Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Ciencias marinas
versão impressa ISSN 0185-3880
Cienc. mar vol.29 no.4b Ensenada Dez. 2003
Artículos
The taxonomic status of Porites sverdrupi, an endemic coral of the Gulf of California
Posición taxonómica de Porites sverdrupi, coral endémico del Golfo de California
R. Andrés López-Pérez1a*, Héctor Reyes-Bonilla2, Ann F. Budd3 and Francisco Correa-Sandoval4
1 Instituto de Recursos Universidad del Mar Puerto Ángel, Oaxaca, México. a Current address: Department of Geoscience, The University of Iowa, Iowa City, IA 52242, USA. *E-mail: rlopezpe@blue.weeg.uiowa.edu
2 Departamento de Biología Marina Universidad Autónoma de Baja California Sur La Paz, Baja California Sur CP 23080, México.
3 Department of Geoscience, University of Iowa Iowa City IA 52242, USA.
4 Instituto de Investigaciones Oceanológicas Universidad Autónoma de Baja California Apartado postal 453 Ensenada, CP 22800, Baja California, México.
Recibido en junio de 2003;
aceptado en septiembre de 2003.
Abstract
Porites sverdrupi has long been considered to be an ecotype of the more abundant and widespread species P. panamensis, but multivariate comparisons of corallite characteristics indicate that P. sverdrupi is morphologically distinct and thus represents a valid species. In these analyses, linear measurements and counts were made on 72 colonies of the two species (53 of P. panamensis and 19 of P. sverdrupi), and analyzed multivariately using canonical discriminant analysis. Generally, corallite characteristics of P. sverdrupi are larger in size than P. panamensis. Important characters in the discriminant function (e.g., number of bifurcate septa, wall thickness, and dorsal septum length) have not been recognized in previous studies of Porites in either the Indo-Pacific or Atlantic regions as being significant in distinguishing among poritid species. In addition to corallite characteristics, P. sverdrupi is unique in its ecological habit, colony form, and geographic distribution.
Key words: Porites sverdrupi, corals, Mexico, morphometrics, Scleractinia
Resumen
Desde mediados del siglo pasado Porites sverdrupi ha sido considerado como ecotipo y, por consiguiente, sinónimo de otra especie muy abundante y de amplia distribución, P. panamensis. Sin embargo, comparaciones morfométricas a nivel corallite demuestran que las colonias de P. sverdrupi son morfológicamente distintas y por lo tanto ésta constituye una especie válida. En este análisis se contaron o midieron 16 caracteres morfológicos en 72 colonias (53 de P. panamensis y 19 de P. sverdrupi) y luego fueron sometidos a un análisis discriminante. Generalmente las estructuras medidas en ambas especies se encuentran mejor desarrolladas en P. sverdrupi que en P. panamensis. Varios de los caracteres con mayor peso en las funciones discriminantes (e.g., número de septos bifurcados, amplitud de la pared del corallite y longitud del septo dorsal) no habían sido previamente identificados como importantes, ni en el Indo-Pacífico ni en la región del Atlántico, para el reconocimiento de especies de Porites. Además de las diferencias a nivel corallite entre ambas especies, P. sverdrupi es única en sus características ecológicas, forma colonial y distribución geográfica.
Palabras clave: Porites sverdrupi, corales, México, morfometría, Scleractinia.
Introduction
The presence of recent species of Porites in the eastern Pacific was first recognized in the 1900s by Grewingk (1848), who recorded a specimen from Isla del Carmen in the Gulf of California; however, it was not until Verrill's monographs (Verrill, 1864, 1866, 1868-1870) that significant advances were made in our knowledge of the systematics of the genus in this region. Today, a total of 14 nominal species of Porites are recognized in the eastern Pacific, but only 9 are considered valid (Reyes-Bonilla, 2002): P. arnaudi Reyes-Bonilla and Carricart-Ganivet, 2000; P. australiensis Vaughan, 1918; P. baueri Squires, 1959; P lobata Dana, 1848; P lichen Dana, 1846; P lutea Milne Edwards and Haime, 1860; P. panamensis Verrill, 1866; P. sverdrupi Durham, 1947; and P. rus Forskaal, 1775 (Glynn, 1997; Reyes-Bonilla, 2002). Except for P rus, all of them occur in the Mexican Pacific (Reyes-Bonilla and López-Pérez, 1998; Reyes-Bonilla and Carricart-Ganivet, 2000). The taxonomy of this genus in the eastern Pacific region is based mainly on gross colony morphology (e.g., Durham and Barnard, 1952; Brusca, 1980; Hodgson, 1995). Although this approach is fast and inexpensive, it is relatively imprecise because colony morphology in this genus varies significantly in response to environmental factors (Brakel, 1977; Foster, 1979a, 1984; Ayre et al., 1991; Knowlton et al., 1992; Potts et al., 1993; Garthwaite et al., 1994; Veron, 1995).
The taxonomy of Porites has been influenced by both "splitting" and "lumping". Initially, due to a lack of knowledge about intra-specific and inter-specific morphologic variation and the common practice of classifying every morph as a new and distinct species, several "species" were recognized and formally described. This tendency was displayed by Verrill (1864, 1866, 1868-1870) and Bernard (1905), and also in later works (Durham, 1947; Durham and Barnard, 1952; Brusca, 1980). At the other extreme, many nominal species were considered to be ecotypes and synonyms of other more abundant and widespread species (Squires, 1959; Wells, 1983), even after they were demonstrated to be genetically distinct and biologically valid (Ayre et al., 1991; Knowlton et al., 1992; Potts et al., 1993; Garthwaite et al., 1994).
The species Porites sverdrupi Durham, 1947, appears to be a victim of the practice of taxonomic lumping. First recorded and described by Durham (1947) from material in the Gulf of California, it was subsequently synonymized with Porites panamensis Verrill, 1866, by Squires (1959), who considered it a deeper-water ecomorph of P panamensis (identified under its synonym, P. californica Verrill, 1870), based on gross corallite comparison and the apparent absence of the former at depths less than 20 m. Other authors have suggested that P. sverdrupi is a valid species because it can be differentiated from P. panamensis in colony morphology and calicular characters (Durham, 1947; Reyes-Bonilla, 1992). In this paper, no re-description of P. sverdrupi and P. panamensis is attempted because both of them have already been extensively described by Durham (1947) and Verrill (1866), respectively. Instead, the objective of this paper is to perform a phenetic corallite-level analysis of P. sverdrupi and P. panamensis in order to compare them and clarify whether the former is a valid species. The basic hypothesis behind corallite-level comparison is the knowledge that colony morphology is highly variable in response to environmental factors (Foster, 1984; Garthwaite et al. , 1994; Veron, 1995), whereas corallite architecture is mainly genetically controlled (Brakel, 1977; Foster, 1979a, 1984; Ayre et al., 1991; Knowlton et al., 1992; Potts et al., 1993; Garthwaite et al., 1994; Veron, 1995).
Material and methods
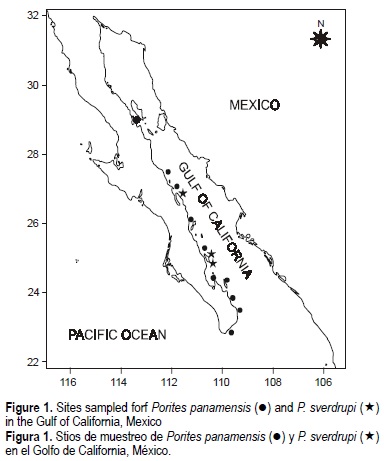
Specimens for this study were collected at various depths (0 to 30 m) from Bahía de los Ángeles (28°55'N) to Bahía Chileno (22°56'N), in the Gulf of California (fig. 1); 53 specimens corresponded to the morphology of P panamensis and 19 to P. sverdrupi. The type specimens of P. sverdrupi (USNM M547362), P. panamensis (YPM 585), P californica (YPM 1599), P. nodulosa Verrill, 1870 (YPM 6844a), and P. porosa Verrill, 1870 (YPM 4068) were also included in the analysis. The differences in the number of colonies under study were due to relative abundances of the two species in the field; whereas P. panamensis is very abundant in the gulf, P. sverdrupi is scarce. In fact, Reyes-Bonilla (2002) considers it a species on the verge of natural extinction. All samples were deposited in the Museo de Historia Natural de la Universidad Autónoma de Baja California Sur (MHNUABCS) at La Paz, Mexico.
Corallite characters and statistical procedures
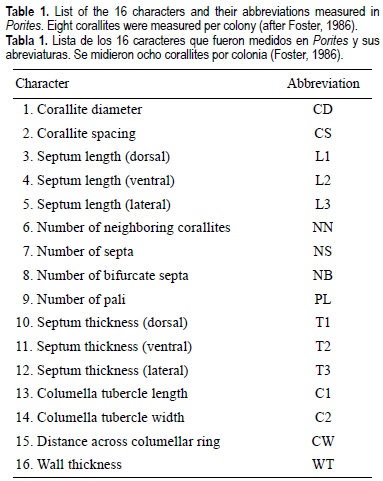
The characters consist of 16 counts and linear measurements made per corallite (table 1). Several of those (e.g., septum development, corallite diameter, and wall thickness) were used by Brakel (1977), Foster (1986), Weil (1992), and Jameson (1997) to distinguish fossil and recent species of Porites from the western Atlantic. Others (e.g., number of pali and degree of development of the columella) are diagnostic characteristics that have been used to identify Atlantic and Indo-Pacific poritid species (Brakel, 1977; Veron and Pichon, 1982; Foster, 1986; Weil, 1992).
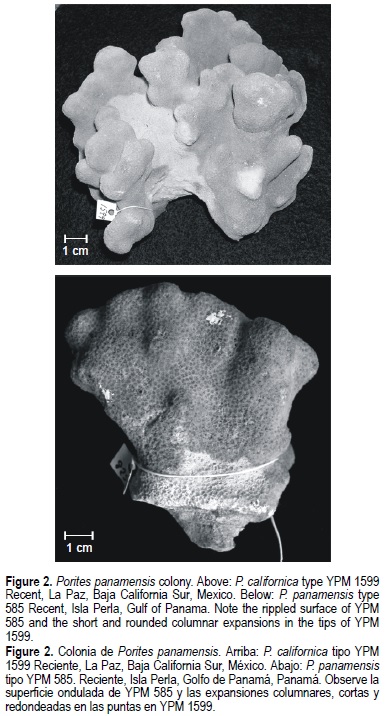
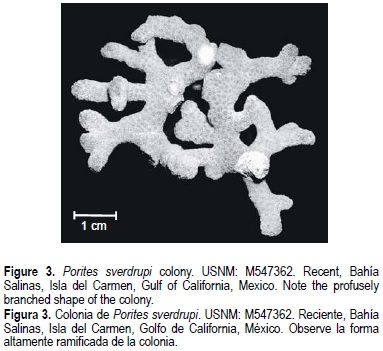
Eight mature corallites from different positions within each colony were measured to the nearest 0.01 mm using a stereoscopic microscope (Foster, 1985), and statistical analyses were performed using colony means. As the major criterion to distinguish between P. panamensis and P. sverdrupi is colony shape (Durham, 1947), this was considered the main criterion to assign specimens to the a priori group (i.e., massive = P panamensis [fig. 2] vs branching = P sverdrupi [fig. 3]). Once the colonies were assigned to the respective group, the method of Foster (1984) was applied to distinguish species. The method basically consists of evaluating colony means using discriminant analyses. The groups must be distinct at a significance level of P < 0.0001 in order to be considered true species (Budd and Coates, 1992).
Univariate statistical analyses (Mann-Whitney U test) were run to test for differences between measured characters (Zar, 1996), using a P value of 0.05.
Results and discussion
Morphological variation
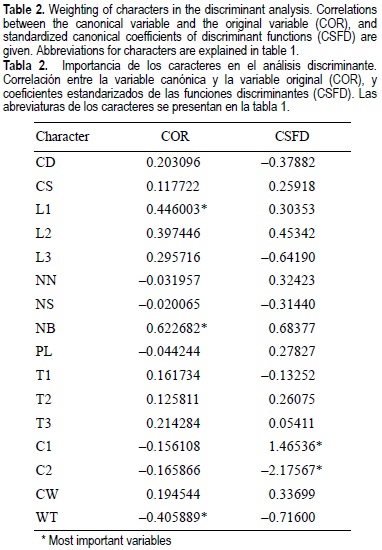
Based on the discriminant analysis (fig. 4; table 2), we recognized two distinct groups that correspond to P. panamensis and P sverdrupi, with 90.56% and 89.47% of all colonies correctly classified. One canonical variable was calculated, and the number of bifurcate septa (NB) was most heavily weighted in the function (table 2). The morphologic differences between the two groups are large enough to statistically consider each one a distinct species (Wilks' lambda = 0.4067; F (16, 55) = 5.014; P < 0.000004), although both overlap in a small area (fig. 4). The occurrence of similar taxonomic characters and/or overlap is common in Porites species, as shown by morphometric comparisons previously done by Brakel (1977), Foster (1986), Weil (1992), and Jameson (1997) for the Porites species in the western Atlantic. This overlap is not necessarily produced by closeness in phylogeny (Weil, 1992; Potts et al., 1993; Garthwaite et al., 1994); for example, Atlantic P colonensis Zlatarski, 1990 is more closely related genetically to P astreoides Lamarck, 1816, but it is morphologically closer to P. branneri Rathbun, 1887. The other option is that the morphological similarity between the studied species results from morphologic variation in response to environmental conditions, a phenomenon displayed not only in Porites but also in many Scleractinia (Brakel, 1977; Foster, 1979b, 1980a, b; Lasker, 1981; Budd, 1988, 1993; Weil, 1992; Budd and Coates, 1992; Amaral, 1994; Jameson, 1997).
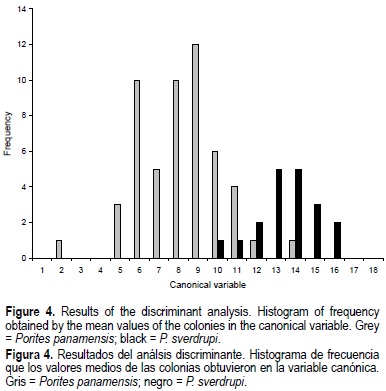
Univariate analyses of 16 characters show that 10 characters are generally larger in P. sverdrupi than in P. panamensis, but meaningful differences exist in only 7 (table 3; fig. 5). Porites sverdrupi has a larger corallite diameter (CD; fig. 5a) and better developed septa (L1, L2, L3, T1, T2, T3; table 3; fig. 3b-d, f) than P. panamensis; also, the number of bifurcate septa (NB; fig. 5e) is higher. In the western Atlantic this characteristic has been associated with decreased calcification rather than with actual forking of trabecular rows (Foster, 1986). Durham (1947) referred to this forking as "abundant perforations". As shown by its importance in the discriminant analysis (table 2), this forking has taxonomic value and is probably the most important character in the distinction of all Mexican Porites species. The only character that appears to be better developed in P. panamensis than in P. sverdrupi is wall thickness (WT; fig. 5g).
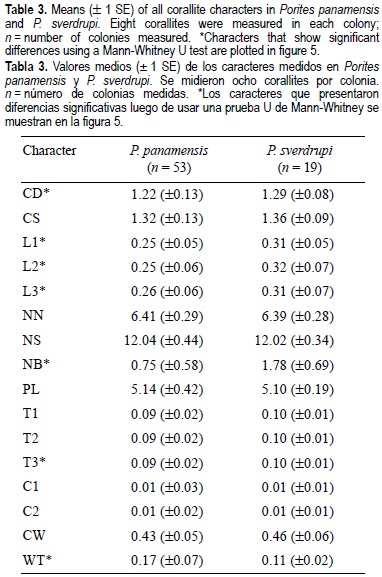
With respect to the remainder of the characters, P. panamensis displays greater morphological variation than P. sverdrupi. For example, P. panamensis usually has 5 pali, but this number varies from 2 to 7. With regard to septa, both species have two cycles and usually a total of 12 septa, but in P. panamensis the number can fluctuate between 10 and 22. On the other hand, there are relevant differences between the frequencies of occurrence of a columella in both species. In P. sverdrupi, 2.63% (n = 152) of the corallites have a columella, compared with 11.79% in P. panamensis (n = 424). Jameson (1997) tested the effect of using characters with missing values such as those involved in the columella development for the Belize Porites. In Jameson's work, the inclusion of columella characters in the analysis failed to distinguish between P. verrilli Rehberg, 1893 and P. astreoides. However the inclusion of the columella character in the present work increases the percentage (3.77%) of correctly classified colonies of P. panamensis, the Mahalanobis distance between group centroids, and the overall performance of the entire analysis.
With qualitative or gross comparisons of corallite architecture of these species it is difficult to detect the slight differences between them, and this may have been in part responsible for their synonymy (Squires, 1959; Wells, 1983). However, colony morphology is the best diagnostic character; P. sverdrupi has ramose colonies, while P. panamensis is massive or, at most, columnar (figs. 2, 3). This difference is conservative in the studied species, and is so important from the taxonomic perspective that its use is recommended to separate species in the Indo-Pacific, where more than 20 taxa can co-exist in the same reef (Veron and Pichon, 1982; Veron 2000).
Different characters have been taken as relevant to distinguish poritid species in the Indo-Pacific and Atlantic-Caribbean region. In the former, the number of pali is the main character used to discriminate species (Veron and Pichon, 1982), whereas on the east coast of the Americas, besides the number of pali, corallite diameter and columella development are important (Foster, 1986; Weil, 1992). These characters turn out to be relatively unimportant in distinguishing the two species analyzed, in which the number of bifurcate septa (NB; fig. 5e; table 2), wall thickness (WT; fig. 5g; table 2), septum dorsal length (L1; fig. 5b), and characters associated with columella development (C1, C2; table 2) are the most useful for taxonomic determinations.
Geographical, ecological and biological remarks
The species have differences in their bathymetric and geographic distribution, which quite possibly influence their ecology. Porites sverdrupi is currently distributed only in the Gulf of California, from Isla San José (25°N) to Isla Ángel de la Guarda (29°N) (Reyes-Bonilla, 1992; Reyes-Bonilla et al., 1997a), but in the Pleistocene it had a more widespread distribution in the southern Gulf of California (La Paz, Cerralvo, and Cabo San Lucas) and south to Islas Marías (21°N), in the Mexican Pacific (Squires, 1959). Squires (1959) reported its presence at Islas Marías (20°N), but surveys at that locality conducted in 1999 produced no positive record. Reyes-Bonilla (1992) indicated that P. sverdrupi was present south to Cabo San Lucas, in the Gulf of California, in the early 1980s, but the local populations become extinct afterwards. This situation is very different from that known for P. panamensis, a species that can be found from western Mexico to Central America, and that has been so successful as to be able to reintroduce itself to Costa Rica and Panama after its local populations were extirpated by the 1982-1983 El Niño (Glynn, 1990; Glynn et al. , 1994). This geographic differentiation supports the hypothesis that both species are valid and sympatric in only part of their range.
Considering vertical distribution, there are also important differences. Squires (1959) suggested that P sverdrupi is restricted to deep water (>20 m), while P panamensis is typical of very shallow areas. Field observations show that the former species can occur almost in the intertidal (2 to 3 m depth) in Bahía Concepción and the northern Gulf of California (Reyes-Bonilla, 1992), although local populations are more common in deep areas. In those locations and others in the gulf, it is possible to find ramose (P. sverdrupi) and massive (P. panamensis) morphs of Porites in the same environments (Reyes-Bonilla, 1993; Reyes-Bonilla et al., 1997a), even exposed to similar conditions of light, currents or predation. Considering that the study of co-occurring colonies of different morphology is a good way to differentiate valid coral species (Veron, 1995), we do not think that the evidence supports the synonymy of P. sverdrupi and P. panamensis. Finally, another argument in favor of their separation is that branching forms in scleractinian zooxanthallate corals are not typical of deep areas, but instead, platy, massive or encrusting (Veron, 1995); hence, if P. sverdrupi were in fact a deep-water variety of P. panamensis it is difficult to explain why the former species presents that unusual form.
Regarding the biology, the polyps of P. sverdrupi are pale cream or colorless and difficult to observe (Squires, 1959), giving the colony a pale-white bleaching appearance through all its vertical distribution, while those of P. panamensis are greenish-yellow to dark brown (Veron, 2000); the first is commonly observed in shallow waters and the second in deep waters. Porites sverdrupi can be distinguished from P. panamensis because its colonies are very small (most of the time <15 cm in diameter; bigger ones are uncommon and never larger than 30 cm) (Reyes-Bonilla et al., 1997a). The mean size of P. panamensis is 13 cm high and more than 30 cm in diameter (Reyes-Bonilla and Calderón-Aguilera, 1994). Other differences occur when both species coexist in rhodolith beds. Coralla of P. sverdrupi did not encrust algae but display a free-living behavior, probably because of the ramose morphology. In contrast, P. panamensis live attached to hard substrata such as dead bivalve shells and dead or living rhodoliths, frequently overgrowing them (Reyes-Bonilla et al., 1997a).
This study concludes that P. sverdrupi is a valid species and endemic of the Gulf of California. Considering that P. sverdrupi and P. panamensis have occurred in the Gulf of California since the Pliocene (Squires, 1959; Reyes-Bonilla, 1992), and apparently were survivors of the mass zooxanthel-late coral extinction in the Pleistocene (Dana, 1975; Heck and McCoy, 1978), this raises the question of their biogeographical origin. A comparison of corallite structure among species of Porites from the Pliocene of Imperial Valley, California (which at the time was the northernmost area of the Gulf of California), and the Neogene Caribbean (Budd, 1989) shows that the hypothesis of a common ancestry cannot be completely rejected. Finally, although gross morphology (colony form, corallite architecture, and dimensions) of P. sverdrupi closely resembles that of the Caribbean P. divaricata Lesueur, 1821 and P. astreoides, it is necessary to perform genetic and morphological comparisons between Atlantic, eastern Pacific and Indo-Pacific species to properly explain the origin of the eastern Pacific coral fauna.
Taxonomy
Phylum Cnidaria Hatschek, 1888
Class Anthozoa Ehrenberg, 1834
Order Scleractinia Bourne, 1900
Family Poritidae Gray, 1842
Genus Porites Link, 1807
Porites sverdrupi Durham, 1947
Figures 3 and 6
Porites sverdrupi Durham, 1947: 23, fig. 4, pl. 12, fig. 2, pl. 13; Durham and Barnard, 1952: 47, fig. 19, pl. 3; Squires, 1959: 420, fig. 5, pl. 33, "discussion"; Reyes-Bonilla et al., 1997a: 331.
Material examined
Two specimens, Isla Blanca, Baja California Sur, Mexico (26°44'N, 111°52'W), MHNUABCS: 1579, 1580; one specimen, Isla Coyote, Baja California Sur, Mexico (26°44'N, 111°53'W), MHNUABCS: 1578; nine specimens, Isla San José, Baja California Sur, Mexico (25°00'N, 110°37'W), MHNUABCS: 751, 752, 753, 754, 755, 758, 756, 759, 775; seven specimens, El Pardito, Baja California Sur, Mexico (24°51'N, 110°38'W), MHNUABCS: 1571, 1572, 1573, 1574, 1575, 1576, 1577; one specimen (type), Bahía Salinas, Isla del Carmen, Baja California Sur, Mexico, USNM M547362.
Description
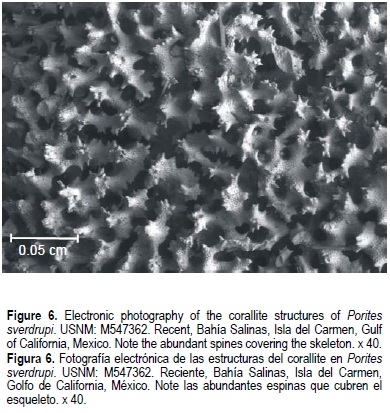
Colonies highly branching. Calices 1.29 mm (± 0.08) in diameter. Corallite walls thin, 0.11 mm (± 0.02). Two septal cycles (12 septa), all equally developed. The origin of the septa, near the corallite walls, often displays evident bifurcations. Five equally developed pali flushing to surface of corallum. Columella usually wanting, or if it appears, poorly developed. All skeletal elements covered with abundant spines (fig. 6).
Distribution
From Isla Ángel de la Guarda, Mexico (29°N), to Isla San José, Mexico (25°N). Islas Marías, Isla Espíritu Santo, La Paz, Isla Cerralvo, Isla San Francisco, Isla Ángel de la Guarda, Isla San Marcos, Roca Lobos, Isla Partida, Isla Coronados, Isla San José, Isla del Carmen, Bahía Agua Verde, Bahía Concepción, Puerto Escondido (Squires, 1959; Reyes-Bonilla, 1992; Reyes-Bonilla et al., 1997a; Reyes-Bonilla and López-Pérez, 1998). Depth range: 2 to 30 m. Although reported for Isla María Madre and Isla María Magdalena, and Islas Marías by Squires (1959), it was not recorded during intensive field searches conducted in 1999.
Fossil distribution
Pliocene: Isla Coronados, Isla del Carmen. Pleistocene: Isla San José, Isla Tiburón, Islas Marías, Isla Coronados, Isla del Carmen (Squires, 1959; Hertlein and Emerson, 1957, 1959).
Type specimen
Porites sverdrupi (USNM M547362), Bahía Salinas, Isla del Carmen, Gulf of California, Mexico.
Porites panamensis Verrill, 1866
Figures 2 and 7

Porites panamensis Verrill, 1866: 329; Bernard, 1905: 108; Reyes-Bonilla et al., 1997a: 330, fig. 3; Reyes-Bonilla et al., 1997b: 1140; Ketchum and Reyes Bonilla, 2001:730, fig. 17a-b [not P panamensis Vaughan, 1919].
Porites californica Verrill, 1870: 503-518; Bernard, 1905: 106; Durham, 1947: 20-22, figs. 3, 5-6, pl. 13, figs. 1,2, 3, 4, 5, pl. 14; Durham and Barnard, 1952: 46, fig. 17a-b, pl. 3; Squires, 1959: 420-421, figs. 3, 4, 5, 6, pl. 32, figs. 3, 4, 5, pl. 33; Durham, 1962: 51; Brusca, 1980: 63.
Porites nodulosa Verrill, 1870: 503-518; Bernard, 1905: 107; Durham, 1947: 22-23, figs. 1-3, pl. 12, fig. 4, pl. 13; Durham and Barnard, 1952: 46-47, fig. 18, pl. 3; Brusca, 1980: 63.
Porites porosa Verrill, 1870: 503-518; Bernard, 1905: 107.
Material examined
Five specimens, Bahía de Los Ángeles, Baja California, Mexico (28°55'N, 113°30'W), MHNUABCS: 1451, 1454, 1456, 1463, 1467; two specimens, Isla Tortuga, Baja California, Mexico (27°27'N, 112°00'W), MHNUABCS: 1567, 1568; five specimens, Punta Chivato, Baja California Sur, Mexico (27°06'N, 111°57'W), MHNUABCS: 1561, 1562, 1565, 1569, 1563; five specimens, El Requesón, Baja California Sur, Mexico (26°38'N, 111°50'W), MHNUABCS: 1480, 1484, 1501, 1508, 1513; five specimens, El Bajo, Baja California Sur, Mexico (26°06'N, 111°20'W), MHNUABCS: 1531, 1538, 1544, 1546, 1558; five specimens, Isla San José, Baja California Sur, Mexico (25°00'N, 110°37'W), MHNUABCS: 760, 761, 762, 766, 768; four specimens, La Catedral (Isla Espíritu Santo), Baja California Sur, Mexico (24°25'N, 110°21'W), MHNUABCS: 868, 869, 870, 871; five specimens, Bahía San Gabriel (Isla Espíritu Santo), Baja California Sur, Mexico (24°25'N, 110°21'W), MHNUABCS: 597, 598, 599, 601, 859; two specimens, Isla Gaviota, Baja California Sur, Mexico (24°17'N, 110°21'W), MHNUABCS: 665, 666; one specimen, Isla Cerralvo, Baja California Sur, Mexico (24°15'N, 110°22'W), MHNUABCS: 929; four specimens, Punta Pericos, Baja California Sur, Mexico (24°02'N, 109°49'W), MHNUABCS: 635, 636, 637, 638; five specimens, Cabo Pulmo, Baja California Sur, Mexico (23°26'N, 109°25'W), MHNUABCS: 1254, 1255, 1256, 1257, 1258; five specimens, Bahía Chileno, Baja California Sur, Mexico (22°56'N, 109°48'W), MHNUABCS: 1320, 1326, 1327, 1394, 1321; one specimen (type), Pearl Island, Gulf of Panama, Panama (P. panamensis YPM 585); one specimen (type), La Paz, Baja California Sur, Mexico (P californica YPM 1599); one specimen (type), La Paz, Baja California Sur, Mexico (P. nodulosa YPM 6844a); one specimen (type), La Paz, Baja California Sur, Mexico (P. porosa YPM 4068).
Description
Colonies massive or encrusting, having an undulated surface or lobes that sometimes extend as columnar expansions, short and rounded at the top (fig. 2). Calices average 1.22 mm (± 0.13) in diameter. Corallites have porous thick walls 0.17 mm (± 0.06) in width. Two cycles of septa, usually producing corallites with 12 septa, although they can display between 22 and 10. Lateral, dorsal and ventral septa equally developed. Usually 5 equally developed pali, but the number can vary from 5 to 7, and sometimes only 2 or 3. Pali do not commonly reache the corallite surface. Columella rudimentary, spongy, poorly developed and often wanting.
Distribution
From San Felipe (30°N), Gulf of California, Mexico, to Isla Gorgona, Colombia (3°N). Also Bahía Magdalena, on the west coast of the Baja California Peninsula (24°N). Bahía Concepción, Isla Margarita, Isla del Carmen, Isla Tiburón, San Luis Gonzaga, Isla Coronado, Bahía las Ánimas, Guaymas, Isla San Marcos, Isla Magdalena, Bahía Santa Inés, Santa Rosalía, Bahía Concepción, Isla San José, La Paz, Isla Espíritu Santo, Isla Ángel de la Guarda, Bahía Agua Verde, Puerto Peñasco, Isla Montserrat, Cabo Pulmo, Los Frailes, Isla Cerralvo. In the Mexican Pacific: Islas Marías, Islas Revillagigedo, Baja California, Baja California Sur, Sonora, Sinaloa, Nayarit, Jalisco, Colima, Guerrero and Oaxaca (Verrill, 1868; Durham, 1947; Squires, 1959; Reyes-Bonilla and López-Pérez, 1998; Reyes-Bonilla et al., in press). In Central America: Sámara, Cabo Blanco, Bahía Culebra, Isla del Caño, and Isla de Coco in Costa Rica; Golfo de Chiriquí, Gulf of Panama, Isla Secas, Isla Perlas, Isla Naranjas, Isla Coiba, Isla Contreras, and Isla Iguana in Panama; Isla Gorgona, Colombia (Glynn and Ault, 2000). Depth range: 0 to 30 m.
Fossil distribution
Pliocene: Isla del Carmen, Isla Cerralvo, Isla Coronados. Pleistocene: Isla Tiburón, Isla del Carmen, San Marcos, Bahía Magdalena, Islas Marías, Oaxaca, Mulegé, La Paz (Palmer, 1928; Squires, 1959; Hertlein and Emerson, 1957; Reyes-Bonilla, 1992).
Type specimens
Porites panamensis (YPM 585), Pearl Island, Gulf of Panama, Panama; P. californica (YPM 1599), La Paz, Baja California Sur, Mexico; P. nodulosa (YPM 6844a), La Paz, Baja California Sur, Mexico; P. porosa (YPM 4068), La Paz, Baja California Sur, Mexico.
Acknowledgements
We thank Luis Herrera (director) of the Museo de Historia Natural de la Universidad Autónoma de Baja California at La Paz, Mexico, for access to the material under their care. Stephen D. Cairns (National Museum of Natural History, Smithsonian Institution) and Eric Lazo (Yale Peabody Museum of Natural History) lent the type specimens of Porites that were analyzed in this paper. Funding was provided by a fellowship to the first author from the Consejo Nacional de Ciencia y Tecnología (CONACYT, Mexico), and by Comisión Nacional para el Conocimiento y Uso de la Biodiversidad (project FB342/H337/96, to HRB). We thank John Dawson (University of Iowa) and Gerardo Leyte (Universidad del Mar) for their comments and suggestions to the manuscript.
References
Amaral, F.D. (1994). Morphological variation in the reef coral Montastraea cavernosa in Brazil. Coral Reefs, 13: 113-117. [ Links ]
Ayre, D.J., Veron, J.E.N. and Dufty, S.L. (1991). The corals Acropora palifera and Acropora cuneata are genetically and ecologically distinct. Coral Reefs, 10: 13-18. [ Links ]
Bernard, H.M. (1905). Porites of the Indo-Pacific region. In: H.M Bernard (ed.), Catalogue of Madreporarian Corals in the British Museum, London, pp. 1-206. [ Links ]
Brakel, W.H. (1977). Corallite variation in the Porites and the species problem in corals. Proc. 3rd Int. Coral Reef Symp., Miami, 1: 457-462. [ Links ]
Brusca, R.C. (1980). Common Intertidal Invertebrates of the Gulf of California. Univ. Arizona Press, Arizona, 513 pp. [ Links ]
Budd, A.F. (1988). Large-scale evolutionary patterns in the reef-coral Montastraea: The role of phenotypic plasticity. Proc. 6th Int. Coral Reef Symp., Townsville, 3: 393-398. [ Links ]
Budd, A.F. (1989). Biogeography of Neogene Caribbean reef corals and its implications for the ancestry of eastern Pacific reef-corals. Mem. Assoc. Australasian Paleontologist, 8: 219-230. [ Links ]
Budd, A.F. (1993). Variation within and among morphospecies of Montastraea. Cour Fors Sencken, 164: 241-254. [ Links ]
Budd, A.F. and Coates, A.G. (1992). Nonprogressive evolution in a clade of Cretaceous Montastraea-like corals. Paleobiology, 18: 425-446. [ Links ]
Dana, T.D. (1975). Development of contemporary eastern Pacific coral reefs. Mar. Biol., 33: 355-374. [ Links ]
Durham, J.W. (1947). Corals from the Gulf of California and the north Pacific coast of America. Mem. Geol. Soc. Am., 20: 1-68. [ Links ]
Durham, J.W. (1962). Corals from the Galapagos and Cocos Islands. Proc. Calif. Acad. Sci., 4th Ser., 32: 41-56. [ Links ]
Durham, J.W. and Barnard, J. L. (1952). Stony corals of the eastern Pacific collected by the Velero III and Velero IV. Allan Hancok Pac. Exped., 16: 1-110. [ Links ]
Foster, A.B. (1979a). Environmental variation in a fossil scleractinian coral. Lethaia, 12: 245-264. [ Links ]
Foster, A.B. (1979b). Phenotypic plasticity in the reef corals Montastraea annularis (Ellis and Solander) and Siderastrea siderea (Ellis and Solander). J. Exp. Mar. Biol. Ecol., 39: 25-54. [ Links ]
Foster, A.B. (1980a). Ecology and morphology of the Caribbean Mio-Pliocene reef-coral Siderastrea. Acta Palaeontol. Pol., 25: 439-450. [ Links ]
Foster, A.B. (1980b). Environmental variation in skeletal morphology within the Caribbean reef corals Montastrea annularis and Siderastrea siderea. Bull. Mar. Sci., 30: 678-709. [ Links ]
Foster, A.B. (1984). The species concept in the fossil hermatypic corals: A statistical approach. Palaeontogr. Americana, 54: 58-69. [ Links ]
Foster, A.B. (1985). Variation within coral colonies and its importance for interpreting fossil species. J. Paleontol., 59: 1359-1381. [ Links ]
Foster, A.B. (1986). Neogene paleontology in the northern Dominican Republic. 3. The family Poritidae (Anthozoa: Scleractinia). Bull. Am. Paleontol., 90: 45-123. [ Links ]
Garthwaite, R.L., Potts, D.C., Veron, J.E.N. and Done, T. J. (1994). Electrophoretic identification of Poritid species (Anthozoa: Scleractinia). Coral Reefs, 13: 49-56. [ Links ]
Grewingk, C. (1848). Beitrag zur Kenntniss der geognostischen Beschaffenheit Californiens. Verhandl Russisch Min Gesell St. Petersburg, 1847: 142-162. [ Links ]
Glynn, P.W. (1990). Coral mortality and disturbances to coral reefs in the tropical eastern Pacific. In: P.W. Glynn (ed.), Global Ecological Consequences of the 1982-83 El Niño Southern Oscillation. Elsevier Oceanographic Series, 52, pp 55-126. [ Links ]
Glynn, P.W. (1997). Eastern Pacific reef coral biogeography and faunal flux: Durham's dilemma revisited. Proc. 8th Int. Coral Reef Symp., Panama, 1: 371-378. [ Links ]
Glynn, P.W and Ault, J.S. (2000). A biogeographic analysis and review of the far eastern Pacific coral reef region. Coral Reefs, 19: 1-23. [ Links ]
Glynn, P.W., Colley, S.B., Eakin, C.M., Smith, D.B., Gassman, N.J., Guzmán, H.M., del Rosario, J.B. and Maté, J.L. (1994). Reef coral reproduction in the eastern Pacific: Costa Rica, Panama and Galapagos Islands (Ecuador). Part II. Poritidae. Mar. Biol., 118: 191-208. [ Links ]
Heck, K.L Jr. and McCoy, E.D. (1978). Long-distance dispersal and the reef-building corals of the eastern Pacific. Mar. Biol., 48: 349-356. [ Links ]
Hertlein, L.G and Emerson, W.K. (1957). Additional notes on the invertebrate fauna of Clipperton Island. Am. Mus. Novit., 1859: 1-9. [ Links ]
Hertlein, L.G and Emerson, W.K. (1959) Pliocene and Pleistocene megafossils from the Tres Marías Islands. Am. Mus. Novit., 1940: 1-15. [ Links ]
Hodgson, G. (1995). Corales pétreos marinos (tipo Cnidaria, orden Scleractinia). En: W. Fischer., F. Krupp., W. Scheider., C. Sommer., K.E. Carpenter y V.H. Niem (eds.), Guia FAO para la Identificación de Especies para los Fines de la Pesca. Algas e Invertebrados. FAO, Roma, pp. 83-97. [ Links ]
Jameson, S.C. (1997). Morphometric analysis of the Poritidae (Anthozoa: Scleractinia) of Belize. Proc. 8th Int. Coral Reef Symp., Panama, 2: 1591-1596. [ Links ]
Ketchum, J.T. y Reyes-Bonilla, H. (2001). Taxonomía y distribución de los corales hermatípicos (Scleractinia) del Archipiélago de Revillagigedo, Pacífico de México. Rev. Biol. Trop., 49(3-4): 803-848. [ Links ]
Knowlton, N., Weil, E., Weigt, L.A. and Guzman, H.M. (1992). Sibling species in Montastraea annularis, coral bleaching and the coral climate record. Science, 225: 330-333. [ Links ]
Lasker, H.R. (1981). Phenotypic variation in the coral Montastraea cavernosa and its effects on colony energetics. Biol. Bull., 160: 292-302. [ Links ]
Palmer, R.H. (1928). Fossils and recent corals and coral reefs of western Mexico. Proc. Am. Philosophical Soc. Philadelphia, 67: 21-37. [ Links ]
Potts, D.C., Budd, A.F. and Garthwaite, R.L. (1993). Soft tissue vs skeletal approaches to species recognition and phylogeny reconstruction in corals. Cour Forsch Institute Senck, 164: 221-231. [ Links ]
Reyes-Bonilla, H. (1992). New records for hermatypic corals (Anthozoa: Scleractinia) in the Gulf of California, Mexico, with an historical and biogeographical discussion. J. Nat. Hist., 26: 1163-1175. [ Links ]
Reyes-Bonilla, H. (1993). Biogeografía y ecología de los corales hermatípicos (Anthozoa: Scleractinia) del Pacífico de México. En: S. Salazar-Vallejo y N.E. González (eds.), Biodiversidad Marina y Costera de México. CONABIO/CIQRO, Chetumal, pp. 207-222. [ Links ]
Reyes-Bonilla, H. (2002). Checklist of valid names and synonyms of stony corals (Anthozoa: Scleractinia) from the eastern Pacific. J. Nat. Hist., 36: 1-13. [ Links ]
Reyes-Bonilla, H. y Calderón-Aguilera, L.E. (1994). Parámetros poblacionales de P. panamensis (Anthozoa: Scleractinia) en el arrecife de Cabo Pulmo, México. Rev. Biol. Trop., 42: 121-128. [ Links ]
Reyes-Bonilla, H. y López-Pérez, R.A. (1998). Biogeografía de los corales pétreos (Scleractinia) del Pacífico de México. Cienc. Mar., 24: 211-224. [ Links ]
Reyes-Bonilla, H. and Carricart-Ganivet, J.P. (2000). Porites arnaudi, a new species of stony coral (Anthozoa: Scleractinia: Poritidae) from oceanic islands of the eastern Pacific Ocean. Proc. Biol. Soc. Washington, 113: 561-571. [ Links ]
Reyes-Bonilla, H., Riosmena-Rodríguez, R. and Foster, M.S. (1997a). Hermatypic corals associated with rhodolith beds in the Gulf of California, Mexico. Pac. Sci., 3: 328-337. [ Links ]
Reyes-Bonilla, H., Duarte, F.S. y Cobarrubias, O.A. (1997b). Gorgonias y corales pétreos (Anthzoa: Gorgonacea y Scleractinia) de Cabo Pulmo, México. Rev. Biol. Trop., 45: 1439-1443. [ Links ]
Reyes-Bonilla, H., Cruz-Piñon, G. y López-Perez, R.A. Lista sistemática, sinonimias y distribución de los corales pétreos (Anthozoa: Scleractinia) del Pacífico Mexicano. Hidrobiologica, (en prensa).
Squires, D.R. (1959). Corals and coral reefs in the Gulf of California. Bull. Am. Mus. Nat. Hist., 118: 370-431. [ Links ]
Veron, J.E.N. (1995). Corals in Space and Time. Comstock/Cornell, Ithaca, 321 pp. [ Links ]
Veron, J.E.N. (2000). Corals of the World. Vols. 1-3. Australian Institute of Marine Science, Townsville, 1382 pp. [ Links ]
Veron, J.E.N and Pichon, M. (1982). Scleractinia of eastern Australia. Part IV. Family Poritidae. Aust. Inst. Mar. Sci. Monogr. Ser., 5: 1-159. [ Links ]
Verrill, A.E. (1864). List of the polyps and corals sent by the Museum of Comparative Zoology to other institutions in exchange, with annotations. Bull. Mus. Comp. Zool. Harvard, 1: 29-60. [ Links ]
Verrill, A.E. (1866). On the polyps and corals of Panama with descriptions of new species. Proc. Boston Soc. Nat. Hist., 10: 325-357. [ Links ]
Verrill, A.E. (1868-1870). Review of the corals and polyps of the west coast of America. Trans. Connecticut Acad. Arts Sci., 1: 377-558. [ Links ]
Verrill, A.E. (1869). Synopsis of the polyps and corals of the North Pacific Exploring Expedition. Madreporaria. Proc. Essex Inst., 6: 83-100. [ Links ]
Weil, E. (1992). Genetic and morphological variation in Caribbean and Eastern Pacific Porites (Anthozoa: Scleractinia). Preliminary results. Proc, 7th Int. Coral Reef Symp., Guam, 2: 643-656. [ Links ]
Wells, J.W. (1983). Annotated list of the scleractinian corals of the Galapagos Islands. In: P.W. Glynn and G.M. Wellington (eds.), Corals and Coral Reefs of the Galapagos Islands. Univ. California Press, Berkeley, pp. 212-295. [ Links ]
Zar, J.H. (1996). Biostatistical Analysis. Prentice Hall, New Jersey, 929 pp. [ Links ]














