Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Ciencias marinas
versão impressa ISSN 0185-3880
Cienc. mar vol.29 no.4 Ensenada Out. 2003
Artículos
Evidence for concentration of anthropogenic mercury in salt marsh sediments
Evidencia de la concentración de mercurio antropogénico en sedimentos de salitrales
C. Micaelo1*, M. Válega2, C. Vale1, E. Pereira2, A. Duarte2 and I. Caçador3
1 Instituto de Investigação das Pescas e do Mar (IPIMAR), Ave. Brasília, 1449-006 Lisboa, Portugal. *E-mail: micaelo@ipimar.pt
2 Departamento de Química, Universidade de Aveiro, 3810-193 Aveiro, Portugal.
3 Instituto de Oceanografia, Faculdade de Ciências, Universidade de Lisboa, R. Ernesto de Vasconcelos, Campo Grande, 1700 Lisboa, Portugal.
Recibido en noviembre de 2000;
aceptado en noviembre de 2002.
Abstract
Sediment cores from two salt marshes, Rosário (Tagus estuary) and Laranjo (Ria de Aveiro), were analyzed for total Hg and Al, and for Fe and Mn extracted with a hydroxylamine-acetic acid solution. Both areas have been contaminated by industrial discharges during the last decades. Vertical distributions of Hg in sediments colonized by Arthrocnemum fruticosum and Halimione portulacoides were compared to profiles in non-vegetated sediments. The same vertical distribution pattern was observed in all situations: Hg enriched in sediment layers with high root density. Mercury concentrations reached 9.3 and 29.1 nmol g-1 in Rosário, and 149.0 and 196.0 nmol g-1 in Laranjo. At both marshes, higher concentrations were found in sediments colonized by H. portulacoides. These values are one order of magnitude above the levels found in non-vegetated sediments. Mercury was enriched in sediment layers containing high concentrations of Fe extracted with a hydroxylamine-acetic acid solution, indicating the importance of Fe (and Mn) oxides formed in the rooting sediments for the retention of anthropogenic Hg.
Key words: mercury, sediment, salt marsh.
Resumen
Se analizaron Hg y Al totales, y Fe y Mn extraídos con solución hidroxilamina-ácido acético de núcleos de sedimento de los salitrales Rosario (Estuario del Tajo) y Laranjo (Ría de Aveiro) en Portugal. Ambas áreas han estado sujetas a contaminación por descargas industriales durante las últimas décadas. Se compararon las distribuciones verticales de Hg en sedimentos colonizados por Arthrocnemum fruticosum y Halimione portulacoides con las de sedimentos sin vegetación. En todos los casos el patrón de distribución fue el mismo: Las capas con gran densidad de raíces se encuentran enriquecidas en Hg. Las concentraciones de Hg alcanzaron 9.3 y 29.1 nmol g-1 en Rosario, y 149.0 y 196.0 nmol g-1 en Laranjo. En ambos salitrales, las mayores concentraciones se encontraron en sedimentos colonizados por H. portulacoides. Estos valores se encuentran un orden de magnitud por encima de los niveles encontrados en sedimentos sin vegetación. Se encontró enriquecimiento de Hg en capas de sedimento con elevadas concentraciones de Fe extraído con solución hidroxilamina-ácido acético, lo que sugiere la importancia que tienen, para la retención del mercurio antropogénico, los óxidos de Fe (y Mn) que se forman en sedimentos poblados con raíces.
Palabras clave: mercurio, sedimento, salitrales.
Introduction
Many estuarine salt marshes in the proximity of industrialized areas receive substantial quantities of anthropogenic material transported by the tidal currents, both in dissolved and particulate forms. Contaminants associated with the suspended particulate matter are trapped by vegetation (Chenhall et al., 1992) and eventually incorporated into the surface sediment of salt marshes (Orson et al., 1992). Plants, by interacting with the surrounding sediment to uptake mineral nutrients (Ernst, 1990), accumulate in their tissues metals retained in the sediments (Alberts et al., 1990). However, the amount taken up by the plants is dependent on the metal availability in the sediment, and this is modified by the root activity (Alloway et al., 1988). Mercury has a great affinity to the particulate phase (Hurley et al., 1994) and in contaminated coastal sediments, vertical profiles of its concentration commonly document the historical evolution of mercury contamination (Gobeil and Cossa, 1993). Post-depositional redistribution of the anthropogenic mercury in contaminated sediment is thus considered small with respect to the mercury in solids, and consequently the toxicity of the buried mercury is limited. Because the sediment environment in salt marshes is exceedingly complicated, with the presence of exudates and intense microbial activity in the interfacial zone between the root and the sediment (Alloway, 1990), it is pertinent to investigate the behaviour of mercury in these peculiar environments.
Ria de Aveiro and the Tagus estuary are the two most mercury-contaminated coastal systems in Portugal (Figuères et al., 1985; Hall et al., 1987). During four decades, a confined area of Ria de Aveiro (Laranjo) has received mercury rejected by a chlo-alkalis plant. Although a fraction of anthropogenic mercury is dispersed through the estuarine system (Pereira et al., 1998a), a considerable amount (25.4 tons) is stored in sediment of Laranjo Bay (Pereira et al., 1998b). Two major sources of industrial mercury were identified in the Tagus estuary (Figuères et al., 1985), the most contaminated areas being the north channel (Canário, 2000) and the channels of the lower southern part. Both estuarine systems contain extensive intertidal flat areas and salt marshes, with homogeneous stands of plants, namely Arthrocnemum fruticosum and Halimione portulacoides. The lower salt marshes of both estuaries are inundated daily by tidal action. The pore water salinity tends to follow the tidal fluctuation in the water column (Madureira, 1997), which varied from mid-salinity values during most of the year to fresh water in winter periods of heavy rain (Pereira, 1996; Vale and Sundby, 1987). The repeated flooding of intertidal flats leads to the transport of suspended sediments enriched in anthropogenic metals, such as Zn, Pb and Cu, to the marshes and their subsequent retention (Caçador et al., 1996). This paper reports the depth distribution of mercury concentration in salt marsh sediments of Ria de Aveiro and the Tagus estuary with different degree of contamination, and relates these profiles to below-ground biomass and reactive iron and manganese concentration in sediments.
Materials and methods
Sampling
Sediment cores were collected in two salt marshes of the Tagus estuary (Rosário) and Ria de Aveiro (Laranjo) from pure areas colonized by A. fruticosum and H. portulacoides and non-vegetated sites (fig. 1). Cores were sampled from the lower marshes that are flooded around high tide. The sampling was done in October 1999 (Laranjo) and in February 2000 (Rosário) with PVC sediment cores of 7-cm diameter down to around 30 to 60 cm. Sediment cores were sliced in loco. In the Tagus, the vertical resolution was 1 cm until the rooting zone and then every 5 cm, and non-vegetated sediments were separated in layers of 0-5, 5-15, 25-35 and 35-45 cm. All sediments from Ria de Aveiro were sliced in 5-cm intervals. After slicing, sediment samples were stored in plastic vials until the top in order to minimize the oxidation effect due to the presence of air bubbles. In duplicate cores, temperature, redox potential and pH were measured in loco using a pH/mV meter. The pH was measured after a short period of stabilization and redox potential was allowed to equilibrate for 15 min.
Grain size and sample preparation
The fraction of fine particles in each sediment layer was determined by wet sieving with a mesh. Before sieving, a portion of each sediment layer was oven-dried at 70°C for 24 h until constant weight and cleaned of roots by coarse sieving using a nylon sieve of 0.5-mm mesh size. Sediments of each layer were then homogenized and the root material washed with demineralized water and oven-dried to constant weight at 70°C.
Analysis of aluminium, iron and manganese
Sediment samples were digested with a mixture of HF and aqua regia at 100°C according to the procedure described in Rantala and Loring (1977). Aluminium, iron and manganese were measured in the solutions by flame atomic absorption spectrometry (Perkin Elmer, model 400). Sediment samples were also digested with 20 cm3 of hydroxylammonium chloride (0.4 mol L-1 in 25% acetic acid solution) for 6 h at room temperature (Chester and Hughes, 1967). Iron and manganese concentrations in the digests were determined.
Analysis of mercury
For mercury determination, acid digestion of the sediments was carried out in duplicate by adding 50 mL of 4 mol L-1 HNO3 to an accurately weighed aliquot of sample (0.5-1 g) in a borosilicate glass beaker covered with a watch glass (Pereira et al., 1998a; Ramalhosa, 2002). The digest was heated in a sand bath for 2 h. Between 0.5 and 1 g of below-ground biomass was acid digested by a mixture of HNO3 and H2SO4 (3:2) (Cajander and Ihantola, 1984) in erlenmeyers, which were covered with a watch glass and then put in a sand bath for 4 h at 60°C. The total mercury in the acid extracts of sediments and biological samples was determined by cold-vapour atomic absorption spectrometry, using a Perkin-Elmer, model 3030B, coupled with a MHS 20 Perkin-Elmer hydride system, with tin chloride as the reducing agent. The system was calibrated with acidified mercury standards. The detection limit for mercury was 50 ng L-1. The accuracy and precision of the mercury determination method for sediment and root samples was evaluated using the estuarine sediment reference material CRM 277 and the biological reference material BCR 60. The values obtained were: 1.73 ± 0.03 µg g-1 (certified value 1.77 ± 0.06 µg g-1) and 0.32 ± 0.02 µg g-1 (certified value 0.34 ± 0.04 µg g-1).
Results
Sediment characteristics
Fine particles were the major constituents of sediments from Rosário, containing in general less than 3% of sand. The sediments from Laranjo had a larger fraction of sand, 40-65% at the vegetated site and 60-85% in non-vegetated sediments. The Al concentrations varied accordingly, being higher at Rosário (7.3-15.2%) than at Laranjo (4.1-8.3%). The values of pH were lower in the vegetated sediments of both salt marshes (6.0-6.7) than in non-vegetated sediments (6.6-7.0). The redox potential varied in broad intervals in the rooting sediments that presented brownish deposits and dark sediments in the vicinity, and EH was always positive (> 100 mV) at the sediment surface. The temperature of upper sediments was relatively uniform with depth, around 13°C at Laranjo (October) and 20°C at Rosário (February).
Root biomass
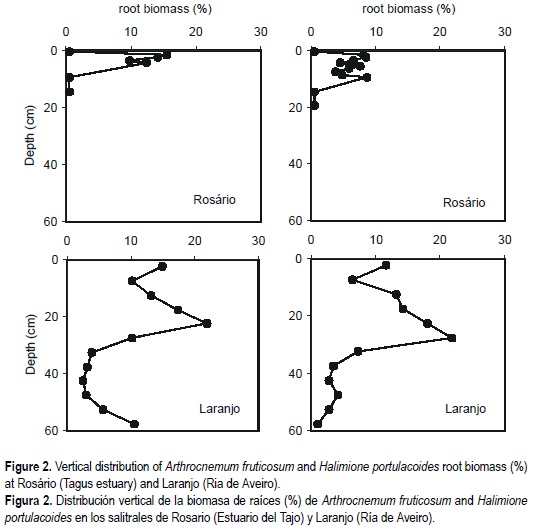
The depth variations of root biomass of A. fruticosum and H. portulacoides showed pronounced increases in sub-surface layers at the two salt marshes (fig. 2). Vegetated sediments at Laranjo presented a maximum of 22% of root biomass around 20-cm depth, and below 40-cm values were much lower. Maximum of root biomass at Rosário occurred closer to the sediment surface. The roots of A. fruticosum constituted 15% of the sediment at 3-cm depth and less than 1% at 10-cm depth. In the case of H. portulacoides, a broad maximum of 8% between 3- and 10-cm depth was found.
Vertical profiles of Fe and Mn
Concentrations of Fe and Mn extracted from colonized sediments by the hydroxylamine-acetic acid solution are compared to levels found in non-vegetated sediments (fig. 3). The vertical distributions of Fe concentrations in vegetated cores from the Rosário marsh largely exceeded the values in non-vegetated sediments. A less contrasting situation was found at Laranjo. Enhancements of extracted Fe were found at around 5-cm depth at Rosário (284 nmol g-1 in A. fruticosum and 365 nmol g-1 in H. portulacoides) and at around 20-cm depth at Laranjo (54 and 96 nmol g-1, respectively). This distribution pattern contrasts with the depth profiles recorded in non-vegetated sediments, with concentrations decreasing progressively with depth (fig. 3). Vegetated cores from Laranjo also showed a sub-surface enrichment of Mn, with a maximum of 0.64 µmol g-1 for A. fruticosum and 0.86 µmol g-1 for H. portulacoides. At Rosário, enhancement (1.83 µmol g-1) was only observed in sub-surface layers of H. portulacoides, but non-vegetated sediments generally contained higher extracted Mn.
The ratio between extractable and total Fe was higher in the same sub-surface layers, with maximum of 39% at Rosário and 16% at Laranjo. In non-vegetated sediments, the fraction of extractable Fe was lower (maximum 6%) and decreased with depth until 1%. At the sediment surface of the Rosário marsh, most of Mn (maximum 98%) was extracted with the hydroxy-lamine solution, whereas at Laranjo, a slight increment was observed in sub-surface layers.
Vertical profiles of Hg
The vertical profiles of Hg/Al ratio in sediments from the two salt marshes colonized by H. portulacoides and A. fruticosum are compared to those found in non-vegetated sediments (fig. 4). Clearly, profiles in vegetated sediments differed considerably from those recorded in non-vegetated areas, and that resulted mainly from the higher levels of total Hg in rooting sediments. Surface layers of non-vegetated sediments at Rosário contained 5 nmol g-1 and values decreased with depth. Otherwise, the vegetated sediments presented higher Hg/Al ratios in sub-surface layers: [Hg] = 29.1 nmol g-1 around 6 cm in the case of H. portulacoides and [Hg] = 9.3 nmol g-1 at 10 cm for A. fruticosum. At Laranjo, both non-vegetated and vegetated sediments presented peak values at around 15 cm, but the Hg concentrations were one order of magnitude higher in the rooting sediments, 149.0 nmol g-1 (A. fruticosum) and 196.0 nmol g-1 (H. portulacoides).
Discussion
The study of Hg in the Tagus estuary and Ria de Aveiro is of particular importance due to the high levels found in sediments of confined areas (Figuères et al., 1985; Pereira et al., 1998a). In general, Hg profiles in coastal sediments reflect mainly the historical evolution of Hg input to the system (Klein and Goldberg, 1970; Breteler et al., 1984), and only a small fraction is recycled within the sediments (Gobeil and Cossa, 1993; Gagnon et al., 1997) or released across the sediment-water interface when oxygen in near-bottom water is depleted (Covelli et al., 1999). Results obtained in our study point to an intense redistribution of anthropogenic Hg in salt marsh sediments. Mercury is highly incorporated in colonized sediments and retention occurs mainly in the rooting sediment layers. This pattern was observed in sediments of two marshes with different grain size, different levels of Hg and colonized by two salt marsh plants eventually at different age stages. The incorporation was more efficient in sediments colonized by H. portulacoides than by A. fruticosum. This process was better observed in the most contaminated area (Laranjo); the intensity of Hg peaks exceeding the values registered in sub-tidal sediments that document the period of higher industrial discharges (Pereira et al., 1998a).
Previous studies in the Tagus salt marshes showed that release of O2 by the roots (Caetano and Vale, in press) oxidizes metal sulphides (Madureira et al., 1997) and precipitates Fe oxides near the roots (Vale, 1990; Sundby et al., 1998). High concentrations of Fe extracted by the hydroxylamine acid solution in the sediments containing higher root biomass, or in contiguous layers, are in line with those findings. Iron oxides (and Mn oxides in the case of Laranjo) should constitute the majority of the precipitates, although metals weakly bound to other sediment phases could also be removed by the hydroxy-lamine solution (Tessier et al., 1979). Besides radial transport of solutes towards the roots by molecular diffusion induced by the concentration gradient, high percentage of Fe in oxide forms is favoured by the root water uptake. This movement brings the pore water solutes to the proximity of the roots, namely nutrients, Fe (II), dissolved mercury and other trace elements.
The lack of correlations between values of root biomass, extractable Fe and total Hg resulted from the fact that precipitates of Fe/Mn and Hg concentrations were not always maximum at layers of higher root biomass (fig. 5). The simplest plausible explanation is smaller roots, with larger active surface areas, delivering occasionally more O2 to the sediment than larger roots (higher biomass), and that causes precipitation of higher quantities of Fe. Maximum of total Hg above the Fe-oxide peak appears to be related to water uptake by roots from upper sediment layers that are daily inundated by the tide. This creates a downward transport of dissolved constituents towards the rooting zone of sediments. Once Fe oxides are formed (Sundby et al., 1998), Hg then may be retained. Iron oxides, which are important scavengers of Hg (Jenne, 1968), thus appear to act as a barrier to the dissolved Hg. At Laranjo, it was not possible to discern whether Mn oxides have a similar role due to the parallelism of Fe and Mn profiles. The influence of precipitation of Fe and Mn on retention of Hg is in line with the explanation for the mobility of Hg in the upper contaminated sediments of Saguenay Fjord, Canada (Gobeil and Cossa, 1993).
It is noticeable that these results were obtained in winter and autumn, since Fe precipitates in other salt marshes of North Europe and North America are consumed in winter due to reduction of plant activity (Mendelssohn et al., 1995; Kostka and Luther, 1995). This is due to the fact that salt marsh plants in the Tagus estuary have a very short period of dormancy or remain active in winter (Catarino and Caçador, 1981). Oxidative conditions tend to stand the entire year and this creates a progressive incorporation of Hg onto Fe oxides.
References
Alberts, J.J., Price, M.T. and Kania, M. (1990). Metal concentrations in tissues of Spartina alterniflora (Loisel) and sediments of Georgia salt marshes. Est. Coast. Shelf Sci., 30: 4-58. [ Links ]
Alloway, B.J. (1990). Soil processes and the behaviour of metals. In: B.J. Alloway (ed.), Heavy Metals in the Soils. John Wiley and Sons, London, pp. 7-27. [ Links ]
Alloway, B.J., Thornton, L., Smart, G., Sherlock, J.C. and Quinn, M.J. (1988). Metal availability. Sci. Tot. Environ., 75: 41-69. [ Links ]
Breteler, R.J., Bowen, V.T., Schneider, D.L. and Henderson, R. (1984). Sedimentological reconstruction of recent pattern of mercury pollution in the Niagara River. Environ. Sci. Tech., 18: 404-409. [ Links ]
Caçador, I., Vale, C. and Catarino, F. (1996). Accumulation of Zn, Pb, Cu and Ni in sediments between roots of the Tagus estuary salt marshes, Portugal. Estuar. Coast. Shelf Sci., 42: 393-403. [ Links ]
Caetano, M. and Vale, C. Trace-elemental composition of seston and plankton along the Portuguese coast. Mar. Chem. (in press).
Cajander, V.R. and Ihantola, R. (1984). Mercury in some higher aquatic plants and plankton in the estuary of the River Kokemaenjoki, southern Finland. Ann. Bot. Fennici, 21: 151-156. [ Links ]
Canário, J. (2000). Mercúrio em sedimentos contaminados e águas intersticiais da Cala do Norte do estuário do Tejo. M.Sc. thesis, Universidade Nova de Lisboa, Portugal, 98 pp. [ Links ]
Catarino, F.M. and Caçador, M.I. (1981). Produção de biomassa e estratégia de desenvolvimento em Spartina maritima e outros elementos da vegetação dos sapais do estuário do Tejo. Bol. Soc. Broteriana, 2a sér. 54: 384-403. [ Links ]
Chenhall, B.E., Yassini, I. and Jones, B.J. (1992). Heavy metal concentration in lagoonal saltmarsh species, Ilhawarra region, southeastern Australia. Sci. Tot. Environ., 125: 203-225. [ Links ]
Chester, R. and Hughes, M.J (1967). A chemical technique for the separation of ferromanganese minerals, carbonate minerals and adsorbed trace metals from pelagic sediments. Chem. Geol., 2: 249-262. [ Links ]
Covelli, S., Faganeli, J., Horvat, M. and Brambati, A. (1999). Porewater distribution and benthic flux measurements of mercury and methylmercury in the Gulf of Trieste. Estuar. Coast. Shelf Sci., 48: 415-428. [ Links ]
Ernst, W.H.O. (1990). Ecophysiology of plants in waterlogged and flooded environments. Aquat. Bot., 38: 73-90. [ Links ]
Figuères, G., Martin, J.M., Meybeck, M. and Seyler, P. (1985). A comparative study of mercury contamination in the Tagus Estuary (Portugal) and major French estuaries (Gironde, Loire, Rhône). Est. Coast. Shelf. Sci., 20: 183-203. [ Links ]
Gagnon, C., Pelletier, É. and Mucci, A. (1997). Behaviour of anthropogenic mercury in coastal marine sediments. Mar. Chem., 59: 159-176. [ Links ]
Gobeil, C. and Cossa, D. (1993). Mercury in sediments and sediment pore water in the Laurentian Trough. Can. J. Fish. Aquat. Sci., 50: 1794-1800. [ Links ]
Hall, A., Duarte, A.C., Caldeira, M.T.M. and Lucas, M.F.B. (1987). Sources and sinks of mercury in the coastal lagoon of Aveiro, Portugal. Sci. Tot. Environ., 64(1-2): 75-87. [ Links ]
Hurley, J.P., Watras, J.C. and Bloom, N.S. (1994). Distribution and flux of particulate mercury in four stratified seepage lakes. In: C. Watras and W. Hunckabee (eds.), Mercury Pollution: Integration and Synthesis. Lewis Publishers, California, 726 pp. [ Links ]
Jenne, E.A. (1968). Controls of Mn, Fe, Co, Ni, Cu and Zn concentrations in soils and water: The significant role of hydrous Mn and Fe oxides. In: R.F. Gould (ed.), Trace Inorganics in Water. Adv. Chem. Ser., 73. Am. Chem. Soc. Washington, DC, pp. 337-387. [ Links ]
Klein, D.H. and Goldberg, E.D. (1970). Mercury in the marine environment. Environ. Sci. Tech., 4: 765-768. [ Links ]
Kostka, J.E. and Luther III, G.W. (1995). Seasonal cycling of Fe in saltmarsh sediments. Biogeochemistry, 29: 159-181. [ Links ]
Madureira, M.J. (1997). Biogeoquímica do enxofre em sedimentos de sapais. Efeitos na química do ferro e do manganês. Ph.D. thesis, Universidade Técnica de Lisboa, Instituto Superior Técnico, Portugal, 219 pp. [ Links ]
Madureira, M.J., Vale, C. and Simões-Gonçalves, M.L. (1997). Effect of plants on sulphur geochemistry in the Tagus salt-marshes sediments. Mar. Chem., 58: 27-37. [ Links ]
Mendelssohn, I.A., Kleiss, B.A. and Wakeley (1995). Factors controlling the formation of oxidized root channels: A review. Wetlands, 15: 37-46. [ Links ]
Orson, R.A., Simpson, R.L. and Good, R.E. (1992). A mechanism for the accumulation and retention of heavy metals in tidal freshwater marshes of the upper Delaware River Estuary. Estuar. Coast. Shelf Sci., 34: 171-186. [ Links ]
Pereira, M.E. (1996). Distribuição, Reactividade e transporte do Mercúrio na Ria de Aveiro. Ph.D. thesis, Universidade de Aveiro, Portugal, 284 pp. [ Links ]
Pereira, M.E., Duarte, A.C., Millward, G.E., Vale, C. and Abreu, S.N., (1998a). Tidal export of particulate mercury from the most contaminated area of Aveiro's Lagoon, Portugal. Sci. Tot. Environ., 213: 157-163. [ Links ]
Pereira, M.E., Duarte, A.C., Millward, G.E., Abreu, S.N. and Vale, C. (1998b). An estimation of industrial mercury stored in sediments of a confined area of the Lagoon of Aveiro (Portugal). Wat. Sci. Tech., 37(6-7): 125-130. [ Links ]
Ramalhosa, E. (2002). Mercúrio na Ria de Aveiro: associações, reactividade e especiação. Ph.D. thesis, Universidade de Aveiro, Portugal, 353 pp. [ Links ]
Rantala, R.T.T. and Loring, D.H. (1977). A rapid determination of 10 elements in marine suspended particulate matter by atomic absorption. Atom. Absorp. News, 16: 51-52. [ Links ]
Sundby, B., Vale, C., Caçador, I., Catarino, F., Madureira, M.J. and Caetano, M. (1998). Metal-rich concretions on the roots of salt marsh plants: Mechanism and rate of formation. Limnol. Oceanogr., 43(2): 245-252. [ Links ]
Tessier, A., Campbell, P.G.C. and Bisson, M. (1979). Sequential extraction procedure for the speciation of particulate trace metals. Anal. Chem., 51(7): 844-851. [ Links ]
Vale, C. (1990). Temporal variations of particulate metals in the Tagus River Estuary. Sci. Tot. Environ., 97/98: 137-154. [ Links ]















