Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Ciencias marinas
versión impresa ISSN 0185-3880
Cienc. mar vol.31 no.1a Ensenada mar. 2005
Artículos
Assessing the potential toxicity of marine sediments found in petroleum industry areas: A new approach based on responses of postlarval shrimp
Evaluación del potencial de toxicidad de sedimentos marinos en áreas de la industria petrolera: Un nuevo método basado en respuestas de postlarvas de camarones
A.J.A. Evangelista1, I.A. Nascimento1*, S.A. Pereira1, M.B.N.L. Lopes1, L.K.P. Martins1 and G. Fillmann2,3
1 Lab. de Bio Marinha e Biomonitoramento, IBIO-UFBA Campus Universitário de Ondina Salvador, BA, 40170-290, Brazil Faculdade de Tecnología e Ciencias - FTC * E-mail: iracema@ftc.br, iraceman@ufba.br
2 Fundacao Universidade do Rio Grande Depto. de Oceanografia C.P. 474 Rio Grande, RS, 96201-900, Brazil.
3 Plymouth Marine Laboratory Prospect Place West Hoe, Plymouth, PL1 3DH, UK.
Recibido en mayo de 2004;
aceptado en agosto de 2004.
Abstract
In this study we tested the toxicity of bulk sediment from the northeastern area of Todos os Santos Bay, Bahia, Brazil, to evaluate environmental impact induced by 50 years of exposure to the local petroleum industry (Petrobras). Sediment samples were collected during one year, at three-month intervals, from four sites in areas of oil extraction (Ilha das Fontes, station 4), transportation (Ilhas de Madre de Deus and Pati, stations 2 and 3) and refinement (RELAM, station 1). Two reference stations (5 and 6) were located outside the petroleum influence area, to the south of the bay. Static bioassays were conducted for 96 h, using 7-8 day old Litopenaeus vannamei postlarvae (PL). The assays were conducted in 2.5 L plastic jars containing 200 g of surface (1 cm deep) bulk sediment covered by 2 L of dilution water (filtered seawater, 28 ppt salinity, 27 + 2°C and DO under saturation). Fifteen exposed PL in each jar were fed daily on 60 recently hatched Artemia salina nauplii. Physico-chemical parameters were monitored. Mortality and dry weight gain were taken as end-points. The PL mortality data obtained for sediment from the Petrobras stations in comparison to the data from the reference stations were not significantly different (P > 0.05); however, the dry weight gain showed significant differences among stations. A maximum value was reached at station 5 (reference area) and a minimum at station 1 (RELAM refinery). Stations 2 and 3 in petroleum transportation areas did not show significant differences (P > 0.05). To evaluate the sensitivity of this bulk-sediment test in detecting contaminant effects generated by the petroleum industry, the toxicity data were considered in terms of the hydrocarbon levels analyzed in sediments from the same Petrobras areas and in one of the control areas, located outside the bay. The results support the assumption that the bulk-sediment bioassay on penaeid PL is a suitable methodology not only to distinguish between impacted and relatively unperturbed environments, but also to separate the different degrees of impact among areas subjected to petroleum industry activities in the coastal environment.
Key words: bulk sediment, biomonitoring, penaeid PL bioassay, petroleum impact, hydrocarbon levels.
Resumen
Se evaluó la toxicidad de sedimentos del área del noreste de la Bahía de Todos os Santos, Bahía, Brasil, para evaluar el impacto ambiental inducido por 50 años de actividad industrial petrolera (Petrobras). Se recolectaron muestras de sedimento durante un año, con intervalos de tres meses, en cuatro sitios relacionados con áreas de extracción (Ilha das Fontes, estación 4), transporte (Ilhas de Madre de Deus y Pati, estaciones 2 y 3) y refinación del petróleo (RELAM, estación 1). Dos estaciones de referencia (5 y 6) se localizaron fuera del área de influencia del petróleo, al sur de la bahía. Se llevaron a cabo bioensayos estáticos por 96 h, usando postlarvas (PL) de Lytopenaeus vannamei con 7 a 8 días de desarrollo. El ensayo fue realizado en contenedores con 200 g de sedimento superficial (1 cm de profundidad) y 2 L de agua (agua de mar filtrada, 28 ppm de salinidad, 27 ± 2°C y DO bajo saturación). Se alimentaron 15 PL en los contenedores diariamente con 60 nauplios de Artemia salina. Se monitorearon parámetros químicos. La mortalidad y la ganancia en peso seco fueron tomadas como parámetros de validación (end point). Los datos de mortalidad obtenidos de los sedimentos de las estaciones de Petrobras no mostraron diferencias significativas, en comparación con las estaciones de referencia; sin embargo, la ganancia en peso seco mostró diferencias significativas entre estaciones. El valor máximo se alcanzó en la estación 1 (refinería RELAM). Las estaciones 2 y 3 de las áreas de transporte de petróleo no mostraron diferencias significativas. Para evaluar la sensibilidad de la prueba del sedimento para detectar contaminación por la industria petrolera, los datos de toxicidad fueron considerados en términos de niveles de hidrocarburos analizados en dichos sedimentos en las áreas de Petrobras y una de las áreas de control, localizada fuera de la bahía. Los resultados indican que el ensayo de sedimentos con PL de peneidos es una metodología adecuada, no sólo para distinguir entre los medios impactados y relativamente no perturbados, sino también para separar los diferentes grados de impacto entre áreas sujetas a actividades de la industria petrolera en el ambiente costero.
Palabras clave: sedimento marino, biomonitoreo, bioensayo con postlarvas de peneidos, impacto por petróleo, niveles de hidrocarburos.
Introduction
Todos os Santos Bay, one of the biggest bays in the world, is an estuarine complex bordered by extensive mangroves; however, it is characterized mainly by oceanic conditions (Nascimento et al., 2000a, 2000b). The intensive water circulation permits high levels of water recycling within the bay, except at some stagnant points along its borders. As shown by Nascimento et al. (2000a), this water exchange does not totally prevent sediment toxicity in some areas of petroleum industry activity.
The Brazilian Petroleum Company (Petrobras) started its activities in the northestern area of Todos os Santos Bay, Bahia, Brazil (38°37'30" W, 12°52'30" S), 50 years ago. It was not until the late 1990s, however, that an environmental diagnosis of the area was first carried out. The study involved a socio-economic, chemical, benthic and ecotoxicological survey to define the extent of the possible impacts generated by the industry (Peso-Aguiar and Almeida, 1996; da Silva et al., 1997), but did not include bulk-sediment toxicity testing.
Sediments are recognized as sinks and sources of contaminants in aquatic ecosystems (Nipper et al., 1998), especially in mudflat areas, and their analysis can indicate long-term effects of low pollution levels. Nevertheless, the ecological consequence of marine sediment contamination is still difficult to demonstrate (Roper et al., 1988; Cairns et al., 1992), as is the interpretation of bulk-sediment toxicity test results in terms of ecological significance.
It has been argued that to assess potential ecological impacts of pollutant contamination, the use of sensitive, local benthic species in toxicity tests can provide results which can be more accurately transferred to actual field conditions (Environment Canada, 1994, 1995). Penaeid shrimps are considered among the most sensitive estuarine crustaceans for toxicity tests (Cripe, 1994). Penaeid postlarvae (PL), after reaching the stage of PL7, develop a burrowing behaviour, making them particularly suitable for bulk-sediment testing. The tests, based on early-life stages, involve end-points that have ecological significance since they reflect population recruitment (McKim, 1985).
Based on the above considerations, the objectives of this study are three-fold: (1) to evaluate, based on a new and complimentary approach, the environmental impact induced by 50 years of exposure to the local petroleum industry; (2) to associate PL responses with the presence of petroleum hydrocarbons in sediments; and (3) to assess the possibility of predicting benthic effects by using bulk sediment as the source of contamination and penaeid PL as indicator organisms.
Materials and methods
Sediment samples (oxic layer, 1 cm deep) were collected during low tide from intertidal mudflats, 50 cm above the water line. The samples were collected using a SS spatula and transported to the laboratory in ice boxes (4°C). Sediment for the chemical analysis was well mixed and kept frozen until analysis. For the bioassays, sampling was carried out at three-month intervals, at six sites, while for the chemical analysis, samples were taken at six-month intervals during the dry and rainy seasons. Four of these sites are areas with petroleum activity, including oil extraction (station 4), transportation lane (station 2), transport terminal (station 3) and refinery (station 1), and are located, respectively, at Ilha das Fontes (12°43' S, 32°38' W), Ilha de Pati, Ilha de Madre de Deus (12°43' S, 32°37' W) and Mataripe (RELAM; 12°43' S; 38°34' W). Two other sites (stations 5 and 6), free from the influence of the petroleum industry, were used as reference stations for the bioassays. Station 5 is located near the entrance of the bay, in the channel between the mainland and Itaparica Island. The chemical analysis only considered Madre Deus Island as a transportation area and one control station outside the bay (Barra dos Carvalhos; 13°39' S, 38°57' W).
For the bioassays, the sediment samples were placed in plastic boxes, homogenized and kept under refrigeration for 48 h prior to the toxicity test. After being homogenized in the laboratory, subsamples of 200 g were taken for bulk-sediment static bioassays, conducted for 96 h, using 7-8 day old Litopenaeus vannamei PL. The assays were carried out in three replicated 2.5-L plastic jars containing 200 g of surface sediment, covered by 2 L of the laboratory dilution water (filtered seawater, collected from a control station, with salinity of 28 ppt). Previously, sediment samples from all stations were comparatively analyzed for particle-size distribution, measured by wet sieving and pipette analysis (Folk, 1968). No significant differences (P > 0.05) were found among stations. The same sediment from reference station 5 was used as control after being treated in accordance with general procedures recommended by Environment Canada (1995). In order to free it from organic matter, the sediment was washed with distilled water, dried at 105°C and treated with 10 vol H2O2 at a temperature of 60°C for 3 h, and boiled for 20 min three times in distilled water; decanting was carried out for 1 h and the supernatant discarded at each interval.
The bulk-sediment toxicity was assessed using survival and dry weight gain by shrimp PL, obtained from a commercial cultivation farm (Maricultura da Bahia S/A). All the PL were the same age (7-8 days old) and, in order to minimize genetic interference of different strains in the test results, they were taken from a pool (n = 6) of females. One sample of 50 PL was randomly taken for dry weight determination on the first day of the test. The remaining PL were randomly distributed in replicated jars at a density of 15 per vial. Penaeid PL exposed in jars were fed twice daily (in the morning and afternoon) on 60 recently hatched Artemia salina nauplii.
Physico-chemical parameters such as salinity, T°C, pH, dissolved oxygen (DO) and ammonia were monitored at the beginning, two days later and at the end of the test period. The PL were maintained throughout the entire test period in seawater of 28 ppt, at 27 + 2°C, under oxygen saturation. The DO never dropped below 6.2 mg L-1 and the pH was 8.1 + 0.4. The unionized ammonia (minimum of 0.017 to a maximum of 0.193 mg L-1) values were considered normal for this type of sediment.
Tests were terminated by sieving the contents from each jar through a 500 mesh net and counting the live and dead organisms. Missing animals were considered dead. The live organisms were then washed in distilled water and dried for dry weight determination. For correction, the mortality results were expressed as a percentage of net risk calculated by Abbott's formula (Finney, 1971), based on the control sediment effects. The data were checked for normality, arc-sin transformed and analyzed by ANOVA, followed by multiple comparison tests (SNK).
Replicate test results were comparatively analyzed within each sampling period but they varied considerably. The linear transformation formula X' = (X-Xmin)/(Xmax-Xmin) employed by Gower (1971) to standardize the characters used in his general similarity coefficient was used to reduce these variations. The results calculated according to the above formula ranged from 0.0 for minimum response to 1.0 for maximum response. Values for the same sampling station were then reduced to a single mean value that was classified on a qualitative scale of relative toxicity: 0.00-0.15 = relatively non-impacted, >0.15-0.30 = minimum impact, >0.30-0.60 = medium impact and >0.60-1.00 = maximum impact. The dry weight data were treated the same way; however, the relative scale of toxicity was inverted, since a higher gain in dry weight should correspond to better environmental conditions.
Total carbon analysis in sediment samples was determined using a Carlo Erba NA-1500 Elemental Analyzer, following the methodology described by Verardo et al. (1990).
Aliphatic and aromatic hydrocarbon analysis was carried out using a technique described by Readman et al. (2002). Briefly, the sediments were freeze-dried and dry/wet ratios were determined. Each sediment sample (10-20 g) was spiked with internal standard: C18-1 (for the aliphatic hydrocarbon fraction) and 9,10-dihydroanthracene (for the aromatic hydrocarbon fraction). The samples were Soxhlet extracted for 16 h into hexano/dichloromethane (250 mL). The extract was then concentrated down to a few milliliters using rotary evaporation followed by a gentle nitrogen "blow down". Sulfur was removed by shaking the extracts with copper turnings until the metal surface remained shiny. Extractable organic matter was determined gravimetrically.
Clean-up and fractionation was performed by passing the extract through a silica/alumina column (silica and alumina were activated at 200°C for 4 h and then partially deactivated with 5% water). Elution was performed using 25 mL of hexane to yield the first fraction (which contains the aliphatic hydrocarbons); then 30 mL of hexane/dichloromethane (90:10) was combined with 20 mL of hexane/dichloromethane (50:50) to obtain the polycyclic aromatic hydrocarbons (PAHs).
Fractions were then analyzed by gas chromatography using a Hewlett Packard HP5890 series II with a flame ionization detector. The capillary column used was a DB-5MS (J&W Scientific, Folsom, CA, USA) with fused silica (crosslinked 5%-phenyl/95%-dimethylpolysiloxane, 30 m length, 0.25 mm i.d., 0.25 µm film thickness). The temperature was programmed from 40°C to 60°C at 40°C min-1, from 60°C to 300°C at 5°C min-1 and subsequently maintained at 300°C for 20 min. Injector and detector temperatures were set at 40°C and 300°C, respectively. Helium was used as carrier gas at a flow of 1.2 mL min-1. Confirmation of peak identification was obtained for selected extracts using gas chromatography with mass spectrometric detection (Hewlett-Packard 5889B MS "Engine"). Appropriate blanks and reference material IAEA-357 were analyzed simultaneously with each batch.
Results and discussion
The mortality rates of shrimp PL shown by the toxicity tests conducted on the bulk sediment are presented in table 1; each value represents one sampling period. There were no significant (P > 0.05) differences among stations. The relative comparison made by the SNK test showed a large variation in the mortality rate (%) among stations and periods. In general, the mortality rate varied between 1.77% and 28.19%, considering all the sampling periods; however, it did not show a consistent pattern of relative toxicity between stations. Consequently, for this kind of short-term test, the mortality rate was not considered a suitable end-point to discriminate among stations.
In contrast, the dry weight gain data showed significant differences among stations (table 2) when analyzed jointly. A maximum average value was obtained at station 5 (reference 1) and a minimum at station 1 (RELAM refinery). The values of dry weight gain ranged from 0.17 to 0.58 g, including all the stations and periods (table 2). For stations associated with petroleum transportation (stations 2 and 3), the data did not show significant differences (P > 0.05) between themselves. Gower's index applied to the net risk values of dry weight gain (fig. 1) clearly showed that this end-point can discriminate areas with different degrees of impact.
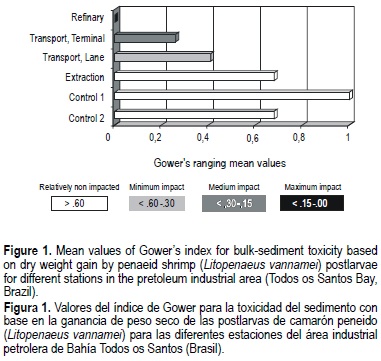
In many pollution studies, benthic communities have been shown to be affected along well-established contamination gradients (Pearson and Rosenberg, 1978; Carr et al., 1996; Nascimento et al., 1998); however, this kind of survey is expensive and time-consuming, and thus unlikely to be possible for the majority of routine programs (Gray et al., 1988). An ecotoxicity test using bulk sediment as substrate, a sensitive species and one relevant end-point would be expected to provide more useful information (ASTM, 1993, 1994) to distinguish impacted from non-impacted areas, but it is especially difficult to detect the effects of pollutant contamination when the level of sediment contamination is low as determined for the sediments used in this bioassay. This explains why the mortality rate, taken as an end-point, failed to show differences among the studied sites. Nevertheless, a gain in dry weight (a sublethal effect), even whilst not showing significant variations among stations during some of the considered periods, was able, when all the data were analyzed together, to discriminate impacted stations (1, 2 and 3) from control stations. This result shows that the test is a good procedure for assessing sediment quality.
The results found using Gower's coefficient (fig. 1) showed that station 1, where the Landulfo Alves refinery is located, suffered maximum impact in relation to reference station 1. Station 2 (transport terminal) was considered to suffer medium impact, while station 3 (transport lane) was considered to suffer minimum impact on the same relative scale. Sediment from station 4 (extraction) did not show detrimental effects to L. vannamei PL.
This qualitative scale, based on the means of ranged values, has an important limitation. When significant differences exist among stations, the most impacted ones will consistently yield high values (near 1.0), while the least impacted stations will yield low values (near 0.0) regardless of the absolute degree of impact. The scale is, therefore, relative to the specific study. The ranged values are more consistent than the original net risk values, but are inappropriate for parametric ANOVA comparisons.
A non-parametric analysis based on ranks (Friedman's test) provided the significance of the differences between means and proved the ranking obtained by Gower's coefficient. Apart from the non-toxic site (extraction area at station 4), these results were confirmed by other toxicity tests (Nascimento et al. , 2000a), using other species as bioindicators and other materials as substrates, such as pore water and transient surface water, from the same sampling stations. The integration of the data from toxicological assays (Nascimento et al., 2000a) using acute mortality of brine shrimp nauplii, and chronic abnormalities of sea-urchin and mangrove oyster larvae permitted a single multispecies ordination of the study sites. Tests with the sediment pore water revealed maximum impact (toxicity) at stations 1 (refinery) and 4 (extraction). Stations 2 and 3 (transport terminal and transport lane) revealed medium impact, and station 5 (reference 1) minimum impact when compared with reference station 6 (control station 2) on a qualitative scale (Nascimento, 1996; Nascimento et al., 1998). These results were also confirmed by other biological studies, based on benthic community responses (Peso-Aguiar and Almeida, 1996).
The sediments in the study sites were mostly muddy or sandy mud. Total organic carbon varied from 0.3% to 5.2%. The highest value was found at the control site. Twenty-seven individual aliphatic hydrocarbons (n-C12 to C36) and twenty-four PAHs were analyzed (tables 3, 4).
For the dry season, the total aliphatic concentration values were 37.0 mg g-1 (dry wt) at the reference site, 33.4 mg g-1 (dry wt) at Madre de Deus (transport lane), 43.8 mg g-1 (dry wt) at RLAM (refinery site) and 184.6 mg g-1 (dry wt) at Ilha das Fontes (oil extraction site). For the rainy season, the concentration values of total aliphatics were 43.0, 58.9, 91.5 and 399.6 mg g-1 (dry wt), respectively, for the reference site (lowest value in terms of organic carbon), Madre de Deus, Ilha das Fontes and RLAM (refinery). These levels indicate comparatively little contamination. Organic-rich marine sediments may contain up to 100 ng g-1 of "total" aliphatic hydrocarbons, but concentrations higher than these are usually associated with petroleum inputs (Volkman et al., 1992; Bouloubassi and Saliot, 1993). Compared with other regions of the world (Readman et al., 2002), the lower levels are comparable to those encountered in places where hydrocarbon contamination was considered relatively low, whereas the higher levels are comparable to those reported for locations known to be chronically contaminated by oil, for example, Odessa, in the Black Sea (110-310 µg g-1 dry wt), Hong Kong (60646 µg g-1 dry wt) and the New York Bight (35-2900 µg g-1 dry wt).
In general, the presence of an unresolved complex mixture (UCM) in aliphatic hydrocarbon chromatograms is considered to be associated with degraded or weathered petroleum residues (Farrington and Tripp, 1977; Le Dréau et al., 1997). Ratios of unresolved to resolved (U/R) >4 confirm the widespread presence of important petroleum-related residues (Mazurek and Simoneit, 1984; Lipiatou and Saliot, 1991). In the oil related sites, the UCM was by far the major component of the "total" sedimentary aliphatic hydrocarbons. UCM concentrations varied from 32to 386 µg g-1 dry wt, which accounted for 84-97% of the "total" aliphatic hydrocarbons (table 3). The U/R ratio ranged from 5.4 to 27 (table 3), indicating chronic petroleum contributions to these sediments, particularly for the refinery site. On the other hand, at the control site, UCM concentrations were 14 and 23 µg g-1 (dry wt), accounting for 38% and 53% of the "total" aliphatic hydrocarbons (dry and rainy seasons, respectively) (table 3). The U/R ratio was about 1, indicating low chronic petroleum contributions to these sediments.
Pristane (C19) and phytane (C20) are common isoprenoids detected in coastal marine sediments. They are often considered good indicators of petroleum contamination. As a rule, a high ratio of pristane to phytane or the predominance of a single isoprenoid (such as pristane) indicate a biogenic source (UNEP/IOC/IAEA, 1992). The pristane to phytane ratios in the sediments were =1 in most of the oil related sites (except for the extraction during the dry season), reflecting contamination originated from petroleum. The highest pristane to phytane ratios (>2.0) were recorded at the reference site, reflecting biogenic origins.
Concentrations of "total" PAHs (the sum of 24 parental and alkylated compounds) in sediments of the oil related sites ranged from 440 to 777 ng g-1 (dry wt) and from 96 to 310 ng g-1 (dry wt) for the dry and rainy seasons, respectively (table 4). These concentrations are comparable to moderately polluted locations worldwide (Readman et al., 2002). Conversely, the concentration observed for the reference site (rainy season) was lower than 50 ng g-1 (dry wt), which is a typical concentration for locations distant from extensive anthropogenic activities (Baumard et al., 1998a). The dry season, however, showed a slightly higher value (83 ng g-1 dry wt) (table 4).
Concentrations of aromatic UCM ranged from 4518 to 63719 ng g-1 (dry wt) and from 7528 to 55167 ng g-1 (dry wt) for the dry and rainy seasons, respectively, which accounted for 73-96% of the "total" aromatic hydrocarbons (table 4) and 11-38% of the "total" (aromatic + aliphatic) hydrocarbons. Conversely, values were lower than 3250 ng g-1 (dry wt) for the reference site, accounting for less than 62% of the "total" aromatic hydrocarbons, and 0.6% (dry) and 6.7% (rainy) of the "total" (aromatic + aliphatic) hydrocarbons.
Among several chemical contaminants, the pollution caused by hydrocarbons is of the greatest concern. This is due to the carcinogenicity and therathogenicity of the highest molecular weight compounds and the toxicity of the most soluble compounds (Baumard et al., 1998a, 1998b). The significance of contamination by hydrocarbons has been recognized considering the hydrophobic nature of most of these compounds (Neff, 1979; Hellow et al., 1993; Djomo et al., 1996; Baumard et al., 1999a, 1999b).
Total PAH levels found for the bulk surficial sediment from the Petrobras sampling locations are below the concentrations proposed by MacDonald et al. (1996) as able to affect the biota; however, due to their characteristic of being bound to organic substrates, it has been proven that hydrocarbons may cause adverse biological effects even though quality criteria are not exceeded (NRC, 1989). The lower shrimp PL dry weight gain provides evidence of a chronic deleterious effect on shrimp PL, which denotes a physiological alteration. This response indicates that the whole-sediment toxicity test on L. vannamei PL is a sensitive tool to discriminate among impacted and non-impacted areas.
In short, the test for determining sediment quality, using bulk sediment as substrate, L. vannamei PL as species test and the dry weight gain as an end-point, was able to discriminate between impacted and non-impacted areas at Todos os Santos Bay (Brazil), where the petroleum industry prevails. The test results indicate that the impact of the petroleum industry has a chronic effect, since the weight gain of shrimp PL, taken as an end-point, succeeded in discriminating between impacted and reference stations, even though the chemical concentrations of PAHs did not exceed quality criteria.
Acknowledgements
This research was financed by Petrobras and by the Brazilian Research Council's (CNPQ) northeast program for research and postgraduate study. All experiments were carried out in accordance with national and institutional guidelines for animal welfare.
References
ASTM (American Society for Testing and Materials) (1993). Standard guide for designing biological tests with sediments. E 1525-93. In: Annual Book of ASTM Standards, Vol 11.4, Philadelphia, pp. 1339-1351. [ Links ]
ASTM (American Society for Testing and Materials) (1994). Standard guide for conducting 10-day static sediment toxicity tests with marine and estuarine amphipods. E 1367-92. In: Annual Book of ASTM Standards, Vol. 11.4, Philadelphia, pp. 1138-1163. [ Links ]
Baumard, P., Budzinski, H., Garrigues, P., Sorbe, J.C., Burgeot, T. and Bellocq, J. (1998a). Concentrations of PAHs (polycyclic aromatic hydrocarbons) in various marine organisms in relation to those in sediments and to trophic level. Mar. Pollut. Bull., 36(12): 951-960 [ Links ]
Baumard, P., Budzinski, H., Mchin, Q., Garrigues, P., Burgeot, T and Bellocq, J. (1998b). Origin and bioavailability of PAHs in the Mediterranean Sea from mussel and sediment records. Estuar. Coast. Shelf Sci., 47: 77-90. [ Links ]
Baumard, P., Budzinski, H., Garrigues, P., Narbonne, J.F., Burgeot, T., Michel, X. and Bellocq, J. (1999a). Polycyclic aromatic hydrocarbon (PAH) burden of mussels (Mytilus sp.) in different marine environments in relation with sediment PAH contamination, and biovailability. Mar. Environ. Res., 47: 415-439. [ Links ]
Baumard, P., Budzinski, H., Garrigues, P., Dizer, H. and Hansen, P.D. (1999b). Polycyclic aromatic hydrocarbons in recent sediments and mussels (Mytilus edulis) from the western Baltic Sea: Occurrence, bioavailability and seasonal variations. Mar. Environ. Res., 47: 17-47. [ Links ]
Bouloubassi, I. and Saliot, A. (1993). Investigation of anthropogenic and natural organic inputs in estuarine sediments using hydrocarbon markers (NAH, LAB, PAH). Oceanol. Acta, 16: 145-161. [ Links ]
Cairns, J.J., Niederlehner, B.R. and Smith, E.P. (1992). The emergence of functional attributes as endpoints in ecotoxicology. In: G.A. Burton Jr. (ed.), Sediment Toxicity Assessment. Lewis, Boca Raton, Florida, pp. 111-128. [ Links ]
Carr, R., Long, E., Windom, H., Chapman, D., Thursby, G., Sloane, G. and Wolfe D. (1996). Sediment quality assessment studies of Tampa Bay, Florida. Environ. Toxicol. Chem., 15: 1218-1231. [ Links ]
Cripe, G. (1994). Comparative acute toxicities of several pesticides and metals to Mysidopsis babia and postlarval Penaeus duorarum. Environ. Toxicol. Chem., 13: 1867-1872. [ Links ]
Da Silva, E.M., Peso-Aguiar, M.C., Navarro, M. de F.T. and Chastinet, C. de B. e A. (1997). Impact of petroleum pollution on aquatic coastal ecosystems in Brazil. Environ. Toxicol. Chem., 16(1): 112-118. [ Links ]
Djomo, J.E., Garrigues, P., and Narbonne, J.F. (1996). Uptake and depuration of polycyclic aromatic hydrocarbons from sediment by the zebrafish (Bracydanio rerio). Environ. Toxicol. Chem., 15: 1177-1181. [ Links ]
Environment Canada (1994). Guidance Document on Collection and Preparation of Sediments for Physicochemical Characterisation and Biological Testing. Environmental Protection Series 1/RM/ 29, 132 pp. [ Links ]
Environment Canada (1995). Guidance Document on Measurement of Toxicity Test Precision using Control Sediment Spiked with a Reference Toxicant. Environmental Protection Series 1/RM/30, 56pp. [ Links ]
Farrington, J.W. and Tripp, B.W. (1977). Hydrocarbons in western North Atlantic surface sediments. Geochim. Cosmochim. Acta, 41: 1627-1641. [ Links ]
Finney, D.J. (1971). Probit Analysis. 3rd ed. Cambridge Univ. Press, Cambridge, 333 pp. [ Links ]
Folk, R. (1968). Petrology of Sedimentary Rocks. Hemphill, Austin. [ Links ]
Gray, J., Aschan, M., Carr, M., Clarke, K., Green, R., Pearson, T., Rosenberg, R. and Warwick, R. (1988). Analysis of community attributes of the benthic macrofauna of Frierfjord/Langesundfjord and in a mesocosm experiment. Mar. Ecol. Prog. Ser., 46: 151-165. [ Links ]
Gower, J.C. (1971). A general coefficient of similarity and some of its properties. Biometrics, 27: 857-871. [ Links ]
Hellow, J., Upshall, C., Payne, J.F., Naidu, S. and Paranjape, M.M. (1993). Total unsaturated compounds and polycyclic aromatic hydrocarbons in molluscs collected from waters around New Foundland. Arch. Environ. Contam. Toxicol., 24: 249-257. [ Links ]
Le Dréau, Y., Jacquot, F., Doumenq, P., Guiliano, M., Bertrand, J.C. and Mille, G. (1997). Hydrocarbon balance of a site which had been highly and chronically contaminated by petroleum wastes of a refinery (from 1956 to 1992). Mar. Pollut. Bull., 34: 456-468. [ Links ]
Lipiatou, E. and Saliot, A. (1991). Hydrocarbon contamination of the Rhone delta and western Mediterranean. Mar. Pollut. Bull., 22: 297-3. [ Links ]
Mazurek, M.A. and Simoneit, B.R.T. (1984). Characterization of biogenic and petroleum-derived organic matter in aerosols over remote rural and urban areas. In: L.H. Keith (ed.), Identification and Analysis of Organic Pollutants in Air. Ann Arbor Science, Butterworth, Boston, pp. 353-370. [ Links ]
MacDonald, D.D., Carr, R.S., Calder, F.D., Long, E.R. and Ingersoll, C.G. (1996). Development and evaluation of sediment quality guidelines for Florida coastal waters. Ecotoxicology, 5: 253-278. [ Links ]
McKim, J.M. (1985). Early life stage toxicity tests. Chapter 3. In: G.M. Rand and S.R. Petrocelli (eds.), Fundamentals of Aquatic Toxicology. Hemisphere Publ. Corp., New York, pp. 58-95. [ Links ]
Nascimento, I.A. (1996). Relatório do subprojeto "Testes Ecotoxicológicos" do programa de Monitoramento dos Ecosistemas ao norte da Baía de Todos os Santos, Convênio Petrobrás/UFBA, July 1996. Convênio PETROBRAS/UFBA. Contracts 220.2.045.93-5 and 220.2.051.94-8. 80 pp. [ Links ]
Nascimento, I.A., Pereira, S.A., Smith, D.H. and Evangelista, A.J.A. (1998). Testes ecotoxicológicos usados para avaliação de impacto ambiental resultante da extração e refino de petróleo na Bahia de Todos os Santos, Bahia, Brasil. Trab. Oceanogr. Univ. Fed. Pe., 26(1): 135-143. [ Links ]
Nascimento, I.A, Smith, D.H., Pereira, S.A., Sampaio de Araújo, M.M. and Mariani, A.M. (2000a). Integration of varying responses of different organisms to water and sediment quality at sites impacted and not impacted by the petroleum industry. In: State of Brazilian Aquatic Ecosystem Health. J. Aquat. Ecosyst. Health Manage. (Canad), Spec. issue, 3(4): 485-197. [ Links ]
Nascimento, I.A., Smith, D.H., Gomes, M.G.S., Santos, G.V. and Pereira, S.A. (2000b). Ecotoxicological diagnosis of Aratu Bay, Bahia, Brazil: A new approach to validate a reactive short-term toxicity end-point by comparison with intertidal benthic activity. In: State of Brazilian Aquatic Ecosystem. J. Aquat. Ecosyst. Health Manage. (Canada), Spec. issue, 3(4): 449-458. [ Links ]
Nipper, M.G., Roper, D.S., Williams, E.K., Martin, M.L., Van Dam, L.F. and Mills, G.N. (1998). Sediment toxicity and benthic communities and mildly contaminated mudflats. Environ. Toxicol. Chem., 17(3): 502-510. [ Links ]
NRC. (1989). Contaminated Marine Sediments - Assessment and Remediation. National Research Council. National Academic Press, 493 pp. [ Links ]
Neff, J.M. (1979). Policyclic aromatic hydrocarbons in the aquatic environment: Sources, fates and biological effects. Applied Science Publishers Ltd., Essex, England. 262 pp. [ Links ]
Pearson, T. and Rosenberg, R. (1978). Macrobenthic succession in relation to organic enrichment pollution of the marine environment. Oceanogr. Mar. Biol. Ann. Rev., 16: 229-311. [ Links ]
Peso-Aguiar, M.C. and Almeida, V.G. (1996). Relatório Final do Programa de Monitoramento dos Ecosistemas ao Norte da Baía de Todos os Santos. Convenio PETROBRAS/UFBA. Contracts 220.2.045.93-5 and 220.2.051.94-8. 80 pp. [ Links ]
Readman, J.W., Fillmann, G., Tolosa, I., Bartocci, J., Villeneuve, J.P., Catinni, C. and Mee, L.D. (2002). Hydrocarbon residues in sediments from the Black Sea. Mar. Pollut. Bull., 44(1): 48-62. [ Links ]
Riebel, P.N. and Percy, J.A. (1991). Acute toxicity of petroleum hydrocarbons to the Arctic shallow-water mysid, Mysis oculata (Fabricius). Sarsia, 75: 223-232. [ Links ]
Roper, D., Thrush, S. and Smith, D.H. (1988). The influence of runoff on intertidal mudflat benthic communities. Mar. Environ. Res., 26: 1-18. [ Links ]
UNEP/IOC/IAEA. 1992. Determination of petroleum hydrocarbons in sediments. Reference methods for marine pollution studies. No. 20. UNEP, Monaco, 75 pp. [ Links ]
Verardo, D.J., Froelich, P.N. and McIntyre, A. (1990). Determination of organic carbon and nitrogen in marine sediments using Carlo Erba NA-1500 Analyser. Deep-Sea Res., 37(1): 157-165. [ Links ]
Volkman, J.K., Holdsworth, D.G., Neill, G.P. and Bavor Jr., H.J. (1992). Identification of natural, anthropogenic and petroleum hydrocarbons in aquatic sediments. Sci. Total Environ., 112: 203-219. [ Links ]














