM were extracted adding 15 mL of 10% HCl to approximately 1.5 g of wet sediment, for 2 h with continuous agitation. After extraction, a centrifuge was performed at 3000 g for 30 min to separate hydrochloric acid extracts from solids (Langston and Spence, 1994).
To determine total metal concentration, approximately 0.20 g of dry weight sediment was digested by microwaves, following the method described by Loring and Rantala (1992). SEM and total metal concentration were analyzed by graphite furnace (GFAAS, PE 4100ZL), with Zeeman background correction and appropriate matrix modifiers. Blanks were run with each batch of analysis. Samples of a reference estuarine sediment (BCR n.277) were also analyzed, simultaneously with all metal analysis, to determine the analytical accuracy. Accuracy for samples ranged from 70% to 99%. Merck Supra-pur reagents were used in all cases. Porosity of the sediment (Φ) was measured as the loss in weight by desiccation.
Pore water analysis
Pore water was extracted by centrifugation at 48,000 g for 1 h at 4°C using a Beckman J2-21 centrifuge. The pore water samples obtained were kept in an acid environment by adding ultra-pure HCl, 1% (v/v). The determination of Zn, Cd, Pb and Cu was made with anodic stripping voltammetry using Metrohm equipment (VA 746 Trace Analyzer) over a 10-mL volume of sample (Ponce et al., 2000). To avoid the interference in the analytical signals due to the presence of the organic matter in the pore water samples, the oxidation of organic matter was conducted with UV radiation digestion (Metrohm, 705 UV), at 80-90°C with 1% H2O2 for 90 min.
Sediment quality criteria
As proposed by Ankley et al. (1996), the bioavailability of metals in the sediments was predicted, following Sediment Quality Criteria (SQC) based on AVS criteria and IWCTU. Because metals act in a competitive manner when binding to AVS, e.g., in the order of increasing solubility, they must be considered together. Likewise, regarding their possible toxic interactions, the interstitial water metal concentrations are converted into toxic units and these are summed. When sediments exceed the SQC and have no effect or biological impact on organisms, all of the following conditions are satisfied:
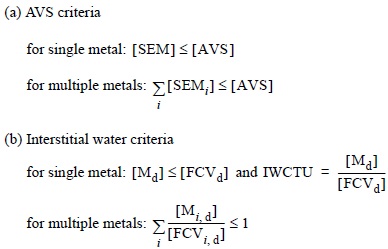
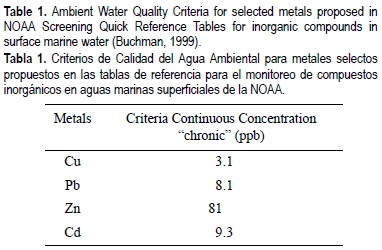
where [SEM] is the concentration of simultaneously extracted metal, [AVS] is the concentration of acid volatile sulfides, [Md] is the total dissolved interstitial water concentration of metal and [FCVd] is the final chronic value of metal applied to total dissolved metal concentration. We calculated the FCV from Criteria Continuous Concentration (CCC) recommended by SQUIRTs, for trace elements in Ambient Water Quality Criteria (AWQC) (Buchman, 1999). The CCC values for Cu, Zn, Pb and Cd are shown in table 1. In order to establish the relative affinity of metals for solid phase, the partition coefficient for each metal (K) was calculated as follows:
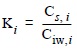
where Cs,i and Ciw,i represent the total concentration of the ith metal in the solid phase and in interstitial water, respectively. Because of the molar stoichiometry of metal binding to AVS, the solid-phase constituents (AVS, SEM) are expressed in µmol g-1 dry weight. The interstitial water metal concentrations are expressed in µmol L-1 and the partition coefficients are in L pore water g-1 dry weight.
SQUIRTs for trace elements in marine sediment
Total metal dry weight concentrations were compared with marine sediment screening values reported by NOAA SQUIRTs, which are associated with several probabilities of adverse biological effects. Threshold Effect Levels (TELs) represent the level below which adverse effects are expected to occur only rarely. Conversely, they do not predict toxicity. The Effect Range Low (ERL) represents the value at which toxicity may begin to be observed in sensitive species. Probable Effect Levels (PELs) are the concentration above which adverse effects are frequently expected (Buchman, 1999).
Diffusive fluxes
Benthic fluxes of a dissolved species through the sediment-water interface, due to molecular diffusion, were estimated applying Fick's first law:
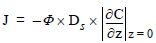
where J is the diffusive flux, Φ is the porosity and  is the concentration gradient between the concentration of the dissolved species in the pore water in the upper layer of the sediment and that in the overlying water column. Since the remobilization took place in the upper layer of the sediment, a linear fit of the concentration of trace metals in the pore water at different depths was used to calculate the concentration gradients of trace metals between the pore waters and the overlying waters. Calculated from Sweerts et al. 's (1991) empirical equation, Ds is the sediment diffusion coefficient:
is the concentration gradient between the concentration of the dissolved species in the pore water in the upper layer of the sediment and that in the overlying water column. Since the remobilization took place in the upper layer of the sediment, a linear fit of the concentration of trace metals in the pore water at different depths was used to calculate the concentration gradients of trace metals between the pore waters and the overlying waters. Calculated from Sweerts et al. 's (1991) empirical equation, Ds is the sediment diffusion coefficient:
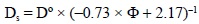
where D° is the diffusion coefficient to infinite dilution. For Zn, Cd, Pb and Cu, the values of D° used were those described by Li and Gregory (1974) for a temperature of 18°C.
Statistics
A p-Pearson correlation was performed with Statistica 5.0 computer software; P < 0.05 was used to statistically determine the significance.
Results and discussion
SEM/AVS ratio
The SEM, AVS and IWCTU concentration values for each metal and for all sites and depths are reported in table 2. The depth variation showed that SEM concentration was lower than AVS concentration, except in the first 5 cm of the superficial sediment of site B, although the difference between AVS and SEM concentrations is very small. Depth profiles of AVS concentrations showed a similar trend. The concentrations of AVS increased with depth, reaching a maximum at approximately 10 cm. Most reports and several studies (Di Toro et al., 1996; Peterson et al., 1996; Yu et al., 2001) indicate that surface concentrations of AVS are smaller than in deeper sediments, because in the upper sediment, AVS, formed by anaerobic oxidation of organic matter, are exposed to bio-irrigation, pore-water mixing and bioturbation that cause aerobic oxidation of the sulfide, thus increasing the rate of AVS depletion. Sites B and C showed similar sediment characteristics for organic matter content and percentage of fine-fraction (silt and clay) (table 3); however, at site C, AVS concentration reached 20.2 µmol g-1, while at site B, the maximum value was 1.3 µmol g-1. This difference may be due to the intense activity of mollusk harvesting that could increase penetration of oxygen and, together with bio-irrigation, may lead to oxidation of the sulfide. Moreover, the lower anoxic condition of the surface sediment at site B (table 3) could control AVS concentration as indicated by Gonzalez (1996), who reported that redox potentials appear to be inversely related to AVS concentrations, even if this covariation is weak, especially when AVS is <4 µmol g-1 dry weight. At site A, the low concentrations of AVS can be associated with low organic matter content, as well as with the low concentrations of total extracted heavy metals (table 3). This could be due to different sedimentary characteristics, since they show a high percentage of the sandy fraction, known to be poor in binding metals compared with silt or clay.
Pore water profiles
The vertical profiles of Zn, Cd, Cu and Pb in the pore water at each site are presented in figure 2. At site B, in the upper sediment that presented the highest SEM/AVS ratios and highest pore water metal concentrations, the concentrations for Zn, Pb and Cu in the interstitial water appeared inversely correlated to the AVS concentrations (at P < 0.05, rZn = -0.89, rPb= -0.74, rCu= -0.66). If SQC are applied, sediments pass the first condition (AVS criteria), except at site B, depending on the degree of accuracy. Nevertheless, even though the binding capacity of AVS with metals was not totally exploited, metals were remobilized into pore water and all three sediments violated the second condition (interstitial water criteria). The IWCTU were especially elevated for Cu and Pb and shot up SIWCTU values (table 2).
SQUIRTs
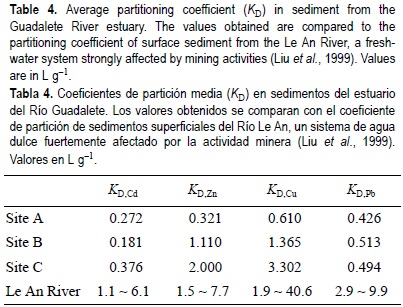
The elevated Cu concentrations in pore water reflected the high Cu levels in sediment, which for sites B and C exceeded the Threshold Effect Levels proposed by NOAA (fig. 3). Also, total dry weight concentration for Ni showed values higher than the Effect Range Low for site B, around 40 µg g-1, and near the Probable Effect Level value proposed for site C (fig. 3). Comparison of Cu, Pb, Cd and Zn total dry weight concentrations, in the solid phase, showed that the concentrations were lower than those reported in others studies for surface sediments located in the southern part of the Bay of Cádiz (Ponce et al., 2000). Nevertheless, metals had higher bioavailability. This large bioavailability may be due to a great affinity of metals for the aqueous phase, reflected by the small values of partitioning coefficients, KD (table 4), lower than those reported by Liu et al. (1999) for fresh-water sediments strongly impacted by local mining activities in the Le An River region. Especially in the case of Pb, KD was below one order of magnitude compared with the minimum value reported by Liu et al. (1999). Based on KD values, the sequence of affinity of the metals for the aqueous-phase, for all three sites, was: Cd > Pb > Zn > Cu. On the other hand, even in sediments where concentrations of AVS are significant, other partitioning phases may provide additional binding capacity for SEM. In anaerobic sediments, organic carbon appears to be an additional binding phase controlling metal partitioning, in particular for Cd, Cu and Pb (Ankley et al., 1996). Consequently, the presence of elevated concentrations of particulate organic carbon in the interstitial water, characteristic of estuarine systems (Morris, 1985), may explain the high concentrations of Cu and Pb for unfiltered pore water samples.
Bioavailability and toxicity
Even considering the relatively large bioavailability of trace metals in pore water, especially Pb and Cu, this does not mean that the sediments are toxic, since the water quality criteria are not expressed based on metal activity but on an approximation to this condition (final chronic value), and a substantial number of experiments suggest that biological effects are often correlated to the divalent metal activity, the only bioavailable form (Ankley et al., 1996). Another reason is associated with equilibrium assumption of SQC. This theory assumes that pore water is in equilibrium with the sediment. If the sediment is anoxic and therefore toxic, pore water does not meet the water quality standards. Also, based on this assumption, in presence of AVS, there is no oxygen available for aerobic life. Except near the sediment-water interface, pore water in most coastal sediments is anoxic. However, these conditions are not lethal to benthic organisms because they develop strategies to obtain this oxygen and water-borne nourishment from the overlying water or they irrigate their environment with such water (O'Connor and Paul, 2000). So, if benthic organisms do not equilibrate with the pore water it is not correct to think that the bioavailability of heavy metals in the pore water is toxic.
Diffusive fluxes
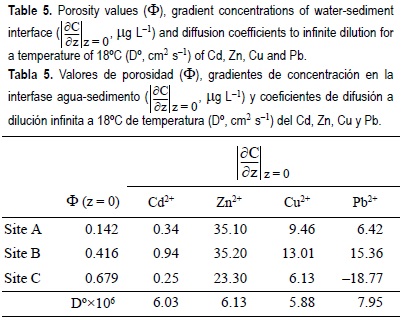
The diffusive fluxes calculated for Cd, Pb, Cu and Zn by pore water profiles (table 5) indicated that the metals migrated downward into the sediment following a net deposition flow (table 6). Since the return diffusion flux is lower than the deposition or burial flux, the water column residential time of the metal decreases, thus decreasing the likelihood that the contaminant will be flushed out of the system and transported offshore. This is true for all sites and metals, except for Pb at site C, where flux was negative, thus producing a net return diffusion flux. The order of magnitude of the estimated diffusive fluxes was Zn > Cu = Pb > Cd, though no significant differences were observed between Cu and Pb at site A. The overall implication is that these sediments are an efficient trap for metals. Due to physical and biological processes, the magnitude of diffusive fluxes is associated with seasonal variability and an evaluation of these fluxes during the year is essential to determine the relative importance of sediment-water fluxes on the cycling of metals. Shine et al. (1998) reported that Cd and Pb were, respectively, more and less sensitive to changes in temperature. For this reason, the fluxes estimated here should be analyzed over more time (Yu et al., 2000).
In conclusion, the analysis of diffusive fluxes through the sediment-water interface demonstrated the efficiency of the estuarine sediment from Guadalete as sink for heavy metals. As proposed by SQC, even if the ratio between SEM and AVS is smaller than one, these should not be used as the only criteria to establish toxicity. Evidence that Cu and Pb concentrations are higher than the Threshold Effect Levels proposed by NOAA seems to indicate that further studies are necessary, with both benthic and pelagic organisms, in order to evaluate the real bioavailability and bioaccumulation of trace metals, and to test acute and chronic toxicity and whether this is caused by metals or other chemicals.
Acknowledgements
This work was carried out within the framework of the project Estudio de las Comunidades Acuáticas del Estuario del Río Guadalete y su Función como Zona de Cría de Especies Marinas, funded by the Consejería de Medio Ambiente (Xunta de Andalucía).
References
Ankley, G.T., Di Toro, D.M., Hansen, D.J. and Berry, W.J. (1996). Technical basis and proposal for deriving sediment quality criteria for metals. Environ. Toxicol. Chem., 15(12): 2056-2066. [ Links ]
Boothman, W.S. and Helmstetter, A. (1992). Vertical and seasonal variability of acid volatile sulfides in marine sediments. Final Research Rep., US Environmental Protection Agency, Narragansett, RI. [ Links ]
Buchman, M.F. (1999). NOAA Screening Quick Reference Tables. NOAA HAZMAT Report 99-1, Seattle, WA. Coastal Protection and Restoration Division, National Oceanic and Atmospheric Administration. [ Links ]
Chapman, P.M. (1995). Ecotoxicology and pollution-key issues. Mar. Pollut. Bull., 31: 167-177. [ Links ]
Di Toro, D.M., Mahony, J.D., Hensen, D. and Berry, W.J. (1996). A model of the oxidation of iron and cadmium sulfide in sediments. Environ. Toxicol. Chem., 15(12): 2168-2186. [ Links ]
Gonzalez, A.M. (1996). A laboratory-formulated sediment incorporating synthetic acid-volatile sulfide. Environ. Toxicol. Chem., 15(12): 2209-2220. [ Links ]
Langston, W.J. and Spence, S.K. (1994). Metal analysis. In: P. Calow (ed.), Handbook of Ecotoxicology. Vol. 2. Blackwell Sci. Publ., pp. 45-78. [ Links ]
Li, Y.H. and Gregory, S. (1974). Diffusion of ions in sea water and in deep-sea sediments. Geochim. Cosmochim. Acta, 38: 703-714. [ Links ]
Liu, W., Wang, Z., Wen, X. and Tang, H. (1999). The application of preliminary sediment quality criteria to metal contamination in the Le An River. Environ. Pollut., 105: 355-366. [ Links ]
Loring, D.H. and Rantala, R.T.T. (1992). Manual for the geochemical analysis of marine sediments and suspended particulate matter. Earth Sci. Rev., 32: 235-283. [ Links ]
Morris, A.W. (1985). Estuarine chemistry and general survey. In: P.C. Head (ed.), Practical Estuarine Chemistry: A Handbook. Cambridge Univ. Press, Cambridge, pp. 1-60. [ Links ]
O'Connor, T.P. and Paul, J.F. (2000). Misfit between sediment toxicity and chemistry. Mar. Pollut. Bull., 40(1): 59-64. [ Links ]
Peterson, G.S., Ankley, G.T. and Leonard, E.N. (1996). Effect of bioturbation on metal-sulfide oxidation in surficial freshwater sediments. Environ. Toxicol. Chem., 15(12): 2147-2155. [ Links ]
Ponce, R., Forja, J.M. and Gómez-Parra, A. (2000). Influencia de la actividad antropogénica en la distribución vertical de Zn, Cd, Pb y Cu en agua intersticial y sedimentos marinos costeros (Bahía de Cádiz, SW de España). Cienc. Mar., 26(3): 479-502. [ Links ]
Shine, J.P., Ika, R. and Ford, T.E. (1998). Relationship between oxygen consumption and sediment-water fluxes of heavy metals in coastal marine sediments. Environ. Toxicol. Chem., 17(11): 2325-2337. [ Links ]
Simpson, S.L. (2001). A rapid screening meted for acid-volatile sulfide in sediments. Environ. Toxicol. Chem., 20(12): 2657-2661. [ Links ]
Sweerts, J.P.R.A., Baer-Gilissen, M.J. and Cornelese, A.A. (1991). Oxygen-consuming processes at the profundal and littoral sediment-water interface of a small meso-eutrophic lake (Lake Vechten, The Netherlands). Limnol. Oceanogr., 36: 1124-1133. [ Links ]
Yu, K.C., Lam, M.H.W., Yen, Y.F. and Leung, A.P.K. (2000). Behavior of trace metals in the sediment pore waters of intertidal mudflats of a tropical wetland. Environ. Toxicol. Chem. 19(3): 535-542. [ Links ]
Yu, K.C., Tsai, L.J., Chen, S.H. and Ho, S.T. (2001). Chemical binding heavy metals in anoxic river sediments. Water Res., 35(17): 4086-4094. [ Links ]














