Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Ciencias marinas
versão impressa ISSN 0185-3880
Cienc. mar vol.29 no.2 Ensenada Jun. 2003
Artículos
Antitumor potential of natural products from marine ascidians of the Gibraltar Strait: A survey
Potencialidad antitumoral de productos naturales marinos de ascidias del Estrecho de Gibraltar: Una panorámica
Eva Zubía, María J. Ortega and Javier Salvá*
Departamento de Química Orgánica, Facultad de Ciencias del Mar y Ambientales, Universidad de Cádiz, 11510 Puerto Real, Cádiz, España. * E-mail: javier.salva@uca.es
Recibido en enero de 2003;
aceptado en marzo de 2003.
Abstract
The chemical study of a selection of marine ascidians from the Gibraltar Strait has led to the characterization of a collection of natural products of diverse biosynthetic origin. The structures were elucidated by spectroscopic methods and, in certain instances, the absolute stereochemistry was determined using chiral auxiliaries following the Mosher method. In particular, the new compounds isolated from the ascidians Phallusia fumigata (Grübe, 1864), Botryllus leachi (Savigny, 1816), Stolonica socialis (Hartmayer, 1903), and Pseudodistoma obscurum (Pères, 1959) are presented and key aspects of the structural elucidation are discussed. In order to anticipate a possible antitumor application, the cytotoxicity of the new compounds was evaluated against cell lines P-388 (suspension culture of a lymphoid neoplasm from a DBA/2 mouse), A-549 (monolayer culture of a human lung carcinoma), HT-29 (monolayer culture of a human colon carcinoma), and MEL-28 (monolayer culture of a human melanoma).
Key words: natural products, ascidians, structure elucidation, cytotoxicity.
Resumen
El estudio químico de una serie de ascidias seleccionadas procedentes del Estrecho de Gibraltar ha llevado a la caracterización de una colección de productos naturales de muy diverso origen biosintético. Las estructuras se establecieron mediante métodos espectroscópicos y, en algunos casos, la estereoquímica absoluta se determinó usando auxiliares quirales siguiendo el método de Mosher. En concreto, se presentan los nuevos compuestos aislados a partir de las ascidias Phallusia fumigata (Grübe, 1864), Botryllus leachi (Savigny, 1816), Stolonica socialis (Hartmayer, 1903) y Pseudodistoma obscurum (Pères, 1959) y se discuten aspectos clave acerca de la elucidación estructural. Para poder anticipar una posible aplicación antitumoral, se ha realizado una evaluación de citotoxicidad de los nuevos compuestos frente a las líneas celulares P-388 (cultivo en suspensión de un neoplasma linfoide de ratón DBA/2), A-549 (cultivo en monocapa de un carcinoma de pulmón humano), HT-29 (cultivo en monocapa de un carcinoma de colon humano) y MEL-28 (cultivo en monocapa de un melanoma humano).
Palabras clave: productos naturales, ascidias, elucidación estructural, citotoxicidad.
Introduction
Marine organisms have proved to be a rich source of bioac-tive natural products. During the past 20 years, marine natural product chemists in collaboration with pharmacologists, both academic and industrial, have described a large number of novel compounds with useful biomedical properties. The number and quality of leads generated more than justify research on marine pharmacology (Faulkner, 2000).
In the area of cancer research, pharmacologically active marine compounds have traditionally played a prominent role. Thus, after the discovery of TaxolR and its stabilizing micro-tubules mode of action, three marine natural compounds have exhibited a similar biological behaviour: discodermolide, eleutherobin and sarcodictyin A. In general, the biological material that has received more attention in this field of research are marine invertebrates of the groups of sponges, molluscs, bryozoans, corals and ascidians. In particular, ascidians are well known producers of nitrogenous metabolites and among the marine-derived compounds that have entered phase I and II trials as antitumor agents, two of them, ecteinascidin 743 and aplidine, are derived from ascidians. Ecteinascidin 743 or ET-743 (YondelisTM) is an alkaloid obtained from the ascidian Ecteinascidia turbinata (Rinehart et al., 1990; Wright et al., 1990), which was licensed to PharmaMar S.A. and which currently undergoes the final steps in the clinical trials to become an anticancer drug exhibiting a unique mode of action (D'Incalci et al., 2002). Aplidine is a cyclic depsipeptide of the well known didemnin family that was isolated from the ascidian Aplidium albicans (Rinehart et al. , 1989) and that is currently undergoing phase I clinical trials under the auspices of PharmaMar S.A.
The projects carried out by our research group in this area have been aimed to the discovery of new cytotoxic metabolites from marine invertebrates, mainly of the southern coast of Spain. In this context, in the present paper we survey the progress achieved in the study of the antitumor agents from a selection of ascidians collected in the Gibraltar Strait. The biological material of this group of invertebrates, after extraction and preliminary chemical analysis, was fractioned following a bioassay guided isolation approach and, finally, the compounds responsible for the activity were identified using spectroscopic methods.
Materials and methods
The organic extracts from each marine ascidian were frac-tioned using column chromatography. The fractions obtained were analyzed using thin layer chromatography and 1H nuclear magnetic resonance (NMR) spectroscopy. Final separations using both normal and reversed phase high performance liquid chromatography (HPLC) enabled us to obtain pure compounds.
Identification and structural elucidation were performed using organic spectroscopy techniques such us 1H and 13C NMR and mass spectrometry, as well as infrared (IR) and ultraviolet (UV) spectroscopy. In particular, 1H and 13C NMR spectra were made at 399.952 and 100.577 MHz, respectively, using CDCl3, CD3OD or pyridine-d5 as solvents. In HPLC separations, LiChrosorb Si 60 was used in normal phase mode and LiChrosorb RP-18 in reversed phase mode, using in both cases a differential refractometer and a UV detector. All solvents used were spectral grade or distilled from glass prior to use.
The antitumor screening assays were performed employing the following cell lines: P-388 (suspension culture of a lymphoid neoplasm from a DBA/2 mouse), A-549 (monolayer culture of a human lung carcinoma), HT-29 (monolayer culture of a human colon carcinoma), and MEL-28 (monolayer culture of a human melanoma). Cells were maintained in logarithmic phase of growth in Eagle's Minimun Essential Medium.
Two methods for inhibition of cell growth have been employed: the first, by counting total cell numbers using an adapted form of the Bergeron method (Bergeron et al., 1984) and 24 well microtiter plates; the second, by counting viable cells using 96 well microtiter plates and the colorimetric method (Mosmann, 1983; Faircloth et al., 1988). The samples assayed have been plated over cell lines in logarithmic growth phase. The length of incubation has depended on each cell line growth rate.
The results of these assays have been used to generate dose-response curves from which the IC50 value has been calculated for each case (sample concentration which produces 50% of cell growth inhibition).
Results
The ascidian Phallusia fumigata (Grübe, 1864) from Tarifa Island, near the Gibraltar Strait, contained a series of new glucosphingolipids, the phallusides 1-4 [1-4] (fig. 1). The phallusides represent the first report of glucosphingolipids isolated from ascidians and three of them [1-3] contain the uncommon sphingoid base 2-amino-9-methyl-D-erythro-(4E, 8E, 10E)-octadeca-4,8,10-triene-1,3-diol (Durán et al., 1998).

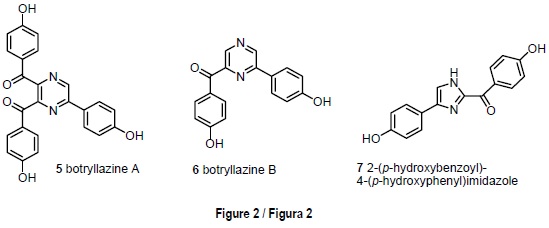
The red ascidian Botryllus leachi (Savigny, 1816), from Tarifa Island, contained two novel pyrazine alkaloids, botrylla-zine A [5] and botryllazine B [6], and the new imidazole alkaloid 2-(p-hydroxybenzoyl)-4-(p-hydroxyphenyl)imidazole [7] (fig. 2). Botryllazine A [5] represents the first example of a marine alkaloid containing a pyrazine nucleus derived from three tyrosine precursors. Two of the new alkaloids exhibited a mild cytotoxicity against the tumor cell lines assayed (Durán et al., 1999).
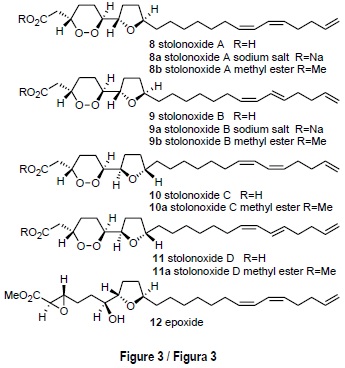
The colonial ascidian Stolonica socialis (Hartmayer, 1903), from Tarifa Island, contained a new class of strongly cytotoxic acetogenins named stolonoxides A-D [8-11] (fig. 3). The structures of the stolonoxides present a 1,2-dioxane ring linked to a tetrahydrofuran nucleus and were established by spectroscopic study of both the natural mixtures, whose components differ in the geometry of a double bond, and of the corresponding methyl esters. Derivatization by treatment with base afforded epoxide 12 and application of the Mosher method allowed unambiguous elucidation of the absolute stereochemistry 3S, 6S, 7S, 10R for stolonoxide A [8]. The stolonoxides exhibited a powerful cytotoxicity in antitumor testing (Durán et al., 2000).
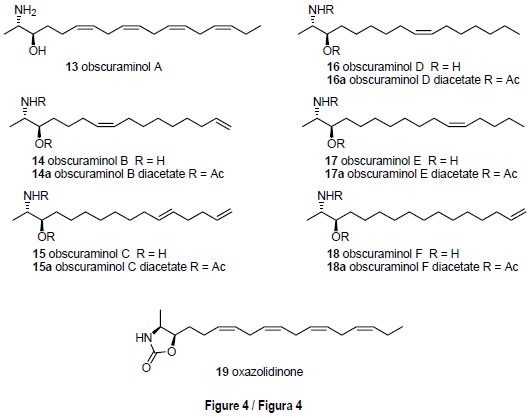
The ascidian Pseudodistoma obscurum (Pères, 1959), from Tarifa Island, has led to the characterization of six new unsaturated 2-amino-3-ol compounds: the obscura-minols A-F [13-18] (fig. 4). Their structures were established by spectro-scopic analysis, their relative configurations by NOEDS study of oxazolidinone derivatives, and their absolute configurations by application of the Mosher method to the N-acetyl derivatives of the natural components. Although the obscuraminols A-F [13-18] were active enough to employ cytotoxicity guided fractionation, no significant activities were encountered in the tests performed (Garrido et al., 2001).
Discussion
In our ongoing project directed toward the search for antitumor compounds from marine ascidians of the Gibraltar Strait, we collected specimens of Phallusia fumigata. The medium polar fraction of an acetone-methanol extract afforded, after separation both on silica gel column and reversed phase HPLC, the new glucosphingolipids phallusides 1-4 [ 1-4] (fig. 1). In general, glycosphingolipids are a large group of amphipathic biomolecules containing two basic structural units: a sugar and a hydrophobic ceramide that involves a sphingoid base amide linked to a fatty acid derived acyl chain.
The IR and NMR spectroscopic data of the phallusides were quite similar. The IR absorptions at 3400, 1650 and 1540 cm-1 indicated the presence of hydroxyl and amide groups. The 13C NMR signal at δ 105.7 (d) together with the 1H NMR doublet around δ 4.9 were assigned to the anomeric methine of a β-glucopyranoside moiety, which, in addition, gave rise to the carbon signals at δ 78.6 (d), 78.5 (d), 75.2 (d), 71.6 (d) and 62.7 (t), and to a series of proton signals in the 1H NMR spectrum at δ 4.5-3.8. The N-alkylamide functionality was ascertained by the 13C NMR signals at δ 175.7 (s) and 54.6 (d). These common spectroscopic features together with the presence of a series of aliphatic and olefinic signals suggested that the phallusides were a series of glucosphingolipids with slight structural differences. Nevertheless the structure of each component could be elucidated by an extensive study of their 1H, 13C NMR and FAB mass spectra, as well as by the study of the derivatives produced by methanolysis of the natural compounds.
The phallusides encountered in P. fumigata are the first account of glycosphingolipids obtained from ascidians. Structurally related ophidiacerebrosides (Jin et al., 1994) have been reported to show strong cytotoxicity against L-1210 leukaemia cells in vitro and the agelasphins (Natori et al., 1994) have shown in vivo antitumor activity against B16 murine melanoma. However, although the acetone-methanol extract of P fumigata had exhibited cytotoxicity against A-549 cell line (IC50 = 5 µg/mL), the phallusides assayed [1,2] were inactive in the cytotoxicity tests performed against the tumor cell lines mentioned above (table 1).
The red ascidian Botryllus leachi from Tarifa Island provided an example of the well known issue that ascidians are a rich source of nitrogen containing metabolites (Faulkner, 2002). Frozen specimens of B. leachi were extracted with an acetone-methanol mixture. The n-butanol soluble material was chromatographed on silica gel and final purification using HPLC allowed the isolation of two novel pyrazine alkaloids, botryllazine A [5] and botryllazine B [6], together with the new imidazole derivative 7 (fig 2). The structures of compounds 5-7 were elucidated by interpretation of spectral data, being especially important the analyses of the 1H-13C couplings to define the nature of the pyrazine nucleus and, therefore, to discard the pyridazine and pyrimidine alternatives in the structures of the botryllazines A and B [5, 6].
The biogenetic pathway through which the botryllazines might derive from tyrosine precursors in B. leachi deserves some remarks. The double condensation of two amino acid units with amide formation leads to a diketopiperazine 3,6-disubstituted by the a-chains of the corresponding precursors. On the other hand, the biosynthesis through the connection of each amine group with C-2 of the other precursor, likely via benzylic oxidation an imine formation, leads to a 2,5-disubstituted piperazine ring. Representative examples (Fahy et al., 1991; Fu et al., 1998) of marine metabolites derived through these two biosynthetic routes are given in scheme 1. Neither of these routes provides a satisfactory explanation to the novel structure of botryllazine B [6]. However, it might be biosynthetically plausible to propose a tyrosine condensation with tyramine to afford an amide followed by cyclization via imine as in the second route mentioned above. The structure of botryllazine A [5] is unprecedented since three tyrosine derived units are involved in the formation of the pyrazine nucleus. However, the way in which these units condense with each other is not apparent (Durán et al., 1999).
Although the crude extract of B. leachi had shown significant cytotoxicity against the tumor cell lines P-388, A-549, HT-29 and MEL-28, botryllazine A [5] was inactive with IC50 values over 10 µg/mL in all cases. Botryllazine B [6] exhibited a mild cytotoxicity against A-549 and MEL-28 cell lines (IC50 = 5 µg/mL). Imidazole compound 7 exhibited a moderate cytotoxicity (IC50 = 5 µg/mL) against the four tumor cell lines tested; however, the close relation of its structure with those of the bis(indolyl)imidazoles topsentines (Bartik et al., 1987), which are well known antiinflamatory agents of marine origin, led us to propose the new alkaloids from B. leachi as promising candidates for antiinflamatory testing (table 1).
Unlike the ascidians mentioned above, the chemical study of Stolonica socialis from Tarifa Island resulted in the isolation of a series of non-nitrogenous metabolites, a less frequent result in the chemistry of ascidians. Frozen specimens of S. socialis were extracted with an acetone-methanol mixture (1:1). After evaporation of the solvent, the aqueous residue was extracted with diethyl ether to yield a cytotoxic extract active against the tumor cell lines panel (IC50 = 5 µg/mL). Bioassay guided separation using both column chromatography and reversed phase HPLC led us to obtain three pairs of inseparable compounds: a mixture (9:1) of stolonoxide A [8] and stolonoxide B [9], a mixture (6:4) of their corresponding sodium salts [8a, 9a], and a mixture (6:4) of the sodium salts of stolonoxides C and D [10, 11] (fig. 3).
The stolonoxides [8-11] are C24 acetogenins characterized for possessing a 1,2-dioxane ring connected to a tetrahydrofuran ring, whose structures differed either in the geometry of the side chain double bonds or in the stereochemistry of the five-membered ring. Since the above-mentioned mixtures proved to be inseparable under all HPLC conditions, a portion of the natural material was converted into the corresponding methyl esters 8b, 9b, 10a and 11a by treatment with diaz-omethane (Durán et al., 2000).
The plane structure of stolonoxide A [8] was established by means of the study of the high resolution mass spectrum and of the NMR data, in particular COSY and LRCOSY. The presence of a peroxide ring was confirmed by the characterization of the corresponding diol produced through dioxane ring opening upon palladium catalyzed hydrogenation of stolon-oxide A [8].
The stereochemistry of each heterocyclic system was deduced by the correlations observed in the ROESY spectrum, while the spatial relation between both rings had to be inferred from the study of epoxide 12 (fig. 3) obtained by treatment of methyl ester 8b with aqueous NaOH followed by re-esterification with diazomethane. The epoxide thus obtained was found to possess a threo stereochemistry about C-6, C-7 and bore a single hydroxyl group, while retaining the absolute configuration at all stereogenic centers of the natural compound. This fact is quite remarkable since it allowed us to apply the Mosher method to epoxide 12 and thus unambiguously determine an absolute stereochemistry 3S, 6S, 7S, 10 R for stolonoxide A [8]. A similar rationale allowed us to establish the structures of the remaining members of the stolonoxide family [9-11].
The cytotoxicity assays against the tumor cell lines showed that the stolonoxides A and B [8/9] are strongly cytotoxic against cell line P-388 (IC50 = 0.01 µg/mL). The mixture of stolonoxides C and D [10/11] was also strongly active against cell lines P-388 and A-549 (IC50 = 0.01 µg/mL). Interestingly, the synthetic derivatives 12 and the hydrogenation diol were inactive, demonstrating the importance of the 1,2-dioxane ring in relation to the antitumor activity (table 1).
Although the genus Pseudodistoma is renown as a prolific source of nitrogenous metabolites, in our expeditions to Tarifa Island we encountered specimens of P. obscurum, a species that had not been previously studied. Cytotoxicity guided isolation of a portion of the medium polar organic extract of lyophilized material led to the isolation of the new unsaturated amino alcohol obscuraminol A [13], which was the major component, together with an inseparable mixture of minor compounds [14-18]. These latter compounds could be isolated only as their corresponding diacetyl derivatives [14a-18a], obtained after acetylation of the remaining natural extract (Garrido et al., 2001) (fig. 4).
The molecular constitution of the major component 13 was obtained by intepretation of high resolution mass spectrometry data and proton and carbon NMR spectra, and the presence of the characteristic 2-amino-3-hydroxypropyl unit was apparent. A careful interpretation of the correlations observed in the COSY spectrum allowed the location of the double bonds.
The stereochemistry was defined as follows. The relative stereochemistry of compound 13 was elucidated by interpretation of a series of NOEDS experiments in the oxazolidinone 19 (fig. 4) obtained by treatment of obscuraminol A [13] with 1',1'-carbonyldiimidazole. These experiments indicated a relative configuration erythro at C-2, C-3. Finally, the absolute configuration R at C-3, and therefore an absolute configuration 2S, 3R, was deduced by application of the Mosher method on the 7V-acetylderivative of obscuraminol A [13]. A similar strategy led to the structural characterization of the remaining members of the obscuraminol series.
From a biogenetic point of view, the absolute configuration determined for the 2-amino-3-ol metabolites of P. obscurum requires L-alanine and the corresponding fatty acids as biosyn-thetic precursors, in agreement with the biogenetic hypothesis proposed by Scheuer for the two epimeric 2-amino-3-ol derivatives isolated from an unidentified species of the genus Xestospongia and with which the obscuraminols share identical enantiomeric series (Gulavita and Scheuer, 1989).
Although the obscuraminols A-F [13-18] were active enough to be isolated using cytotoxicity guided fractionation, only obscuraminol A [13] could be tested in its natural form (table 1). In general the activities found can be considered mild since no significant IC50 values were found either for obscuraminol A [13] or the diacetyl derivatives [14a-18a].
In conclusion, the survey carried out by our research group on the antitumor properties of ascidians from the Gibraltar Strait shows the outstanding ability of these invertebrates to produce new compounds, some of them with promising pharmaceutical potential (Durán et al., 2001). Among the compounds which we have described, the stolonoxides [8-11] seem to be the most prominent cytotoxic agents. However, the testing of the obscuraminols [14-18] in their natural form rather than diacetyl derivatives together with the antitinflama-tory testing of the alkaloids [5-7] from B. leachi, based on their structural similarities to the members of the topsentin family, are instances that should not be disregarded in the future.
Acknowledgements
This research was supported by grants from MCYT (research projects MAR98-0834 and PPQ2001-0020) and Junta de Andalucía (FQM-285). The biological material was collected and identified by S. Naranjo. Cytotoxicity assays were performed through a cooperation agreement with Instituto Biomar, S.A.
References
Bartik, K., Braekman, J.C., Daloze, D., Stoller, C., Huysecom, J., Vandevyver, G. and Ottinger, P. (1987). Topsentins, new toxic bisindole alkaloids from the marine sponge Topsentia genitrix. Can. J. Chem., 65: 2118-2121. [ Links ]
Bergeron, R.J., Cavanaugh, P.F. Jr., Kline, S.J., Hughes, R.G. Jr., Elliot, G.T. and Porter, C.W. (1984). Antineoplastic and antiherpetic activity of spermidine catecholamide irons chelators. Biochem. Biophys. Res. Commun., 121(3): 848-854. [ Links ]
D'Incalci, M., Erba, E., Damia, G., Galliera, E., Carrassa, L., Marchini, S., Mantovani, R., Tognon, G., Fruscio, R., Jimeno, J. and Faircloth, G.T. (2002). Unique features of the mode of action of ET-743. Oncologist, 7: 210-216. [ Links ]
Durán, R., Zubia, E., Ortega, M.J., Naranjo, S. and Salvá, J. (1998). Phallusides, new glucosphingolipids from the ascidian Phallusia fumigata. Tetrahedron, 54: 14597-14602. [ Links ]
Durán, R., Zubia, E., Ortega, M.J., Naranjo, S. and Salvá, J. (1999). Novel alkaloids from the red ascidian Botryllus leachi. Tetrahedron, 55: 13225-13232. [ Links ]
Durán, R., Zubia, E., Ortega, M.J., Naranjo, S. and Salvá, J. (2000). Minor metabolites from the ascidian Stolonica socialis and cytotoxicity of stolonoxides. Tetrahedron, 56: 6031-6037. [ Links ]
Durán, R., Zubia, E., Ortega, M.J., Salvá, J., Fernández-Puentes, J.L., García-Grávalos, D. and Naranjo-Lozano, S. (2001). Stolonoxides. British Patent Application #WO 01/83477 A1, November 8, 2001. [ Links ]
Fahy, E., Potts, B.C.M., Faulkner, D.J. and Smith, K. (1991). 6- Bromotryptamine derivatives from the Gulf of California tunicate Didemnum candidum. J. Nat. Prod., 54: 564-569. [ Links ]
Faircloth, G.T., Stewart, D. and Clement, J.J. (1988). A simple screening procedure for the quantitative measurement of cytotoxicity assay. J. Tissue Culture Methods. 11(4): 201-205. [ Links ]
Faulkner, D.J. (2000). Marine pharmacology. Antonie van Leeuvenhoek, 77: 135-145. [ Links ]
Faulkner, D.J. (2002). Marine natural products. Nat. Prod. Rep., 19: 1-49. [ Links ]
Fu, X., Ferreira, M.L.G., Schmitz, F.J. and Kelly-Borges, M. (1998). New diketopiperazines from the sponge Dysidea chlorea. J. Nat. Prod., 61: 1226-1231. [ Links ]
Garrido, L., Zubia, E., Ortega, M.J., Naranjo, S. and Salvá, J. (2001). Obscuraminols, new unsaturated amino alcohols from the ascidian Pseudodistoma obscurum. Structure and absolute configuration. Tetrahedron, 57: 4579-4588. [ Links ]
Gulavita, N.K. and Scheuer, P.J. (1989). 2 Epimeric aminoalcohols from a sponge, Xestospongia sp. J. Org. Chem., 54: 366-369. [ Links ]
Jin, W., Rinehart, K.L. and Jares-Erijman, E.A. (1994). Ophidiacerebrosides: Cytotoxic glycosphigolipids containing a novel sphingosine from a sea star. J. Org. Chem., 59: 144-147. [ Links ]
Mosmann, T. (1983). Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunol. Methods, 65: 55-63. [ Links ]
Natori, T., Morita, M., Akimoto, K. and Koezuka, Y. (1994). Agelasphins, novel antitumor and immunostimulatory cerebrosides from the marine sponge Agelas mauritanus. Tetrahedron, 50: 2771-2784. [ Links ]
Rinehart, K.L. (1989). Novel anti-viral and cytotoxic agent. British Patent Application # 8922026.3, September 29, 1989. [ Links ]
Rinehart, K.L., Holt, T.G., Fregean, N.L., Stroh, J.G., Kiefer, P.A., Sun, F., Li, L.H. and Martin, D.G. (1990). Ecteinascidins 729, 743, 745, 759B and 770: Potent antitumor agents from the Caribbean tunicate Ecteinascidia turbinata. J. Org. Chem., 55: 4512-4515. [ Links ]
Wright, A.E., Forleo, D.A., Gunawardana, G.P., Gunasekera, S.P., Koehn, F.E. and McConnell, O.J. (1990). Antitumor tetrahydroisoquinoline alkaloids from the colonial ascidian Ecteinascidia turbinata. J. Org. Chem., 55: 4508-4512. [ Links ]














