Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Ciencias marinas
versão impressa ISSN 0185-3880
Cienc. mar vol.30 no.1b Ensenada Mar. 2004
Artículos
Sodium and potassium alginates extracted from Macrocystis pyrifera algae for use in dental impression materials
Alginatos de sodio y potasio extraídos del alga Macrocystis pyrifera para usos en materiales para impresión dental
Raúl Reyes-Tisnado1*, Gustavo Hernández-Carmona2,a, Francisco López-Gutiérrez2, Eduardo Jaime Vernon-Cartera and Pastor Castro-Moroyoqui1
1 Centro Regional de Investigación Pesquera Km 1 Carretera a Pichilingue La Paz, Baja California Sur, México. *E-mail: rulreyes@prodigy.net.mx
2 Centro Interdisciplinario de Ciencias Marinas. Apartado postal 592 La Paz, CP 23000, Baja California Sur, México.
3 Universidad Autónoma Metropolitana Iztapalapa DCBI (IPH) México, CP 09340, DF.
Recibido en octubre de 2002;
aceptado en octubre de 2003.
Abstract
Sodium and potassium alginates were extracted at the pilot plant scale from the giant kelp, Macrocystis pyrifera, from Bahía Tortugas, Baja California Sur, Mexico. Alginates were coded as S1, S2 and S3 for sodium alginate, and as P1, P2 and P3 for potassium alginate. The average viscosities of the sodium and potassium alginates in aqueous 1% solution were 58, 145 and 506 mPa s, and 48, 155 and 200 mPa s, respectively. Results showed that dental impresion material prepared with sodium alginate with extra low viscosities (S1 = 58 mPa s) and low viscosities (S2 = 145 m Pa s) did not form gels, the material prepared with medium viscosity (S3 = 506 mPa s) produced a gel type II (regular set) in 70% of the 10 formulations experimented, and the material prepared with potassium alginate with extra low viscosity (P1 = 48 mPa s) produced a gel type II in 90% of the formulations. Using potassium alginate with low viscosity (P2 = 155 mPa s), 90% of the formulations were type I (fast set), and using potassium alginate with medium viscosity (P3 = 200 mPa s), 80% of the formulations were type I. The highest compressive strength was obtained using sodium alginate of 506 mPa s and the three potassium alginates experimented at 25% concentration, with values of 2474, 1209, 2101 and 2124 g cm-2 for S3, P1, P2 and P3 alginates, respectively. The elasticity order of formulation with 25% alginates compared with a commercial product (Jeltrate®) was as follows: Jeltrate® > P2 > S3 > P3 > P1. It was concluded that S3, P1, P2 and P3 alginates have good potential for use in the production of dental impression materials.
Key words: alginates, Macrocystis pyrifera, algae, dental impression material.
Resumen
Se extrajeron alginatos de sodio y de potasio a escala piloto del alga Macrocystis pyrifera proveniente de Bahía Tortugas, Baja California Sur, México. Los alginatos fueron codificados como alginatos de sodio (S1, S2 y S3) y alginatos de potasio (P1, P2 y P3). Las viscosidades medias de los alginatos de sodio y de potasio en solución acuosa al 1% fueron de 58, 145 y 506 mPa s, y 48, 155 y 200 mPa s, respectivamente. Los resultados mostraron que el material para impresiones dentales preparado con los alginatos de sodio con viscosidades extra bajas (S1= 58 mPa s) y viscosidades bajas (S2 = 145 mPa s) no formó geles, el material preparado con viscosidades medias (S3 = 506 mPa s) produjo geles tipo II (fraguado normal) en 70% de las 10 formulaciones experimentadas, y el material preparado con alginatos de potasio con viscosidad extra baja (P1 = 48 mPa s) produjo geles tipo II en 90% de las formulaciones. Usando alginatos de potasio con viscosidad baja (P2 = 155 mPa s), 90% de las formulaciones fueron de tipo I (fraguado rápido), y usando alginato de potasio con viscosidad media (P3 = 200 mPa s), 80% de las formulaciones fueron de tipo I. La más alta resistencia a la compresión fue obtenida usando alginato de sodio de 506 mPa s y los tres alginatos de potasio experimentados a concentración de 25%, con valores de 2474, 1209, 2101 y 2124 g cm-2 para los alginatos S3, P1, P2 y P3, respectivamente. El orden de elasticidad de las formulaciones con 25% de alginatos comparados a un producto comercial (Jeltrate®) fue el siguiente: Jeltrate® > P2 > S3 > P3 > P1. Se concluye que los alginatos S3, P1, P2 y P3 tienen un buen potencial para usarse en la producción de material de impresión dental.
Palabras clave: alginatos, Macrocystis pyrifera, algas, impresiones dentales.
Introduction
Alginate is the salt of alginic acid, a polysaccharide constituted by the unbranched binary copolymer of P-D-manuronic (M) and a-L-guluronic (G) acids, structured in sequences of MM, MG blocks joined by β(1-4) bonds, and GG, GM blocks joined by α(1-4) bonds. Alginates are structural polysaccha-rides of the amorphous matrix of the cellular wall in brown seaweeds such as Macrocystis pyrifera (L.) C. Agardh. Their function is to give strength and flexibility to the algal tissue (Skjäk-Braek and Martinsen, 1991; Smidsrod and Draget, 1996).
Sodium and potassium alginates are hydrocolloid materials commonly used in dental impressions. Dentists use them in powder form together with other components to prepare the material with the appropriate viscosity, which is transported to the patient's mouth by means of an impression tray. The gelling process takes place in the mouth and, after a few minutes, the impression is removed. There are several methods of producing the gel, but one of the easiest is to use a soluble alginate, which reacts with calcium sulfate to produce an insoluble calcium alginate. In fact, this reaction should occur in the mouth, and it is therefore necessary to slow down the reaction with trisodium phosphate, while the dental material is mixing with water. The paste is placed on the impression tray and then in the mouth. At present, alginates are widely used in dental impression materials because they are easy to handle, comfortable for patients and do not require sophisticated equipment. They are also used in dental prostheses, as models for studying jagged and toothless mouth impressions (Skinner and Phillips, 1982; Osborne et al., 1987).
The use of alginates in dental impression materials is based on the aqueous reaction between a sparingly soluble calcium salt (usually calcium sulfate dihydrate) and an alginate. A common formula for a dental impression material, based on the above description, is as follows: 12% of sodium or potassium alginate, 12% of calcium sulfate, 2% of trisodium phosphate, and 74% of diatomaceous earth. The exact proportions of the formula vary accordingly to the type of alginate used. The chemical composition of the alginates can greatly affect the gel strength. Alginates with high content of guluronic acids characteristically yield materials with high gel strength. The quantity of retarder should be adjusted carefully in order to provide the appropriate gelling time. Trisodium phosphate is a retardant, while calcium sulfate is the reactant used to promote gel formation. In this way, if appropriate quantities of potassium alginate, calcium sulfate and trisodium phosphate are mixed with water, the following reaction takes place: 2Na3PO4 + 3CaSO4 → Ca3(PO4)2 + 3Na2SO4. When there is no more trisodium phosphate available, the calcium ions of calcium sulfate begin to react with the potassium alginate to form a calcium alginate gel (dental impression) as follows: Kn Alg + n/2CaSO4 → n/2K2SO4 + Can/2 Alg.
According to the gelling time, two types of dental impression material are specified: type I (fast set), with a gelling time of 0-120 s, and type II (regular set), with a gelling time of around 121-420 s. Diatomaceous earth is a filling material that increases the resistance and rigidity of alginate gel when used in appropriate amounts, providing a uniform texture and lack of superficial adhesion (Skinner and Phillips, 1982; Onsoyen, 1996).
The giant kelp, Macrocystis pyrifera, is one of the brown seaweeds that are commercially exploited as a source of alginates (McHugh, 1987; Reyes-Tisnado et al., 1992). In Mexico, giant kelp distribution extends from the border with the United States of America to Punta San Hipólito, Baja California Sur. The estimated harvestable biomass (1 m below the sea surface) varies seasonally between 36,520 t in winter and 99,626 t in summer (Hernández-Carmona et al., 1989a, 1989b, 1991).
At the present time in Mexico, M. pyrifera is harvested and exported to the United States as raw material for alginate production; however, all alginates and dental impression materials used in the country are imported mainly from the United States, Norway, England, Germany, Italy and the Netherlands (Reyes-Tisnado et al., 2000). Efforts are being made to develop an alginate production industry in Mexico based on the rational and efficient exploitation of M. pyrifera, thus contributing to the regional development of Baja California Sur and to decrease imports of these materials.
To determine the potential use of alginates from M. pyrifera, it was necessary to carry out studies related to their properties and applications. The objective of this research was to produce sodium and potassium alginates from M. pyrifera at the pilot plant level, and to determine their general properties and test their use for the preparation of dental impression material. Another objective was to analyze the gelling time of the dental impression materials, the compressive strength, and the rheological characteristics (storage modulus, loss modulus and loss factors) of the dental impression materials.
Material and methods
1. Extraction of sodium and potassium alginates at the pilot plant scale
Ten kilograms of dried and milled alga (M. pyrifera), harvested from Bahía Tortugas, Baja California Sur, Mexico, were placed in a tank to hydrate overnight with 90 L of 0.1% formalin solution. The residual solution was drained off and the alga was washed with 100 L of hydrochloric acid solution at pH 4 in the same tank for 15 min, with constant agitation (Hernández-Carmona et al., 1999a). The alga was transferred to an extraction kettle containing 166 L of water; the pH was adjusted to 10 using powdered sodium carbonate and the solution was heated to 80°C for 2 h, with constant stirring. The resulting paste was then diluted to 45 mPa s and filtered in a rotary vacuum filter, using diatomaceous earth as the filter aid (Hernández-Carmona et al., 1999b). During filtration, the alginate solution was pumped into the precipitation tank, while a solution of 10% calcium chloride was added simultaneously to precipitate it as calcium alginate, using a proportion of 2.2 parts of calcium chloride to 1 part of alginate in solution. The calcium alginate fibers were bleached using 700 mL of 5% sodium hypochlorite solution and converted to alginic acid, with three acid washings at pH 2, 1.8 and 1.8. The alginic acid fibers obtained were pressed using a hydraulic press to remove as much water as possible (McHugh et al., 2001). To obtain the sodium or potassium alginate, alginic acid fibers were neutralized with sodium or potassium carbonate until pH 7.5, in a double planetary mixer during 40 min. The sodium or potassium alginate fibers were separated and dried at 60°C for enough time to get a product with 12% moisture content (Hernández-Carmona et al., in press). Samples of three types of sodium and potassium alginates were extracted from plants collected on different dates in order to have alginate samples with different viscosities, and they were coded, according to the viscosity, as S1 = 58, S2 = 145 and S3 = 506 mPa s, and P1 = 48, P2 = 155 and P3 = 200 mPa s, respectively.
2. Viscosity and pH of 1% (w/v) alginate aqueous solutions
Three grams of sodium or potassium alginate were dissolved in 300 mL of distilled water and the viscosity was measured with a Brookfield viscometer model DV-I at 60 rpm, at 25°C with the LV spindle No. 2 (18.72 mm diameter, 6.86 mm high). The viscosity was measured again after adding sodium hexametaphosphate (SHMP) (0.5 g per 100 mL of alginate solution) and stirred for 15 min to sequestrate the calcium ions present in the solution. The percentage of viscosity reduction was calculated as follows: (viscosity without SHMP-viscosity with SHMP) x 100/viscosity without SHMP (Cottrell and Kovacs, 1980); pH was determined with a pHmeter.
3. Moisture content of the alginate powders
Seven grams of sodium or potassium alginate were placed in a moisture balance at 110°C for 30 min. The moisture content was estimated from the resultant weight loss.
4. Production of dental impression material using sodium and potassium alginates
Several 100 g samples of dental impression material were prepared using S1, S2 and S3 sodium alginates, and P1, P2 and P3 potassium alginates in 10 different formulations as show in table 1. A total of 60 experimental formulations were prepared. Formulation a1 corresponded to the basic formulation proposed by Skinner and Phillips (1982).
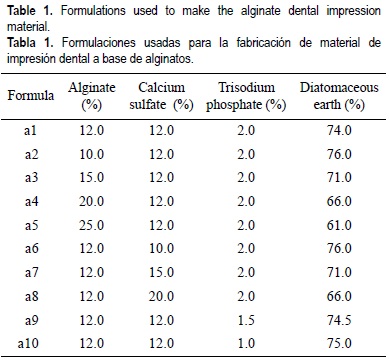
5. Determination of gelling time
Seven grams of each dental impression formulation (table 1) were mixed with 18 mL of distilled water for 1 min. The mixture was placed in a dental impression tray and the gelling time was recorded with a chronometer. This time was obtained at the moment when the material stopped being sticky or adherent, and it is of critical importance because it determines the time available to mix the material, fill the impression tray and place it in the patient's mouth by the dentist.
6. Determination of compressive strength
Samples of dental impression formulations (table 1) that formed gels using the previous procedure, were placed in a cylindrical geometry (3 cm diameter, 3 cm high) and placed in a Chatillon Materials Tester, model TCM200, for determining compressive strengths (g cm-2) using a compressive speed of 2.54 cm min-1.
7. Dynamic rheological measurements
The storage modulus, loss modulus and loss factor (Thomas, 1991) of the dental impression formulations that exhibited higher compressive strength in the previous procedure were determined at a frequency of 1 Hz over a strain sweep of 1-200% and a temperature of 25 ± 0.5°C with a high dynamic rheometer Physica DSR 4000. A cone and plate measuring system was used with a cone diameter of 2.5 cm, and a gap between cone and plate of 50 microns. Temperature control was achieved with a Peltier element. None of the formulations below 1% strain sweep were measured because the equipment does not have the sensitivity to do it. For these rheological measurements, all samples were allowed to gel and the determinations were immediately carried out.
Results
Table 2 shows the quality profiles of sodium and potassium alginates extracted from M. pyrifera that were used in the elaboration of dental impression formulations. The average viscosities of the 1% solutions varied between 58 and 506 mPa s for the sodium alginates and between 48 and200 mPa s for the potassium alginates. The average viscosity reductions after adding SHMP were never higher than 20%. The pH of 1% alginate solutions ranged from 6.4 to 8.2 and moisture content was lower than 14% in all cases.
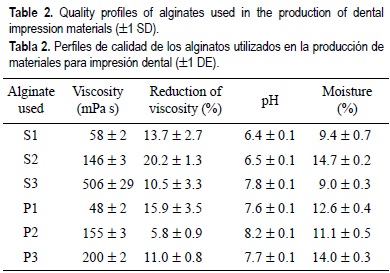
Table 3 presents the gelling time of the dental impression materials produced. Dental formulations with S1 and S2 alginates did not form gels; therefore, no data are shown in table 3. Formulations with S3 alginate were type II (regular set), with a gelling time of 121-420 s, except for a1, a8 and a10 that were type I (Fast Set), with a gelling time of 0-120 s. Formulations using the P1 alginate were type II, except for a10 that was type I. Formulations with P2 alginate were type I, except for a6 that was type II. Formulations with P3 alginate were type I, except for a8 and a10 that were type II.
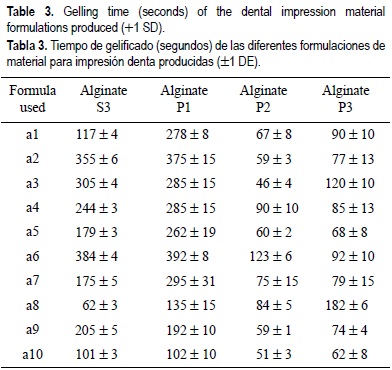
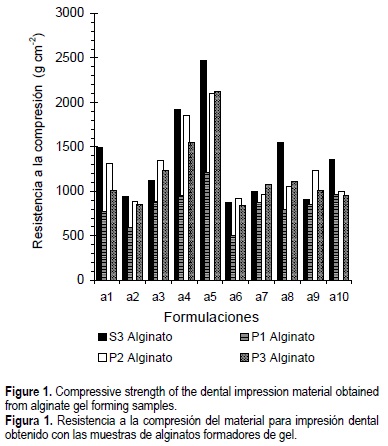
Figure 1 shows the compressive strength of dental impressions produced with the alginates that formed gels. The compressive strength of the gels increased as the percentage of alginate in the formulation was increased to 15%, 20% and 25% (a3, a4 and a5 formulations) in comparison to the basic formulation (a1) of Skinner and Phillips (1982). The compressive strength within a given formulation varied with the type of alginate used. Thus, in formulation a5 the compressive strengths from higher to lower were 2474, 2124, 2101 and 1209 g cm-2 for the S3, P1, P2 and P3 alginates respectively. Also, a slight increasing trend in the compressive strength occurred when the percentage of calcium was increased to 15 and 20% (a7 and a8 formulations). Conversely, the a2 and a6 formulations having the lowest alginate and calcium sulfate concentrations showed the lowest compressive strengths.
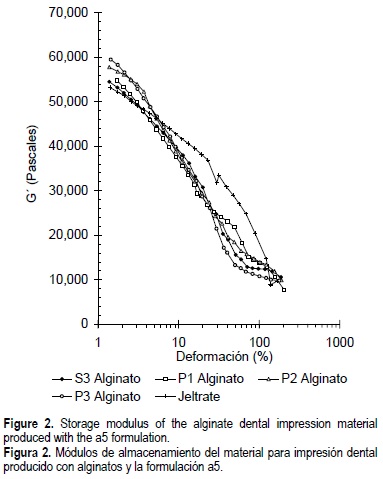
The storage modulus (G') decreased sharply with the increase in strain percent (ϒ) in all the formulations, from about 59,500 Pa at 1% strain to 7,800 Pa at 200% strain (fig. 2). In all cases, the gels did not exhibit linear viscoelastic behavior and as the strain percent increased, a partial breakdown of the elastic structure occurred. The dental impression material prepared with P3, P2, P1 and S3 alginates had a higher storage modulus than the commercial control (Jeltrate®) up to 5% strain. After this value, a crossover of the G'-ϒ curves occurred and Jeltrate® exhibited a slightly higher G'. The curves obtained got closer at strains of around 100%. No significant differences were obtained between the experimental formulations.
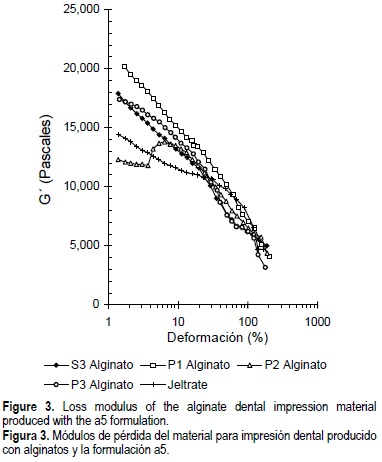
The loss modulus values (G") decreased with the increase in strain percent. The highest G'' value was obtained with P1 alginate, followed by P3, S3 and P2 alginates, and Jeltrate® with strains up to 25%, at which a crossover in the G''-ϒ curves occurred (fig. 3).
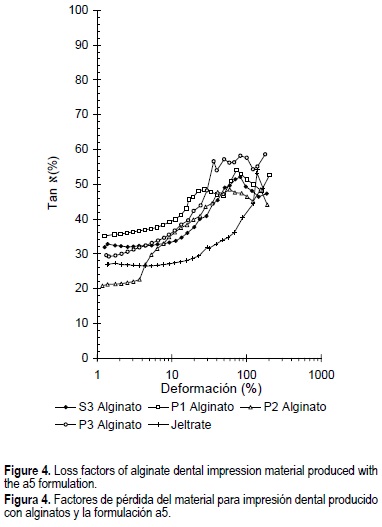
The loss factor relates the amount of dissipated energy to stored energy. Tan 8 values of all the formulations tended to maintain constant up to strains of about 5%, indicating that the elastic nature of the formulations predominates over their viscous nature (fig. 4). At 5% strain the formulation with the highest elastic behavior was Jeltrate®, followed in decreasing order by P2, P3, S3 and P1. After 5%, no significant differences were obtained.
Discussion
The alginates obtained with the process selected produced a range of products with viscosities from 50 to 506 mPa s (sodium alginate) and 48 to 200 mPa s (potassium alginate). One of the quality characteristics of the alginates is their calcium content. This amount is related to the viscosity reduction after one sequestrates the calcium with sodium hexametaphosphate. The maximum reduction allowed by alginate users is 10-40%. This reduction represents about 0.3-1.2% of the calcium content. In our study, all the samples had less than 20% calcium; thus, the alginates used in this study fulfilled the commercial quality profiles.
The alginate viscosities are not affected over the pH range of 5-11. When the alginate is in solution, pH below 5 causes the -COO- ions in the alginate chain to become protonated to -COOH, so the electrostatic repulsion between chain segments is reduced, being able to come closer and form hydrogen bonds, until a gelatinous precipitate is formed. Above pH 11, slow depolymerization occurs in stored alginate solutions, causing a decrease in the apparent viscosity (King, 1983). In our case, the pH of the alginate solutions was between 6.4 and 8.2 and, therefore, a high depolymerization is not likely to occur during the storage period.
Most of the commercial alginates had moisture contents of less than 15%, because the rate of depolymerization of the product increases when the moisture content is higher than 15% (McHugh, 1987). All the alginates produced in this study had moisture contents of less than 14%.
The dental impression formulations produced experimentally using the P1, P2 and P3 potassium alginates and the S3 sodium alginate formed gels. In contrast, the formulations made with the S1 and S2 alginates did not gel, because of the low polymerization degree (low viscosity) of the sodium alginate. This fact was confirmed since the a5 formulation of the S3 sodium alginate, which had a high viscosity, presented the highest compressive strength value (2474 g cm-2). This formulation contained 25% sodium alginate, 12% calcium sulfate, 2% trisodium phosphate, and 61% diatomaceous earth. This formulation was the closest to the compressive strength specified by the Dental American Association (3500 g cm-2) for dental impression alginates (Skinner and Phillips, 1982).
Compressive strength of dental impression materials may depend on the alginate composition (algal species), alginate concentration, its polymerization degree, and calcium concentration. Also, alginates from different seaweeds have differing proportions of manuronic acid and guluronic acid in their structures and different proportions of M, G and MG blocks. For example, the alginates from Laminaria hyperborea have a high percentage of G blocks, and they can form stronger gels with slight syneresis or water loss. On the contrary, the alginates from M. pyrifera or Ascophyllum nodosum have a high proportion of M blocks, and form softer and more elastic gels, but with higher syneresis (Smidsrod and Draget, 1996). In our case, the most important factor to increase the compressive strength of the dental impression material formulated was the polymerization degree; however, it could be improved if the alginate viscosity is increased to values of 600-700 mPa s. The alginate viscosity can be increased if the extraction is carried out from fresh harvested kelp, as is generally done by alginate factories (personal observation). The commercial sodium alginate viscosity produced by ISP in USA from M. pyrifera can be as high as 1300 mPa s (1% solution).
The storage modulus represents the elastic behavior of the gel in the dental impression material prepared. The G' decreased when the strain was increased in all the experimental formulations and controls. Although the gels did not show a linear viscoelastic behavior, the relation was similar for all cases and the breakdown of the elastic structure occurred at about the same strain percent.
The loss modulus (G") represents the viscous behavior of the formulation. As expected, G" also decreased when the strain was increased and after 25% all curves were similar, indicating properties similar to the control. The loss factor relates the amount of dissipated energy to stored energy. Tan 8 values of all the formulations tended to maintain constant up to strains of about 5%, indicating that the elastic nature of the formulations predominates over their viscous nature. However, at larger strains value, Tan 8 increased for all formulations, suggesting that as the partial breakdown of the elastic structure proceeds with increasing strain, a change to a relatively more viscous behavior of the formulations takes place. This viscous nature reaches a peak for most formulations at a strain of about 80%. Although the formulation that presented the largest elastic behavior was Jeltrate®, the values obtained for P2 were similar to the Jeltrate control.
The rheological characterization of the dental impression alginates indicates that the elastic behavior of all the a5 formulations was slightly lower than the commercial product (Jeltrate®); however, this could be improved by using higher molecular weight potassium or sodium alginates, with viscosities higher than 500 mPa s. Also, it could be improved by combining the use of modifiers such as magnesium oxide, potassium sulfate, sodium silicoflouride and other types of diatomaceous earths. The effect of these proposed modifications will be discussed in another paper. A further study using alginates extracted from Sargassum algae is also recommended, as they have a high percentage of guluronic acid and may form stronger gels (Shyamali et al., 1984; Wedlock et al., 1986). The total biomass of Sargassum in the Gulf of California, Mexico, has been estimated to be 31,000 ± 3,200 dry tons (Pacheco-Ruíz et al., 1998).
We concluded that the P1, P2 and P3 potassium alginates, as well as the S3 sodium alginate extracted from M. pyrifera algae at the pilot plant scale, have a good potential to be used in the production of dental impression materials.
Acknowledgements
We acknowledge the United Nations Development Program (FAO) for the financial support to construct the pilot plant for alginate production. Thanks to Rubén Ortega (Paar Physica Company) for technical assistance in determining the rheological properties of the samples.
Referencias
Cottrell, I.W. and Kovacs, P. (1980). Alginates. In: R.L. Davidson (ed.), Handbook of Water-soluble Gums and Resins. McGraw-Hill, pp. 2.1-2.42. [ Links ]
Hernández-Carmona, G., Rodríguez-Montesinos, Y.E., Torres-Villegas, J.R., Sánchez-Rodríguez, I. y Vilchis MA (1989a). Evaluación de los mantos de Macrocystis pyrifera (Phaeophyta-Laminariales) en Baja California, México. I. Invierno 1985-1986. Cienc. Mar., 15(2): 1-27. [ Links ]
Hernández-Carmona, G., Rodríguez-Montesinos, Y.E., Torres-Villegas, J.R., Sánchez-Rodríguez, I., Vilchis, M.A. y García-de la Rosa, O. (1989b). Evaluación de los mantos de Macrocystis pyrifera (Phaeophyta, Laminariales) en Baja California, México. II. Primavera 1986. Cienc. Mar., 15(4): 117-140. [ Links ]
Hernández-Carmona, G., Rodríguez-Montesinos, Y.E., Casas-Valdez, M.M., Vilchis, M.A. y Sánchez-Rodríguez, I. (1991). Evaluación de los mantos de Macrocystis pyrifera (Phaeophyta, Laminariales) en la península de Baja California, México. III. Verano 1986 y variación estacional. Cienc. Mar., 17(4): 121-145. [ Links ]
Hernández-Carmona, G., McHugh, D.J., Arvizu-Higuera, D.L. and Rodríguez-Montesinos, Y.E. (1999a). Pilot plant scale extraction of alginate from Macrocystis pyrifera. I. The effect of pre-extraction treatments on the yield and quality of alginate. J. Appl. Phycol., 10: 507-513. [ Links ]
Hernández-Carmona, G., McHugh, D.J. and López-Gutiérrez, F. (1999b). Pilot plant scale extraction of alginate from Macrocystis pyrifera. 2. Studies on extraction conditions and methods of separating the alkaline-insoluble residue. J. Appl. Phycol., 11: 493-502. [ Links ]
Hernández-Carmona, G., McHugh, D.J., Arvizu-Higuera, D.L. and Rodríguez-Montesinos, Y.E. (2002). Pilot plant scale extraction of alginates from Macrocystis pyrifera. 4. Conversion of alginic acid to sodium alginate, drying and milling. J. Appl. Phycol (in press).
King, A.H. (1983). Brown seaweed extracts (alginates). In: Food Hydrocolloids. CRC Press, pp. 115-188. [ Links ]
McHugh, D.J. (1987). Production, properties, and uses of alginates. In: D.J. McHugh (ed.), Production and Utilization of Products from Commercial Seaweeds. FAO Fish. Tech. Pap., 288: 58-115. [ Links ]
McHugh, D.J., Hernández-Carmona, G., Arvizu-Higuera, D.L. and Rodríguez-Montesinos, Y.E. (2001). Pilot plant scale extraction of alginate from Macrocystis pyrifera. 3. Precipitation, bleaching and conversion of calcium alginate to algnic acid. J. Appl. Phycol., 13: 471-479. [ Links ]
Osborne, J., Wilson, H.J. y Mansfield, M.A. (1987). Tecnologías y Materiales Dentales. Editorial Limusa, México, 520 pp. [ Links ]
Onsoyen, E. (1996). Commercial applications of alginates. Carbohydrates in Europe, 14: 30-31. [ Links ]
Pacheco-Ruíz, I., Zertuche-González, J.A., Chee-Barragán, A. and Blanco-Betancourt, R. (1998). Distribution and quantification of Sargassum beds along the west coast of the Gulf of California, Mexico. Walter de Gruyter, Berlin, 41: 203-208.
Reyes-Tisnado, R., Hernández-Carmona, G. y Hernández-Valenzuela, R. (1992). Reducción del consumo de agua dulce en el proceso de extracción de alginatos a partir de Macrocystis pyrifera (Phaeophyta, Laminariales), mediante recirculaciones de los líquidos residuales de la pre-extracción y precipitación. Cienc. Mar., 18(3): 105-124. [ Links ]
Reyes-Tisnado, R., López-Gutiérrez, F., Hernández-Carmona, G. y Castro-Moroyoqui, P. (2000). Propiedades Fisicoquímicas de los Alginatos, Polisacáridos de las Algas Phaeophytas. SEMARNAP-INP-CRIP, La Paz, México, 72 pp. [ Links ]
Shyamali, S., de Silva, M. and Savitri Kumar, N. (1984). Composition and sequence of uronate residues in alginates from some brown seaweeds. J. Nat. Sci. Counc. Sri Lanka, 12: 161-166. [ Links ]
Skinner, EW. y Phillips, R.W. (1982). La ciencia de los materiales dentales. Editorial Mundi, México, pp. 124-142. [ Links ]
Skjäk-Braek, G. and Martinsen, A. (1991). Application of some algal polysaccharides in biotechnology. In: Seaweed Resources in Europe: Uses and Potential. John Wiley, pp. 219-258. [ Links ]
Smidsrod, O. and Draget, K.I. (1996). Chemistry and physical properties of alginate. In: Carbohydrates in Europe, 14: 6-11. [ Links ]
Thomas, M. (1991). Oscillation Test. In: Course in Rheology. Paar Physica Co., Germany, pp. 4-21. [ Links ]
Wedlock, D.J., Fasihuddin, B.A. and Phillips, G.O. (1986). Characterization of alginates from Malaysia. In: G.O. Phillips, D.J. Wedlock and P.A. Williams (eds.), Gums and Stabilizers for the Food Industry. Elsevier, London, pp. 47-67. [ Links ]
a Becario por exclusividad de la COFAA-IPN y EDI-IPN.














