Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Ciencias marinas
versión impresa ISSN 0185-3880
Cienc. mar vol.30 no.1b Ensenada mar. 2004
Artículos
Intensive culture of Litopenaeus vannamei Boone 1931, in a recirculating seawater system
Cultivo intensivo de Litopenaeus vannamei Boone 1931, en un sistema de agua de mar recirculada
Benjamín Barón-Sevilla*, L. Fernando Bückle-Ramírez and Mónica Hernández-Rodríguez
* Laboratorio de Ecofisiología, Departamento de Acuicultura. Centro de Investigación Científica y de Educación Superior de Ensenada, Km 107 carretera Tijuana-Ensenada Apartado postal 2732 Ensenada, CP 22800, Baja California, México. * E-mail: bbaron@cicese.mx
Recibido en abril de 2003;
aceptado en octubre de 2003.
Abstract
The intensive culture of Litopenaeus vannamei continues to expand and their study in recirculating rearing tanks with limited water volume is scarce. The aim of this research was to cultivate white shrimp postlarvae (PL 13) for five months in a 5.85-m3 rearing unit. Vertical mosquito screen curtains augmented the tank area of the rearing unit from 13.9 (wall surface) to 84.6 m2. The total water volume (11 m3) was recirculated at a rate of 7.2 m3 h-1. Shrimp postlarvae were fed with fine-graded Camaronina mixed with water for one month and then replaced with Camaronina pellets distributed automatically twice a day. During the trial, water temperature ranged from 23.0°C to 25.8°C, oxygen from 5.3 to 7.5 mg L-1, and salinity from 27.5%o to 28.4%o. At the end of the experiment, water quality was pH 7.4, NH3-N = 0.8 mg L-1, NO2-N = 0.287 mg L-1, PO4 = 7.9 mg L-1, and total PO4 = 1.71 mg L-1. The final crop for the unit was 56.7 kg, with a shrimp mean mass of 4.001 g, and a maximum and minimum of 8.484 and 0.384 g, respectively. In terms of the total recirculated seawater volume (11 m3), the production was 5.15 kg m-3, and in relation to the tank volume, 9.7 kg m-3; considering the tank surface without curtains, production was 4.1 kg m-2, and with curtains, 0.67 kg m-2. The design and installation of the curtains is a viable tank modification to increase the available space in a reduced water volume. Our results indicate that the mean residence time (1.23 h) of the water recirculation in the rearing unit can sustain high water quality. Another extension of our results is to make use of the design to culture postlarvae as a nursery unit to raise juveniles less than 4 g for subsequent transfer to culture facilities. However, it is much more important to implicate in the analysis the use of land that in some countries, such as Mexico, constitutes vast regions of useful agricultural land, transformed into culture ponds to increase the national shrimp production. In this sense, shrimp production of the experiment in terms of the base area (7 m2) of the culture tank was 8.1 kg m-2.
Key words: intensive culture, production, recirculating seawater system, Litopenaeus vannamei.
Resumen
El cultivo intensivo de Litopenaeus vannamei continúa expandiéndose y los estudios en estanques de cultivo con sistemas de recirculación con volúmenes limitados de agua son muy escasos. El objetivo de esta investigación fue cultivar postlarvas (PL 13) del camarón blanco durante 168 días en un estanque de 5.85 m3. Se utilizaron cortinas verticales de tela de mosquitero para aumentar la superficie de la unidad de cultivo de 13.9 a 84.6 m2. El volumen total de agua (11 m3) fue recirculada a una tasa de 7.2 m3 h-1. Las postlarvas fueron alimentadas durante un mes con Camaronina molida, mezclada con agua, y remplazada después con Camaronina peletizada que se distribuyó automáticamente en el estanque dos veces al día. Durante el experimento, la temperatura del agua fluctuó entre 23°C y 25.8°C, el oxígeno entre 5.3 y 7.5 mg L-1 y la salinidad entre 27.5%o y 28.4%o. Al final del experimento, la calidad del agua fue de pH 7.4, NH3-N = 0.8 mg L-1, NO2-N = 0.287 mg L-1, PO4 = 7.9 mg L-1 y PO4 total = 1.71 mg L-1. La cosecha final de esta unidad de cultivo fue de 56.7 kg y una masa media de los camarones de 4.001 g, con un máximo y mínimo de 8.484 y 0.384 g, respectivamente. En términos del volumen total de agua recirculada (11 m3), la producción fue de 5.15 kg m-3 y, en relación con el volumen del estanque, de 9.7 kg m-3; considerando la superficie de la unidad de cultivo sin cortinas, la producción fue de 4.1 kg m-2 y con cortinas, de 0.67 kg m-2. El diseño y la instalación de las cortinas es una modificación viable para incrementar el espacio disponible en un volumen reducido de agua para aquellos organismos que pueden desplazarse a lo largo de las cortinas. Nuestros resultados indican que el tiempo (1.23 h) de residencia del agua recirculada en la unidad de cultivo es adecuado para mantener una alta calidad de los parámetros fisicoquímicos. Otra aplicación de nuestros resultados es utilizar el diseño para cultivar postlarvas como una unidad para crecer juveniles de menos de 4 g y transferirlos después a estanques de cultivo. Sin embargo, es mucho más importante implicar en el análisis la utilización de la tierra que, en algunos países como México, constituye vastas regiones de tierra agrícola transformadas en estanques para incrementar la producción de camarón. En este sentido, la producción de camarón en nuestro experimento con base en el área del estanque de cultivo (7 m2) fue de 8.1 kg m-2.
Palabras clave: cultivo intenso, producción, sistema de agua de mar recirculada, Litopenaeus vannamei.
Introduction
Litopenaeus vannamei Boone 1931 is native to the eastern Pacific Ocean, from the Gulf of California to Peru. This species is studied for its shrimp farming potential in the United States and several countries of Asia and Oceania because it grows faster than some native species. In 1994, the total world production of penaeid shrimp by farms was 741,638 metric tons, of which the giant tiger prawn Penaeus monodon Fabricius 1798 comprised 68.2% and L. vannamei, 14.8% (Anonymous, 1996). Aquaculture is one of the fastest growing food-producing sectors, providing an acceptable supplement to substitute for wild fish and plants. From 1984 to 1995, the cultured shrimp and prawn group grew at an average annual compounded rate (APR) of 16.8%, compared with 2.6% for fisheries capture. In 1995, 96.5% of shrimp and prawn production was penaeids. From 1990 to 1995, however, the production rate and expansion of this activity decreased as a consequence of environmental degradation and mismanagement (Anonymous, 1997).
Intensive culture causes waste that increases environmental concerns about coastal contamination, and pond and raceway shrimp culture depends on high water volume with variable effluent water quality. Alternatively, closed water systems offer the possibility to control exposure to extreme environmental changes (thus avoiding the loss of investments) and to improve pollution control.
Great efforts have been made to grow and reproduce shrimp in complete recirculation systems (Ogle, 1991; Ricque et al., 1993; Tirado et al., 1993, 1996; Porchas et al., 1995; Davis and Arnold, 1998), or in floating cages installed in estuarine zones (Casillas and Villarreal, 1995a; Paquotte et al., 1998). However, information on L. vannamei culture in recirculating systems is scarce because commercial farm production dominates the state of the art and continues to expand.
The aim of this work was to study L. vannamei growth in a reduced water volume, at an optimum temperature and salinity, and with tank surface area increased above that found in typical tanks. For aquaculture expansion, it is necessary to continue exploring alternative closed system production to improve water reuse, though conventional production will remain until the former reaches economic viability.
Materials and methods
Culture system unit description and operation
The culture system comprises a rearing tank (3.0 m diameter x 1.1 m deep, 7 m3) connected to a recirculated seawater system that was purified consecutively through a biological filter, a mechanical filter filled with silica sand, a zeolite filter, and an ultraviolet purifying system (fig. 1) (Spotte, 1979; Anonymous, 1986; Huguenin and Colt, 1989; Timmons and Losordo, 1994). Before drainage from the rearing tank enters the biological filter, seawater passes through a mechanical filter (1.5 m3) divided into five chambers and filled with plastic wool to retain waste material, food particles and faeces (Bückle et al., 1990). The total amount of recirculated seawater was 11 m3 and included minor tanks used for other culture purposes. The mean residence time of the fluid of the large culture tank was 1.23 h (tank water quantity m3 h-1/tank volume m3; Anonymous, 1986).
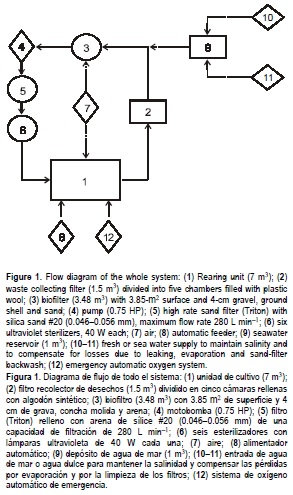
The wall surface and bottom area (13.9 m2) of the 7.0-m3 rearing unit was augmented to 84.6 m2 with 31 vertical mosquito screen curtains upheld from the tank border to an acrylic ring held by the central pipe (figs. 2a-c, 3). The water depth and internal diameter were 0.84 and 2.98 m, respectively, and the total effective volume was 5.85 m3. An irrigator soaker (1.3 cm diameter) was installed around the internal base perimeter of the tank as an air diffuser to maintain appropriate aeration generated by air blowers (fig. 2d). Additionally, an automatic system with pure oxygen was installed to cope with electricity shortage (fig. 1).
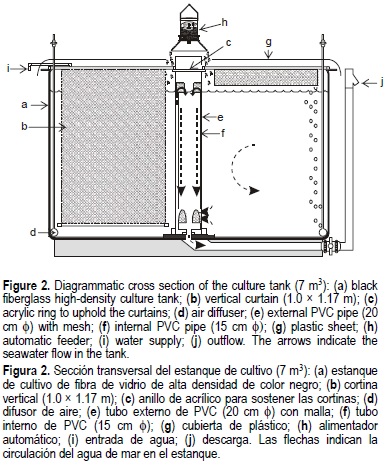

The central PVC pipe (20 cm diameter) of the tank had mesh screens at the base and at the water level in order to keep shrimp from escaping (fig. 2e). The internal PVC pipe (15 cm diameter) maintained the water level of the culture tank (fig. 2f). The water recirculation rate was 7.2 m3 h-1. A transparent plastic sheet was installed over the tank surface to prevent shrimp from jumping out (fig. 2g).
Culture trial of Litopenaeus vannamei
Shrimp postlarvae (PL 13), with an average individual mass of 4.8 mg (according to the provider), were acquired from the Maricultura del Pacifico aquaculture facility in the state of Sinaloa, Mexico, in the southern part of the Gulf of California (23°13' N, 106°25' W). They were raised at 20°C and 24%%.
Immediately after arrival to our laboratory, they were slowly transferred over 2 h to the culture tank under initial conditions of 24°C and 28%. Shrimp (approximately 33,400 PL) were cultivated during 168 days in a 5.85-m3 rearing unit. Afterwards, the temperature was increased over five days to 28°C using two 1000-W stainless steel heaters regulated with an electronic device. During the first month, shrimp were fed twice a day with finely ground Camaronina (Obregón, Mexico; 35% protein content) at a rate of 170 g mixed with 1.0 L of sea-water per day, and then continuing with Camaronina pellets with the same protein content and at the same rate apportioned through an automatic feeder twice a day at 09:00 and 14:00 h (fig. 2h). Different species of algae grew on the light-exposed mosquito screen and on the internal wall of the rearing unit throughout the experiment, and were apparently used by shrimp as supplementary food. The bottom of the rearing unit was periodically inspected with a 6.0 cm PVC pipe covered at one end with clear acrylic, and bottom samples were also taken in order to examine them for waste accumulation. The culture unit was exposed to natural spring and summer photoperiods.
Salinity was measured with a refractometer (Vista model A366ATC ±1.0%) on a daily basis and adjusted to 28% by adding either sea or tap water to the recirculation system. Temperature and oxygen concentration were measured with an oxygen meter (YSI model 50B). At the end of the experiment (October), water quality in the different units that comprised the culture system was measured as follows: ammonia with the salicylate method, nitrite with the diazotization method, nitrate with the cadmium reduction method, and phosphate and total phosphorus with the molybdovanadate and acid persulfate digestion methods (Anonymous, 1992).
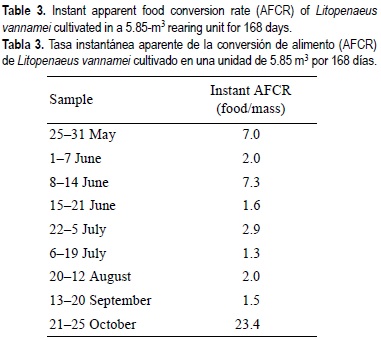
Shrimp samples were taken at random from the rearing unit nine times during the experiment. Shrimp length was measured with an electronic caliper (Max-Cal ±0.03 mm) from the tip of the rostrum to the tip of the telson, and each organism was weighed in an electronic Ohaus balance (±0.001 g). Instantaneous apparent feed conversion ratio (AFCR) (Hepher, 1988) was calculated as the apportioned food/mass gained for each sampling period throughout the experiment.
Results
Culture system operation
The design of the reservoir for shrimp culture provided good performance. The amount of water flow through the rearing units was excellent in terms of the maintenance and cleanliness of the bottom. Ammonia (NH3) in the rearing tank effluent was 0.8 mg L-1 NH3-N and, after water passed through the biological filter, it was reduced to 0.3 mg L-1 NH3-N (63%) (table 1). Seawater at 28%, 28°C and pH 7.4 contains 0.012 mg L-1 NH3-N (Anonymous, 1986).
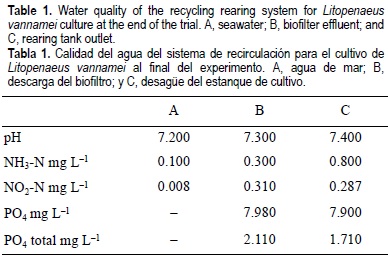
The installation of an air circulation unit made with water soaker-tubing maintained a high oxygen level (5.3-7.5 mg L-1; table 2) and excellent water circulation, preventing excreta and waste material from building up. Periodic inspection of the tank bottom revealed that neither faeces nor food had accumulated.

During the experiment, the temperature, although stable, could not be maintained at the planned 28°C because the spring-summer ambient temperature was lower compared to other years. As a result, the two 1000-W heaters were only able to maintain 23°C-25.7°C (table 2). Salinity of the seawater was maintained at our target level of 28% (table 2). In September, an electricity shortage caused the oxygen content to drop to 0.72 mg L-1, but we did not find any dead shrimp. After this extreme event, we installed an emergency pure oxygen automatic system to compensate for electrical failure.
Production of Litopenaeus vannamei, apparent food conversion rate, and growth
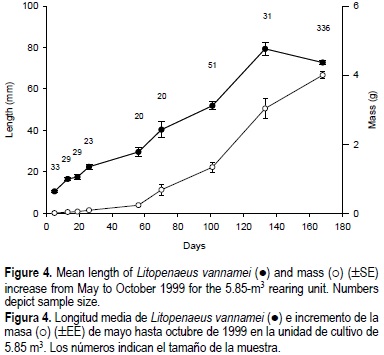
The final crop for the rearing unit was 56.7 kg, with a shrimp mean mass of 4.0 g, and a minimum and maximum of 0.4 and 8.5 g, respectively. Dead or predated organisms during 168 days represented 42.5%. In terms of the total recirculating seawater volume, the production was 5.15 kg m-3. If only the culture tank volume (5.85 m3) is considered, the production was 9.7 kg m-3. In this rearing unit, the initial density of post-larvae was 395 shrimp m-2. Calculated from the initial shrimp mean mass (4 g) of the crop and the effective surface area with curtains, the final density was 200 shrimp m-2.
Although shrimp mass at the end of the culture had increased (fig. 4), the mean length revealed that the organisms did not grow at the normal rate. The last sample, taken 32 days after the extreme oxygen drop, indicated that this event produced stress in the shrimp culture, particularly because previous samples indicated that the culture was growing well. In addition, we consider that the food quantity apportioned to the culture was insufficient because the last sample showed that the instantaneous AFCR increased from 1.5 to 23.4 (table 3). The total amount of food apportioned to the culture was 130.9 kg and the AFCR was 2.3. Part of the food was lost because the mechanical filter always contained uneaten food particles. Five species of macroalgae that the shrimp could consume as a supplemental food source developed on the walls of the rearing unit; we observed shrimp apparently scraping the algae covering the curtain surface, but we did not test for algal consumption. Growth was no affected by disease, since we did not detect infected organisms.
Discussion
The design and installation of mesh curtains is a viable tank modification to increase available space in a reduced water volume. The mesh provides a large surface where shrimp can walk or rest. Shrimp on both surfaces of a curtain were well distributed and for that reason we consider that stress in the culture was reduced.
Zelaya et al. (2001) described a similar culture system utilizing one L. vannamei production pond with water recirculated through a treatment pond. This is the same basic system as in the present trial. The treatment pond corresponds to the biofilter where water is filtered and purified before returning to the culture tank. To avoid pathogenic disease invasion, Shiau et al. (2001) conducted outdoor pond culture using integrated water treatments comprising a sedimentation area, a submerged biofilter, and a pump base with airlift. In our 168-day trial, we did not have any infection, probably due to complete control of water quality and the small amount of water refill to the culture.
Velasco et al. (2001) emphasized water management and nutrition strategy for postlarvae reared in static and recirculating culture systems. Our results indicate that a 1.23-h mean residence time of the recirculated water in the rearing unit can sustain high water quality. During the final stage of the trial, concentrations of total and un-ionized ammonia (0.8 and 0.012 mg L-1, respectively) were lower than the lethal concentrations (LC50) of 1.69, 1.2 and 1.1 mg NH3-N L-1 measured for Penaeus monodon (Allan et al., 1990), P. paulensis Pérez-Farfante 1967 (Ostrensky and Wasielesky, 1995), and P. setiferus Linnaeus 1767 (Alcaraz et al., 1999), respectively. In the present study, L. vannamei grew in a very low ammonia concentration environment compared with the concentrations of 0.21 and 1.39 mg NH3-N L-1 that reduced the growth of P monodon and P. japonicus Bate 1888 to 5% and 50%, respectively (Allan et al., 1990; Chen and Kou, 1992).
Troell et al. (1999) co-cultivated Gracilaria with salmon where macroalgae could remove 50% and 90-95% of the dissolved ammonium released in winter and spring, respectively. Furthermore, eelgrasses have been added to P. chinensis Osbeck 1765 culture ponds to improve water quality and shrimp production (Ren et al., 1991).
Porchas et al. (1995) studied the relation between survival of tank-cultured juvenile P. californiensis (Holmes, 1900) and the growth of live Caulerpa sertularioides. Mass and survival of shrimp increased with the presence of algae over that observed without algae. Porchas et al. (1995) proposed that a biological association could exist between the organisms and macroalgal growth. In this trial, we believe that the growth of algae in the rearing tanks helped to maintain water quality at a safe level and was probably used as an additional food source. We found various species of macroalgae, including Enteromorpha compressa (Linnaeus) Greville, E. linza (Linnaeus) J. Agardh, Ulva lactuca Linnaeus, Cladophora sakai Abbott, and Oscillatoria sp., that are eaten by P. japonicus (Reymond and Lagardère, 1990). Our curtains have a similar effect to that observed for Aqua-Mats™ (Marin et al., 1999) in supporting natural feed for postlarvae and juveniles.
Tacon et al. (2002) contrasted eight-week feeding trials with juvenile shrimp cultivated indoors to outdoor "green water" culture conditions. They observed higher growth and feed performance outdoors, probably caused by the food produced endogenously. Bradvolt and Browdy (2001) stocked tanks with postlarval shrimp, sand and hanging mats as additional vertical surface. Their experiments suggested benefits from natural production during early growth stages. In this trial, we consider that the natural tank feed production was directly integrated as part of the shrimp-feeding regime. Therefore, the calculated food conversion efficiency for this test is only an estimate (table 3).
Each successive sample removed from the rearing unit indicated that the mass of the culture was doubling and the shrimp growth trend was adequate, up to the penultimate sample where the AFCR increased apparently because of stress caused by food shortage and anoxia. The AFCR (2.41) is high compared with that observed by Aragón and Calderón (1997) using earthen pond intensive culture of white shrimp stocked at 92-109 m-2 (AFCR = 1.11). Casillas and Villarreal (1995b) obtained a mean food conversion ratio of 1.8-2.0 for a three-year study in a 44 ha experimental farm. Paquotte et al. (1998) obtained a food conversion efficiency average of 2.58-3.15 using growing cages installed in the estuarine zone. None of these authors discusses probable growth enhancement by macroalgae or biofouling in the cages.
Literature concerning recycling systems for the culture of L. vannamei is scarce, and the systems and conditions of culturing are very diversified. It is only possible to make a comparison taking into account production per kg m-2 crop-1. In this sense, shrimp production has improved from around 1 to 7-8 kg m-2 crop-1 in the last few years (table 4).
In this experiment, shrimp production was 8.1 kg m-2 of base area (excluding mesh curtains). This production was very similar to that obtained by Davis and Arnold (1998), although these authors worked with a raceway system of 33.5 m2.
Another extension of our results is to make use of the system design to culture postlarvae to juveniles less than 4 g for subsequent transfer to culture facilities. However, more research is needed to assess the culture of shrimp and other crustaceans in recirculating systems with our modifications to ascertain the most inexpensive and secure production.
The water recirculation systems for intensive shrimp cultivation have several advantages compared with the open systems. One can have total control of production, of water quality and temperature, of illnesses, and in providing the best environment for the cultured species. Another improvement in this system is the natural food growth in the tank and the huge space increase with the installation of vertical curtains.
However, it is much more important to consider potential reduction in the use of agricultural land that in some countries, such as Mexico, has been transformed into culture ponds in order to increase shrimp production.
Acknowledgements
This work was supported by the Mexican Federal Government, through regular funding of the Centro de Investigación Científica y de Educación Superior de Ensenada, and the Consejo Nacional de Ciencia y Tecnología, grant 4050-B. We extend our thanks to Raúl Aguilar-Rosas and Luis E. Aguilar-Rosas for the scientific names of the macroalgae.
References
Alcaraz, G., Chiapa, C.X., Espinoza, V. and Vanegas, C. (1999). Acute toxicity of ammonia and nitrite to white shrimp Penaeus setiferus postlarvae. J. World Aquacult. Soc., 30: 90-96. [ Links ]
Allan, G.L., Maguire, G.B. and Hopkins, S.J. (1990). Acute and chronic toxicity of ammonia to juvenile Matapenaeus macleayi and Penaeus monodon and the influence of low dissolved-oxygen levels. Aquaculture, 91: 265-280. [ Links ]
Anonymous (1986). Flow-through and recirculation systems. Food and Agriculture Organization of the United Nations. Report of the working group on terminology, format and units of measurement. FAO EIFAC Tech. Pap., 49: 1-100. [ Links ]
Anonymous (1992). Hatch Water Analysis Handbook. Loveland, Colorado, 831 pp. [ Links ]
Anonymous (1996). List of animal species used in aquaculture. FAO Fish. Circ. No. 914, FIRI/C914. [ Links ]
Anonymous (1997). Review of the state of world aquaculture. FAO Fish. Circ. No. 886, FIRI/C886 (Rev. 1) [ Links ]
Aragón, N.E.A. and Calderón, A.L.E. (1997). Feasibility of intensive shrimp culture in Sinaloa, Mexico. World Aquacult. '97: 64.
Bradvolt, D. and Browdy, C.L. (2001). Effects on sand sediment and vertical surfaces (AquaMats™) on production, water quality, and microbial ecology in an intensive Litopenaeus vannamei culture system. Aquaculture, 195: 81-94. [ Links ]
Bückle, R.L.F., Morales, G.E., Valenzuela, B.F. y Flores, A.N. (1990). Guía general del laboratorio de Acuicultura. Centro de Investigación Científica y Educación Superior de Ensenada (Mexico). Informe Especial CAACNC9001. [ Links ]
Casillas, R. and Villarreal, H. (1995a). Effect of density on the cage culture of the white shrimp Penaeus vannamei in Bahía Guásimas, Sonora, Mexico. Aquaculture, 95, San Diego, California: 236-237. [ Links ]
Casillas, R. and Villarreal, H. (1995b). Semi-intensive culture of the white shrimp Penaeus vannamei in the northwest of Mexico: South coast of Sonora. Aquaculture, 95, San Diego, California: 235. [ Links ]
Chen, J.C. and Kou, Y.Z. (1992). Effects of ammonia on growth and molting of Pennaeus japonicus juveniles. Aquaculture, 104: 249-260. [ Links ]
Davis, D.A. and Arnold, C.R. (1998). The design, management and production of a recirculating raceway system for the production of marine shrimp. Aquacult. Eng., 17: 193-211. [ Links ]
Hepher, B. (1988). Nutrición de Peces Comerciales en Estanque. Limusa, México, 406 pp. [ Links ]
Huguenin, J.E. and Colt, J. (1989). Design and Operating Guide for Aquaculture Seawater Systems. Elsevier Interscience, Amsterdam, 264 pp. [ Links ]
Marin, R.E., Buitrago, E. y Cabrera, T. (1999). Generación de alimento natural en piscinas de precría del camarón blanco Litopenaeus vannamei mediante el uso de los AquaMats™. Acuicultura 99, T. Cabrera, D. Jory y M. Silva (eds.), 1: 328-333. [ Links ]
Ogle, T.J. (1991). Design and operation of small tank system for ovarian maturation and spawning of Penaeus vannamei. Gulf Res. Rep., 8: 285-289. [ Links ]
Ostrensky, A. and Wasielesky, W. Jr. (1995). Acute toxicity of ammonia to various life stages of the São Paulo shrimp, Penaeus paulensis Pérez-Farfante, 1967. Aquaculture, 132: 339-347. [ Links ]
Paquotte, P., Chim, L., Martin, J.L.M., Lemos, E., Stern, M. and Tosta, G. (1998). Intensive culture of shrimp Penaeus vannamei in floating cages: Zootechnical, economic and environmental aspects. Aquaculture, 164: 151-166. [ Links ]
Porchas, A.M., Magallon, F., Portillo, G., Naranjo, J., Campos, R. and Villarreal, H. (1995). Direct and indirect effect of the macroalgae Caulerpa sertularioides in the growth and survival of brown shrimp Penaeus californiensis reared at reduced temperatures. Aquaculture 95, San Diego, California: 234-235. [ Links ]
Reid, B. and Arnold, C.R. (1992). The intensive culture of the penaeid shrimp Penaeus vannamei Boone in a recirculating raceway system. J. World Aquacult. Soc., 23: 146-153. [ Links ]
Ren, G., Wang, J., Zhang, Q., and Wang, D. (1991). Transplant eelgrasses in shrimp ponds to increase products of Penaeus chinensis O'sbeck. Mar. Sci./Haiyang Kexue, 1: 52-57. [ Links ]
Reymond, H. and Lagardère, J.P. (1990). Feeding rhythms and food of Penaeus japonicus Bate (Crustacea: Penaeidae) in salt marsh ponds: Role of halophilic entomofauna. Aquaculture, 84: 125-143. [ Links ]
Ricque, D., Martinez, V.J.A. and Aguirre, G.G. (1993). A low cost recirculation synthetic seawater system for nutritional assays in penaeid shrimp. Spec. Publ. Eur. Aquacult. Soc., 19: 161. [ Links ]
Robertson, L., Samocha, T., Gregg, K. y Lawrence, A. (1992). Potencial de engorda postcriadero de Penaeus vannamei en un sistema intensivo tipo "raceway". Cienc. Mar., 18(4): 47-56. [ Links ]
Sandifer, A.P., Hokins, S.J., Stokes, A.D. and Browdy, C.L. (1993). Preliminary comparisons of the native Penaeus setiferus and Pacific P. vannamei white shrimp for pond culture in South Carolina, USA. J. World Aquacult. Soc., 24: 295-303. [ Links ]
Shiau, L.J., Chen, I.M. and Chang, M.C. (2001). Design an outdoor recirculating system for the culture of white shrimp Litopenaeus vannamei broodstock. 6th Asian Fisheries Forum, Philippines. Asian Fisheries Society, 304 pp. [ Links ]
Spotte, S. (1979). Fish and Invertebrate Culture. Water Management in Closed Systems. John Wiley, New York, 179 pp. [ Links ]
Tacon, A.G.J., Cody, J.J., Conquest, L.D., Divakaran, S., Forster, I.P. and Decamp, O.E. (2002). Effect of culture system on the nutrition and growth performance of the Pacific white shrimp Litopenaeus vannamei (Boone) fed different diets. Aquacult. Nutr., 8: 121-137. [ Links ]
Timmons, M.B. and Losordo, T.M. (1994). Development in Aquaculture and Fisheries Science, 27. Aquaculture water reuse systems: Engineering design and management. Elsevier, New York, 333 pp. [ Links ]
Tirado, M.C., Lotz, J.M., Ogle, J.T. and Youngs, D.W. (1993). Effect of temperature on the growth and survival of Penaeus vannamei postlarvae in closed systems. Spec. Publ. Eur. Aquacult. Soc., 19: 176. [ Links ]
Tirado, M.C., Youngs, W.D., Lotz, J.M. and Ogle, J.T. (1996). Reproduction of the marine shrimp Penaeus vannamei in closed systems. Proc. PACON Conference on Sustainable Aquaculture 95, p. 383. [ Links ]
Troell, M., Roennbaeck, P., Halling, C., Kautsky, N. and Buschman, A. (1999). Ecological engineering in aquaculture: Use of seaweeds for removing nutrients from intensive mariculture. J. Appl. Phycol., 11: 89-97. [ Links ]
Velasco, M., Lawrence, A. and Neill, W.H. (2001). Comparison of survival and growth of Litopenaeus vannamei (Crustacea: Decapoda) postlarvae reared in static and recirculating culture systems. Texas J. Sci., 53: 227-238. [ Links ]
Zelaya, O., Boyd, C.E., Teichert-Coddington, D.R. and Rouse, D.B. (2001). Effects of water recirculation on water quality and bottom soil in shrimp ponds. Aquaculture 2001. World Aquaculture Society, 711 pp. [ Links ]














