Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Ciencias marinas
versión impresa ISSN 0185-3880
Cienc. mar vol.29 no.2 Ensenada jun. 2003
Artículos
Agar from two coexisting species of Gracilaria (Gracilariaceae) from the Mexican Caribbean
Agar de dos especies coexistentes de Gracilaria (Gracilariaceae) del Caribe mexicano
Julio Espinoza-Avalos1 Enrique Hernández-Garibay2 José A. Zertuche-González3 and Ma. Esther Meave del Castillo4
1 ECOSUR, Apartado postal 424, Chetumal, CP 77000, Quintana Roo, México. E-mail: jespino@ecosur-qroo.mx
2 Centro Regional de Investigación Pesquera, Instituto Nacional de la Pesca Apartado postal 1306 Ensenada, CP 22800, Baja California, México.
3 Instituto de Investigaciones Oceanológicas, Universidad Autónoma de Baja California, Apartado postal 453, Ensenada, CP 22800, Baja California, México.
4 Departamento de Hidrobiología, Universidad Autónoma Metropolitana - Unidad Iztapalapa, Apartado postal 55-535, México, DF, CP 09340, México.
Recibido en marzo de 2002;
aceptado en febrero de 2003.
Abstract
Gracilaria cornea and G. crassissima are similar species that coexist at Bajo Pepito in the Mexican Caribbean. Differences in agar properties from both species were determined for two reproductive categories: carposporic and undetermined, the latter mainly composed of tetrasporophytes. Agar yield (AY), agar gel strength (GS), 3,6-anhydrogalactose content (AG) and sulfate content (S) of native and alkali-treated agar were determined for both reproductive categories. Significant differences in native and alkali-treated agar between the reproductive categories were recorded for AY, GS and S from G. cornea and G. crassissima, as well as for AG of native agar from the latter species. Our results, and previous studies, demonstrate that neither reproductive stage was predominant over the other(s) in terms of having greater or lower values of agar properties. The potential economic use of agar differences from plants of different reproductive stage remains very limited. Lower AY and GS were found for G. cornea from the Caribbean side of the Yucatan peninsula (this study), in comparison to agar values reported for the Gulf of Mexico side of the peninsula. Warmer and nutrient-poorer waters on the Caribbean side could cause those differences. No pattern for GS or S was found when these two and other tropical species of Gracilariaceae were compared to temperate species. When plants of both reproductive categories were pooled together for each Gracilaria species, significant differences were found in all agar properties. Interspecific and intraspecific differences between and within species of Gracilariaceae have also been found for phenological events. We suggest that the coexistence of G. cornea and G. crassissima requires different phenological responses by each species to the environment at Bajo Pepito, which in turn could be reflected in differences in the agar properties we measured, both at the interspecific and intraspecific levels.
Key words: Gracilaria spp., agar, reproductive phases, seasonal changes, Yucatan Peninsula.
Resumen
Gracilaria cornea y G. crassissima son especies muy similares que coexisten en el Bajo Pepito, Caribe mexicano. Para ambas especies se determinaron propiedades del agar de dos categorías reproductivas: carpospórica e indeterminada, la última compuesta principalmente por tetrasporofitos. Las propiedades del agar medidas fueron el rendimiento de agar (RA), fuerza de gel (FG), contenido de 3,6-anhidrogalactosa (AG) y sulfatos (S) de agar nativo y con tratamiento alcalino. Para agar nativo y alcalino se registraron diferencias significativas de RA, FG y S entre las categorías reproductivas de G. cornea y G. crassissima, lo mismo que para AG del agar nativo de la segunda especie. Nuestros resultados y estudios previos demuestran que ninguna fase reproductiva predomina sobre otra(s), con relación a tener valores mayores o menores en las propiedades del agar. El valor económico potencial de las diferencias del agar de plantas en distinta fase reproductiva permanece muy limitado. Encontramos valores menores de RA y FG para G. cornea del litoral Caribe de la Península de Yucatán, en comparación con aquellos reportados para el litoral del Golfo de México de la península. Aguas más cálidas y oligotróficas en el litoral del Caribe pueden causar estas diferencias. No se encontró un patrón en la FG y el S cuando estas dos y otras especies tropicales se compararon con especies de Gracilariaceae templadas. Se registraron diferencias significativas entre especies en todas las propiedades del agar, cuando se juntaron plantas de las dos categorías reproductivas de cada especie de Gracilaria. También se han encontrado diferencias interespecíficas e intraespecíficas en especies de Gracilariaceae en eventos fenológicos. Se sugiere que la coexistencia de G. cornea y G. crassissima requiere que éstas posean respuestas fenológicas diferentes al ambiente de el Bajo Pepito, lo que a su vez se refleja en diferencias del agar, a niveles interespecífico e intraespecífico.
Palabras clave: Gracilaria spp., agar, fases reproductivas, cambios estacionales, Península de Yucatán.
Introduction
Gracilaria cornea J. Agardh and G. crassissima P. Crouan et H. Crouan in Schramm et Mazé, the two species we investigated, coexist in the study area, sometimes using the same rock as substrate (fig. 1). The anatomic and reproductive similarities in these species suggest that the first species could be merely a rough-water ecad of the second (Bird et al., 1986). However, G. crassissima has been found in relatively calm waters near Cuba, Puerto Rico, and in various localities in Quintana Roo (Díaz-Piferrer, 1964; Díaz-Piferrer and Caballer-de-Pérez, 1964; J. Espinoza-Avalos, pers. obs.). Both species have similar reproductive features, such as the development and form of the spermatangia, cystocarp and tetrasporangia (as Polycavernosa species; Fredericq and Norris, 1985). Nonetheless, the species are dissimilar in other external characteristics. The thallus of G. cornea is erect, generally terete (occasionally flattened in some parts), attached to a single disc-like holdfast, without anastomosis of branches. The color of the plants of this species from the study area is a very uniform, pale yellow (fig. 1). In contrast, the thallus of G. crassissima is prostrate, semiprostrate, decumbent to assurgent, with branches cylindrical or somewhat flattened and broad (fig. 1). Larger plants are sometimes attached to the substratum by more than one point of the thallus, with the presence of anastomosis between branches. The color of the plants of G. crassissima from the study zone is variable, from almost colorless to dark red (as Gracilaria, Polycavernosa or Hydropuntia species; G. cornea also as G. debilis; Taylor, 1960; Chapman, 1963; Díaz-Piferrer and Caballer-de-Pérez, 1964; Norris, 1985; Littler et al., 1989; Littler and Littler, 1997; J. Espinoza-Avalos, pers.obs.).

The agar properties of G. cornea and G. crassissima (G. cornea also as G. debilis) have been studied by Humm and Williams (1948), Díaz-Piferrer and Caballer-de-Pérez (1964), Hong et al. (1969), Durairatnam (1980), Rincones-León (1990) and Marinho-Soriano et al. (2001), and by Díaz-Piferrer and Caballer-de-Pérez (1964) and Lahaye et al. (1988), respectively. In those studies, both Gracilaria species were sampled separately. In contrast, we have investigated two coexisting species collected from the same site at the same time. Freile-Pelegrín and Robledo (1997a, 1997b) have also studied agar characteristics of G. cornea from the Gulf of Mexico side of the Yucatan peninsula. Agarophytes exhibit different agar properties depending on the origin of the seaweeds (Armisen and Galatas, 1987). Thus, we expected to find different agar properties in our population, since our study site is located on the Caribbean Sea side of that peninsula, where contrasting oceanographic and biotic features are present (Díaz-Martín and Espinoza-Avalos, 2000).
The studies carried out to date with G. cornea and G. crassissima have not described the agar characteristics of plants from different reproductive phases. It has been a matter of controversy whether or not plants of Gracilariaceae in different stages of reproduction have differences in agar characteristics. Hoyle (1978) did not find differences in the yield or in the gel strength of the agar from male, female and tetrasporic plants of Gracilaria bursapastoris (Gmelin) Silva and G. coronopifolia J. Agardh (names as in the original paper). Similar findings were reported by Yao et al. (1984) and Minghou et al. (1985) for tetrasporic vs cystocarpic plants of G. verrucosa (Hudson) Papenfuss, and by Durairatnam and Nascimento (1985) for Gelidiopsis sjoestedtii Kylin. Neither Yao et al. (1984) nor Minghou et al. (1985) reported major differences in 3,6-anhydrogalactose and sulfate contents of the agar extracted from reproductive phases of Gracilaria species. However, Kim and Henríquez (1979) registered differences in agar yield from cystocarpic and tetrasporic plants of G. verrucosa, while Whyte et al. (1981) found a lack of synchrony between seasonal fluctuations in the chemical composition of the agar from G. verrucosa-type depending on the reproductive stage of the plants. Durairatnam and Nascimento (1985) recorded that carposporic and tetrasporic plants of G. cylindrica Bergesen yielded comparable amounts of agar, but with unequal gel strength.
Perhaps the contrasting results of these studies up to the first half of the 1980s led McLachlan and Bird (1986) to judge that the evidence for differences in agar composition between nuclear phases within the Gracilariaceae was equivocal. In more recent studies, Pickering et al. (1990), Gerung et al. (1997) and Marinho-Soriano et al. (1999) reported differences in yield and/or gel strength of the agar from life stages of three Gracilaria species. Penniman and Mathieson (1987) and Brito-L. and Lemus-C. (1996) showed that the reproductive stages of two Gracilaria species did not differ in agar yield and gel strength.
The objective of this study was to determine the differences in agar characteristics of two coexisting Gracilaria species, and the agar differences of both species at the intraspecific level. Also, to compare agar properties of G. cornea from both (Gulf of Mexico and Caribbean) sides of the Yucatan peninsula. Because alkali treatment increases the gel strength (and the economic importance) of the agar (Armisen and Galatas, 1987), native and alkali-treated agar from G. cornea and G. crassissima were compared.
Materials and methods
Study area
The study site, Bajo Pepito, is located in northeastern Quintana Roo, in the Mexican Caribbean, approximately 2 km west of Isla Mujeres (fig. 2). This tropical site is part of the National Marine Park "Costa Occidental de Isla Mujeres, Punta Cancún y Punta Nizuc". The sea bottom is very regular, 3-4 m in depth, with coarse sand and calcareous rocks usually less than 50 cm in diameter. Bajo Pepito is located within the Yucatan Strait, where there are strong northward currents (4 knots ≈ 2 m s-1) (Merino-Ibarra, 1992). The water is clear, oligotrophic, with mean nitrate + nitrite and phosphate concentrations less than 0.7 µM year round (data not shown).

Collection of algal material
The study site was visited on February 7, March 10, April 14, May 16, June 20, August 29, October 7 and November 25, 1997, and January 14, 1998. On each visit, all Gracilaria spp. plants found along a 25 m long transect were collected using an aluminum 1 m2 quadrat. The monthly samples (25 quadrats) were also used to measure algal biomass, reported elsewhere. The transects were prefixed and oriented (NW) at the sea bottom with metal stakes and a plastic chain. Plants of both species were sorted into two reproductive categories underwater: carposporic, recognized by the presence of cystocarps, and undetermined (other than carposporic) plants. Plants of each reproductive category and species were introduced underwater into plastic bags. The collected material was sun-dried in the field, then dried at 60°C for three days in a convection oven. Dried tissue was ground using a Thomas-Wiley® mill and sieved through a 0.355-mm sieve. In order to use uniform samples, only the algal particles that did not pass through the sieve were used for agar analysis. The limited number of car-posporic plants of G. crassissima only permitted the analysis of agar for this reproductive stage from May to August 1997. For the same reason, data are not provided for G. cornea carposporic plants from October 1997 and January 1998. The proportion of nuclear phases was determined from independent plant samples for a phenological study, using 32 to 50 (mean = 47) fragments of adult plants collected monthly in the same area, but outside of the above-mentioned transects. Observation under a microscope of these plants revealed that the undetermined reproductive category (URC) was mainly composed of tetrasporophytes: a large percentage (mean 74.3%, n = 752) of the reproductive plants of both species analyzed during the study period corresponded to that reproductive stage (data not shown).
Agar extraction (native)
Triplicate samples of dry and milled algal tissue (4 g) were soaked in 40 mL of HCl 0.05 N for 3 min, thoroughly washed with distilled water, placed in 45 mL of distilled water and the pH adjusted to 6.5-7.0 with 0.1 N NaOH. Extraction was performed by autoclaving at 90°C for 1 h, and completed on a hot plate stirrer until all algal tissue (mixed with ~3 g of Celite) had disintegrated. The extracted agar was vacuum-filtered through Whatman® 40 filters. Agar solution was collected in aluminum trays and kept at room temperature (approximately 23°C) until gel formation. Agar gel was frozen and thawed two to three times, then transferred to 30 mL of ethanol 96% for 30 min and washed twice in 70% ethanol (v/v) and once in 96% ethanol. Agar was subsequently dried at 60°C for three days in a convection oven. Dried agar was weighed and yield calculated from the original dried sample weight. The procedure was a modification of that described by Roleda et al. (1997).
Alkali treatment (modified from Freile-Pelegrín and Robledo, 1997a)
Algal samples of 3 g were soaked overnight (in triplicate) in 60 mL of 3% NaOH (w/v) at room temperature. The next day samples were placed in a water bath at 85°C for 2 h. Algal particles were removed from the bath, cooled, and thoroughly washed with running tap water and then with distilled water. Samples were washed with 60 mL 0.025% H2SO4 for 10 min, and then placed in 90 mL of phosphate buffer solution at pH 6.3. Extraction was carried out just as for the native agar. Data on alkali-treated agar of both species are not presented for February and March 1997, because unknown conditions of alkali treatment did not allow an efficient recovery of the agar in those months.
Gel strength (modified from Miller and Furneaux, 1987)
Agar gels (1.0%, w/w) were prepared by dissolving 0.1 g of the dried agar in 9.9 g of distilled water. The 1.0% solution was selected for analysis (i.e., not 1.5%), in order to use the most number of samples of (the few) carposporic plants. Agar solutions were heated at 100°C for 15 min, and then more water was added to compensate for evaporation. Hot solution (5 mL) was poured into two transparent cylindrical polycarbonate jars of 2 cm diameter and 5 cm height. The agar was allowed to gel overnight at room temperature (approximately 23°C), with the jars placed upside down to prevent surface gel drying. Gel strength (g cm-2) was determined using a two-plate balance and a 0.196 cm2 plunger. A loading digital balance was placed on one side of the two-plate balance. Weight was added as distilled water (~40 g min-1) using a burette. Determination of 1.5% (w/w) agar strength was also performed for alkali-treated agar, only for those months when the highest gel strengths at 1.0% were registered. These gels were prepared by dissolving 0.15 g of the dried agar in 9.85 g of distilled water.
3,6-anhydrogalactose content
Percent 3,6-anhydrogalactose was determined by the resorcinol-acetal method of Yaphe and Arsenault (1965), as modified by Craigie and Leigh (1978), using D-fructose standards. Standards were prepared every time samples were analyzed. Absorbance was read at 555 nm.
Sulfate content
Percent sulfate was determined by the BaCl2 method of Tabatabai (1974), as modified by Craigie et al. (1984). Hydrolysis of 15-20 mg of agar was carried out for 2 h at 100°C in four drops of 95% ethanol (to moisten the agar) and 0.5 mL 2 M HCl, using sealed Ependorf tubes. Standard K2SO4 solutions were used as reference.
Statistical analyses
Data were tested for normality (Kolmogorov-Smirnov) and subjected to Bartlett's test for homogeneity of group variances. Two-way analyses of variance (factors = reproductive category and time) for each species data were performed using the software Statistica, release 4.3, for Windows (Statsoft, Inc., Tusla, USA). Three-way analyses of variance (third factor = species) were not carried out because carposporic plant material of G. crassissima was collected only in three out of nine sampling months. Therefore, two-way analyses of variance were performed to compare the agar properties at the species level by considering together all the data obtained within each species regardless of reproductive categories. Heterogeneous data groups were transformed using log (x + 1) and arcsin square root of x. Pearson's product moment correlation test was used to determine the linear relationship between agar properties.
Results
Agar yield (AY)
Carposporic and URC plants of G. cornea and G. crassissima were significantly different in native and alkali-treated AY (figs. 3a, b, 4a, b; tables 1, 2). Significant differences in native and alkali-treated AY existed between G. cornea and G. crassissima when carposporic and URC plants were pooled together within each species (fig. 5a, b; table 3). No significant interaction was registered in alkali-treated AY of the two species through time (table 3).



Gel strength (GS)
Significant differences were found in GS of native and alkali-treated agar 1.0% of G. cornea and G. crassissima at the intraspecific level (figs. 3c, d, 4c, d; tables 1, 2). GS for 1.5% gels was also performed for alkali-treated agar of undetermined plants of G. cornea collected in August, and carposporic and undetermined plants of G. crassissima collected in June and August, when the highest gel strengths at 1.0% were registered (fig. 4c, d). Mean gel strength values of 1.5% alkali-treated agar solutions were 1281 and 1266 g cm-2 for carposporic and URC plants, respectively, of G. crassissima, and 1020 g cm 2 for the latter category of plants of G. cornea. Significant differences in GS for 1.0% gels occurred between G. cornea and G. crassissima when plants of the two reproductive categories were pooled together within each species (fig. 5c, d; table 3).
3,6-anhydrogalactose content (AG)
Percent AG was significantly different between carposporic and URC plants for native agar of G. crassissima, not for alkali-treated agar of this species or the two types of agar from G. cornea (figs. 3e, f, 4e, f; tables 1, 2). However, when car-posporic and URC plants were pooled together within each species, AG content of both native and alkali-treated agar was significantly different between G. cornea and G. crassissima (fig. 5e, f; table 3).
Sulfate content (S)
Percent S of native and alkali-treated agar from carposporic and URC plants of G. cornea and G. crassissima exhibited significant differences (figs. 3g, h, 4g, h; tables 1, 2). Interspecific significant differences, however, were only recorded for alkali-treated agar, not for native agar (fig. 5g, h; table 3).
In general, differences in properties of native and alkali-treated agar between reproductive categories (figs. 3, 4) were not a result of values that were consistently larger or smaller in one reproductive category than in the other, since significant interactions through time occurred in most agar-property values (tables 1, 2). The only exception was in S of native agar from G. cornea, where carposporic plants always contained more sulfate than plants from the URC group (fig. 3g). Nonetheless, in this case the range values were comparable in both reproductive categories. Larger GS for native agar (fig. 3) and smaller S for alkali-treated agar (fig. 4) from carposporic plants of G. crassissima were also registered. However, only three pairs of data were included on which to base these two comparisons.
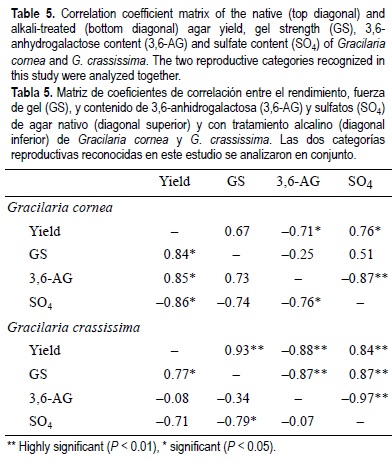
Relationships between properties of native and alkali-treated agar of reproductive categories from G. cornea and G. crassissima did not follow consistent patterns (table 4). For example, a significant positive correlation between AG and S was found for alkali-treated agar from carposporic plants of G. cornea, but a negative one was found between the same properties for native agar from URC plants of G. crassissima (table 4). High correlation values found between agar properties of carposporic plants of G. crassissima (table 4) were not significant because of the small number of pairs (three) of the data that were analyzed. Relationships between agar properties of pooled reproductive categories within each species also varied depending on the factor being evaluated (table 5).
Discussion
Agar yield
In the following paragraphs, agar properties are discussed separately, when possible. Our results on native (20.8-34.6%) and alkali-treated (10.1-21.2%) AY from G. cornea (20.8-34.6%) were comparable to the ranges of native (17.6-42.1%) and alkali-treated (14.5-31.6%) values of AY reported by other authors (Humm and Williams, 1948; Díaz-Piferrer and Caballer-de-Pérez, 1964; Hong et al., 1969; Rincones-León, 1990; Freile-Pelegrín and Robledo, 1997b; Marinho-Soriano et al., 2001). However, those values are much lower than the 50.2%, 52.0% and 75.7% values of AY reported by Díaz-Piferrer and Caballer-de-Pérez (1964), Durairatnam (1980) and Garza-Barrientos and González-Alanís (1981), respectively. The 50.2% and 52.0% AY figures were obtained when plant material was repeatedly rinsed and dried prior to extraction. The latter value (75.7%) seems irregular since the other measurements differ greatly from this solitary value. Mean values of AY from native G. crassissima plants obtained in our study (13.2-35.7%) were comparable to the values (28.2-44.6%) registered by Díaz-Piferrer and Caballer-de-Pérez (1964) and Lahaye et al. (1988).
Significant positive correlations found in this study between AY and GS at the intraspecific and interspecific levels for native and alkali-treated agar were similar to the results of Friedlander (1991). On four occasions, AY and S were also positively correlated at the intraspecific and interspecific levels, similar to that reported by Sasikumar et al. (1997). However, in contrast, a significant negative correlation was also found between the last two agar properties at the interspecific level (table 5).
Gel strength
Gel strength records for native (13-130 g cm-2) and some alkali-treated (335-447 g cm-2) 1.5% agar solutions from G. cornea found by other authors (Humm and Williams, 1948; Díaz-Piferrer and Caballer-de-Pérez, 1964; Hong et al., 1969; Durairatnam, 1980; Rincones-León, 1990; Freile-Pelegrín and Robledo, 1997a), are within the range or lower than our 1.0% agar solution, native (40-213 g cm-2) and alkali-treated (97-722 g cm-2), indicating that agar from G. cornea from the Mexican Caribbean is stronger. In addition, using 1.5% agar solutions, Freile-Pelegrín and Robledo (1997a, 1997b) reported maximum mean GS values of 1653 and 1758 g cm-2, respectively. These values are higher than the mean maximum value recorded in the present study for 1.5% solutions (1020 g cm-2). Freile-Pelegrín and Robledo (1997a) also found higher native AY values (35.6-42.1%) than we did. Both studies were carried out in the Yucatan peninsula, but on the Gulf of Mexico and the Caribbean sides, respectively. Upwelled cold and nutrient-rich waters on the Gulf of Mexico side of the peninsula (Merino, 1997) could have increased tissue nitrogen of G. cornea with concomitant higher agar GS (Patwary and van der Meer, 1983a; Craigie et al., 1984; Bird, 1988; Martínez and Buschmann, 1996). Conversely, relatively warmer waters along the Caribbean side of the peninsula could have caused lower agar GS (Craigie and Wen, 1984), as well as lower AY (Christiaen et al., 1987; Bird, 1988; Castro, 1996) values for the plants of our study site.
Díaz-Piferrer and Caballer-de-Pérez (1964) reported GS values from native agar of G. crassissima comparable (103-132 g cm-2) to that of our 1.0% agar gel (22-185 g cm-2). Also, we registered mean GS values for 1.5% agar solutions of G. crassissima between 1266 and 1281 g cm-2, which are higher (P < 0.006 ) than the mean value (1020 g cm 2) obtained for G. cornea. Lahaye et al. (1988) mentioned that the GS of G. crassissima they measured (in N cm-2) was comparable or better than that of commercial agar or agarose. Thus, the GS values reported herein indicate that the two species are a potential source for commercial use.
A common negative correlation between GS and S (Yaphe and Duckworth, 1972) was found twice in this study at the intraspecific and interspecific levels. However, positive correlations between those agar properties were also found on three occasions, as also reported by Bird et al. (1981) and Castro (1996). Like Bird et al. (1981), we also found negative correlations between GS and AG for G. crassissima.
3,6-anhydrogalactose and sulfate content
Ranges of recorded AG (Hong et al., 1969; Garza-Barrientos and González-Alanís, 1981; Freile-Pelegrín and Robledo, 1997a) from native (31.6-40.0%) and alkali-treated (32.6-47.3%) agar, as well as S from native (2.9-5.5%) and alkali-treated (1.2-4.25%) agar of G. cornea, are comparable to our results (AG of 36.6-44.7% and 39.2-45.9%, and S of 2.6-5.2% and 1.4-2.8%, for native and alkali-treated agar, respectively). Nonetheless, we measured one of the highest S values from native agar (5.2%) known for a tropical species of Gracilariaceae (ranging from 1.5% to 5.5%; Sasikumar et al., 1997, and Freile-Pelegrín and Robledo, 1997a, respectively). Oyieke (1994) suggested that agar from tropical Gracilariaceae generally contained more sulfates than species from temperate waters. This hypothesis was not supported for native agar, for which one of the largest values of S (10.0%) has been measured for a temperate species (Whyte et al., 1981). Rebello et al. (1997) inferred that agar GS from tropical Gracilariaceae would be lower than those from temperate species. However, particular tropical species may contain stronger agar gels than particular temperate ones. Thus, one of the largest GS values for alkali-treated agar from a tropical species (1758 g cm-2), reported by Freile-Pelegrín and Robledo (1997b), was not equaled by agars extracted from temperate species (Abbott, 1980; Christeller and Laing, 1989; Matsuhiro and Urzúa, 1990; Martínez and Buschmann, 1996).
The lack of consistent patterns and the contrasting correlations found in the present study within the native and alkali-treated agar properties at the intraspecific and interspecific levels have no simple explanations (Bird et al., 1981; Ekman and Pedersen, 1990) and are beyond the aims of this study.
Intraspecific agar differences
Native and alkali-treated agar of carposporic vs URC plants of G. cornea and G. crassissima were significantly different in AY, GS and S. Native AG was also different between the reproductive category plants of G. crassissima. Despite the fact that URC plants were mainly composed of tetrasporic plants, our results clearly indicate that the agar properties from carposporic plants were different from the rest of the reproductive phases. Thus, intraspecific agar differences existed within G. cornea and G. crassissima. The difference for the second species must be taken with caution since it was based only on three pairs of mean value comparisons. Significant interactions existed in most factors, indicating that the differences were more a matter of uncoupled cycles in agar properties rather than in the range values. In other words, range values of agar properties of both reproductive categories were equivalent, but their low and high values were not necessarily coincident. These findings are similar to those of Whyte et al. (1981), who reported differences in agar properties between life stages depending upon seasonal fluctuations, but not in absolute values. Growth and biomass differences at the intraspecific level have been found between G. cornea and G. crassissima (data not shown), as has also been found for several species of Gracilariaceae (Whyte et al., 1981; Pickering et al., 1990; Gerung et al., 1997). Thus, differences in underlying biology at the intraspecific level may have lead to inequalities in agar properties at this level.
Our results indicate that it is not appropriate to attribute generally higher or lower values for all agar properties of a particular reproductive stage. For example, carposporic plants of a given Gracilariaceae species may have higher (fig. 3g) or lower S (Marinho-Soriano et al., 1999), while tetrasporic plants may have harder (Kim and Henríquez, 1979) or equal strength (Pickering et al., 1990) agar gels than other life stages. Also, the number of agar properties that is different between nuclear phases depends on the particular species. For example, some species present differences in AY and/or GS (tables 1, 2), but not other species (Yao et al., 1984; Marinho-Soriano et al., 1999). Thus, no general pattern is apparent for agar property differences between reproductive stages of Gracilariaceae species.
The potential for economic use of different reproductive stages of Gracilariaceae species depending on its agar properties is very limited. When differences have been reported, range values have been comparable. Larger differences than those registered between reproductive phases have been obtained from particular mutants. Values from both native and alkali-treated agar GS from the MP40 mutant were one order of magnitude greater than those from other mutants and wild types of G. tikvahiae (Patwary and van der Meer, 1983b). Therefore, up to now, commercially meaningful agar differences can better be researched by examining different species or various mutants rather than within the various reproductive stages of Gracilariaceae. Thus, differences in agar (and biological) measurements between reproductive phases, when present, may have more ecological than economic relevance.
Interspecific agar differences
Native and alkali-treated agar of G. cornea and G. crassissima were significantly different in AY, GS and AG. Sulfate in alkali-treated agar was also different between the two species. Similar interspecific differences in agar characteristics have also been reported for other coexisting Gracilari-aceae species (Pondevida and Hurtado-Ponce, 1996; Falshaw et al. , 1999). As mentioned above, the two Gracilaria species included in this study also exhibited differences in phenologi-cal and ecological aspects (Espinoza-Avalos, unpublished). Similarly, Marinho-Soriano et al. (1998) reported different phenologies for two coexisting Gracilariaceae species from the Mediterranean. Thus, biological differences reflect unequal
physiological responses, which may explain the divergences in quantity and quality of agar between both species of Gracilaria measured herein. Bengtsson et al. (1994) suggested that different phenological and demographic processes between terrestrial plant species could explain their ability to coexist in sympatry (Huntly et al., 1996; Pickering et al., 1996). This hypothesis has been supported for some congeneric terrestrial plant species (Pyke, 1990; Shibata and Nakashizuka, 1995). Thus, the two congeneric species of Gracilaria examined herein may be able to coexist because of species-specific differences in phenological and ecological responses to the environment at Bajo Pepito. In turn, those phenological differences are registered as differences in agar properties, both at the interspecific and the intraspecific level. We conclude that divergences in agar properties between G. cornea and G. crassissima are a consequence of biological divergence of the two taxa.
Acknowledgements
We thank M.A. Díaz-Martín, L.I. Quan-Young and R.A. Herrera-Solís for their help with the field work, and H. Bahena-Basave for providing the photomontage. Isaí Pacheco-Ruíz, Alberto Gálvez and José Guzmán provided warm hospitality to one of us (J.E.A.) while staying at Ensenada, Baja California. Oscar Pedrín-Osuna facilitated the participation of E.H.G. Very special thanks to Scott Monks for his valuable comments on two drafts of the manuscript and for correcting the English. This work is part of a Ph.D. thesis (J.E.A.) at the Universidad Autónoma Metropolitana. This study was made possible with financial support from CONACYT, project 0418P-T.
References
Abbott, I.A. (1980). Some field and laboratory studies on colloid-producing red algae in central California. Aquat. Bot., 8: 255-266. [ Links ]
Armisen, R. and Galatas, F. (1987). Production, properties and uses of agar. FAO Fish. Tech. Paper, 288: 1-57. [ Links ]
Bengtsson, J., Fagerstrõm, T. and Rydin, H. (1994). Competition and coexistence in plant communities. TREE, 9: 246-250. [ Links ]
Bird, C.J., de Oliveira, E.C. and McLachlan, J. (1986). Gracilaria cornea, the correct name for the western Atlantic alga hitherto known as G. debilis (Rhodophyta, Gigartinales). Can. J. Bot., 64: 2045-2051. [ Links ]
Bird, K.T. (1988). Agar production and quality from Gracilaria sp. strain G-16: Effects of environmental factors. Bot. Mar., 31: 33-39. [ Links ]
Bird, K.T., Hanisak, M.D. and Ryther, J. (1981). Chemical quality and production of agars extracted from Gracilaria tikvahiae grown in different nitrogen enrichment conditions. Bot. Mar., 24: 441-444. [ Links ]
Brito-L., L. and Lemus-C., A.J. (1996). Rendimiento y consistencia del agar de Gracilaria damaecornis J. Agardh (Gracilariales, Rhodophyta). Bol. Inst. Oceanogr. Venezuela, Univ. Oriente, 35: 57-62. [ Links ]
Castro, T.R. (1996). Agar yield, gel strength and sulfate content in Gracilariopsis heteroclada farmed in brackishwater canals. Israeli J. Aquacul. Bamidgeh, 48: 94-98. [ Links ]
Chapman, V.J. (1963). The marine algae of Jamaica. Part 2. Phaeophyceae and Rhodophyceae. Bull. Inst. Jamaica, 12: 1-201. [ Links ]
Christeller, J.T. and Laing, W.A. (1989). The effect of environment on the agar yield and gel characteristics of Gracilaria sordida Nelson (Rhodophyta). Bot. Mar., 32: 447-455. [ Links ]
Christiaen, D., Stadler, T., Ondarza, M. and Verdus, M.C. (1987). Structures and functions of the polysaccharides from the cell wall of Gracilaria verrucosa (Rhodophyceae, Gigartinales). Hydrobiologia, 151/152: 139-146. [ Links ]
Craigie, J.S. and Leigh, C. (1978). Carrageenans and agars. In: J.A. Hellebust and J.S. Craigie (eds.), Handbook of Phycological Methods. Physiological and Biochemical Methods. Cambridge Univ. Press, London, pp. 109-131. [ Links ]
Craigie, J.S. and Wen, Z.C. (1984). Effects of temperature and tissue age on gel strength and composition of agar from Gracilaria tikvahiae (Rhodophyceae). Can. J. Bot., 62: 1665-1670. [ Links ]
Craigie, J.S., Wen, Z.C. and van der Meer, J.P. (1984). Interspecific, intraspecific and nutritionally-determined variations in the composition of agars from Gracilaria spp. Bot. Mar., 27: 55-61. [ Links ]
Díaz-Martín, M.A. and Espinoza-Avalos, J. (2000). Distribution of brown seaweeds (Phaeophyta) in the Yucatán peninsula, Mexico. Bull. Mar. Sci., 66: 279-289. [ Links ]
Díaz-Piferrer, M. (1964). Adiciones a la flora marina de Cuba. Carib. J. Sci., 4: 353-371. [ Links ]
Díaz-Piferrer, M. and Caballer-de-Pérez, C. (1964). Taxonomía, Ecología y Valor Nutrimental de Algas Marinas de Puerto Rico.Inst. Biol. Mar., CAAM, Univ. Puerto Rico, Mayaguez, Puerto Rico, 145 pp. [ Links ]
Durairatnam, M. (1980). Studies on the agar producing seaweeds and their distribution in northeast Brazil. Cienc. Cult. (Brazil), 32: 1358-1372. [ Links ]
Durairatnam, M. and Nascimento, H.C. (1985). Agar-agar from vegetative, cystocarpic and tetrasporic plants of Gracilaria sjoestedtii Kylin and Gracilaria cylindrica Boergesen. Seaweed Res. Utiln., 8: 19-22. [ Links ]
Ekman, P. and Pedersn, M. (1990). The influence of photon irradiance, day length, dark treatment, temperature, and growth rate on the agar composition of Gracilaria sordida W. Nelson and Gracilaria verrucosa (Hudson) Papenfuss (Gigartinales, Rhodophyta). Bot. Mar., 33: 483-495. [ Links ]
Falshaw, R., Furneaux, R.H., Pickering, T.D. and Stevenson, D.E. (1999). Agars from three Fijian Gracilaria species. Bot. Mar., 42: 51-59. [ Links ]
Fredericq, S. and Norris, J.N. (1985). Morphological studies on some tropical species of Gracilaria Grev. (Gracilariaceae, Rhodophyta): Taxonomic concepts based on reproductive morphology. Taxon. Econ. Seaweeds, 1: 137-155. [ Links ]
Freile-Pelegrín, Y. and Robledo, D. (1997a). Effects of season on the agar content and chemical characteristics of Gracilaria cornea from Yucatán, Mexico. Bot. Mar., 40: 285-290. [ Links ]
Freile-Pelegrín, Y. and Robledo, D. (1997b). Influence of alkali treatment on agar from Gracilaria cornea from Yucatán, Mexico. J. Appl. Phycol., 9: 533-539. [ Links ]
Friedlander, M. (1991). Growth rate, epiphyte biomass and agar yield of Gracilaria conferta in an annual outdoor experiment. 1. Irradiance and nitrogen. Biores. Tech., 38: 203-208. [ Links ]
Garza-Barrientos, M.A. y González-Alanís, R. (1981). Agar procesado de la planta agarofita Gracilaria debilis (Forsskål) Bergesen, de Yucatán, México. VII Simposio Latinoamericano de Oceanografía Biológica. Instituto de Biología, UNAM, México, p. 87. [ Links ]
Gerung, G.S., Kamura, S. and Ohno, M. (1997). Phenology and agar yield of Gracilaria blodgetii in the tropical water, Okinawa, Japan. Bull. Mar. Sci. Fish., Kochi Univ., 17: 23-28. [ Links ]
Hong, K.C., Goldstein, M.E. and Yaphe, W. (1969). A chemical and enzymic analysis of the polysaccharides from Gracilaria. Int. Seaweed Symp., 6: 473-482. [ Links ]
Hoyle, M.D. (1978). Agar studies in two Gracilaria species (G. bursapastoris (Gmelin) Silva and G. coronopifolia J. Ag.) from Hawaii. I. Yield and gel strength in the gametophyte and tetrasporophyte generations. Bot. Mar., 21: 343-345. [ Links ]
Humm, H.J. and Williams, L.G. (1948). A study of agar from two Brazilian seaweeds. Am. J. Bot., 35: 287-292. [ Links ]
Huntly, N., Chesson, P. and Pickering, C.M. (1996). Germination phenology and the coexistence of desert annual plants. Bull. Ecol. Soc. Am., 77: 209. [ Links ]
Kim, D.H. and Henríquez, N.P. (1979). Yields and gel strengths of agar from cystocarpic and tetrasporic plants of Gracilaria verrucosa (Florideophyceae). Int. Seaweed Symp., 9: 257-262. [ Links ]
Lahaye, M., Revol, J.F., Rochas, C., McLachlan, J. and Yaphe, W. (1988). The chemical structure of Gracilaria crassissima (P. et H. Crouan in Schramm et Mazé) P. et H. Crouan in Schramm et Mazé and G. tikvahiae McLachlan (Gigartinales, Rhodophyta) cell-wall polysaccharides. Bot. Mar., 31: 491-501. [ Links ]
Littler, D.S. and Littler, M.M. (1997). An illustrated marine flora of the Pelican Cays, Belize. Bull. Biol. Soc. Wash., 9: 1-149. [ Links ]
Littler, D.S., Littler, M.M., Bucher, K.E. and Norris, J.N. (1989). Marine Plants of the Caribbean. A Field Guide from Florida to Brazil. Airlife, England, 263 pp. [ Links ]
Marinho-Soriano, E., Laugier, T. and de Casabianca, M.L. (1998). Reproductive strategy of two Gracilaria species, G. bursa-pastoris and G. gracilis, in a Mediterranean lagoon (Thau, France). Bot. Mar., 41: 559-564. [ Links ]
Marinho-Soriano, E., Bourret, E., de Casabianca, M.L. and Maury, L. (1999). Agar from the reproductive and vegetative stages of Gracilaria bursa-pastoris. Biores. Technol., 67: 1-5. [ Links ]
Marinho-Soriano, E., Silva, T.S.F. and Moreira, W.S.C. (2001). Seasonal variation in the biomass and agar yield from Gracilaria cervicornis and Hydropuntia cornea from Brazil. Biores. Technol., 717: 115-120. [ Links ]
Martínez, L.A. and Buschmann, A.H. (1996). Agar yield and quality of Gracilaria chilensis (Gigartinales, Rhodophyta) in tank culture using fish effluents. Hydrobiologia, 326/327: 341-345. [ Links ]
Matsuhiro, B. and Urzúa, C.C. (1990). Agars from Gelidium rex (Gelidiales, Rhodophyta). Hydrobiologia, 204/205: 545-549. [ Links ]
McLachlan, J. and Bird, C.J. (1986). Gracilaria (Gigartinales, Rhodophyta) and productivity. Aquat. Bot., 26: 27-49. [ Links ]
Merino, M. (1997). Upwelling on the Yucatan Shelf: Hydrographic evidence. J. Mar. Syst., 13: 101-121. [ Links ]
Merino-Ibarra, M. (1992). Afloramiento en la plataforma de Yucatán: Estructura y fertilización. Tesis de doctorado, UNAM, Inst. Cien. Mar Limnol., México, 255 pp. [ Links ]
Miller, I.J. and Furneaux, R.H. (1987). Chemical characteristics of the galactans from the formas of Gracilaria secundata from New Zealand. Bot. Mar., 30: 427-435. [ Links ]
Minghou, J., Lahaye, M. and Yaphe, W. (1985). Structure of agar from Gracilaria spp. (Rhodophyta) collected in the People's Republic of China. Bot. Mar., 28: 521-528. [ Links ]
Norris, J.N. (1985). Gracilaria and Polycavernosa from the Caribbean and Florida: Key and list of the species of economic potential. Taxon. Econ. Seaweeds, 1: 101-113. [ Links ]
Oyieke, H.A. (1994). The effect of phenotypic plasticity on agar from Gracilaria salicornia (J. Ag.) Dawson (Gracilariales, Rhodophyta) in Kenya. Biores. Technol., 49: 267-271. [ Links ]
Patwary, M.U. and van der Meer, J.P. (1983a). Growth experiments on morphological mutants of Gracilaria tikvahiae (Rhodophyceae). Can. J. Bot., 61: 1654-1659. [ Links ]
Patwary, M.U. and van der Meer, J.P. (1983b). Genetics of Gracilaria tikvahiae (Rhodophyceae). IX. Some properties of agars extracted from morphological mutants. Bot. Mar., 26: 295-299. [ Links ]
Penniman, C.A. and Mathieson, A.C. (1987). Variation in chemical composition of Gracilaria tikvahiae McLachlan (Gigartinales, Rhodophyta) in the Great Bay Estuary, New Hampshire. Bot. Mar., 30: 525-534. [ Links ]
Pickering, T.D., Gordon, M.E. and Tong, L.J. (1990). Seasonal growth, density, reproductive phenology and agar quality of Gracilaria sordida (Gracilariales, Rhodophyta) at Mokomoko Inlet, New Zealand. Hydrobiologia, 204/205: 253-262. [ Links ]
Pickering, C.M., Huntly, N. and Chesson, P. (1996). Variation found in the growth phenology of desert winter annual plants indicates that temporal environmental variation within a growing season contributes to species coexistence. Bull. Ecol. Soc. Am., 77: 353. [ Links ]
Pondevida, H.B. and Hurtado-Ponce, A.Q. (1996). Assessment of some agarophytes from the coastal areas of Iloilo, Philippines. II. Seasonal variations in the agar quality of Gracilaria changii, Gracilaria manilaensis and Gracilariopsis bailinae (Gracilariales, Rhodophyta). Bot. Mar., 39: 123-127. [ Links ]
Pyke, D.A. (1990). Comparative demography of co-occurring introduced and native tussock grasses: Persistence and potential expansion. Oecologia, 82: 537-543. [ Links ]
Rebello, J., Ohno, M., Ukeda, H. and Sawamura, M. (1997). Agar quality of commercial agarophytes from different geographical origins. 1. Physical and rheological properties. J. Appl. Phycol., 8: 517-521. [ Links ]
Rincones-León, R.E. (1990). Experimental cultivation of an agarophyte alga: Gracilaria cornea in the northwest coast of Venezuela. In: E.C. Oliveira and N. Kautsky (eds.), Cultivation of Seaweeds in Latin America. Univ. São Paulo, Brazil, pp. 65-67. [ Links ]
Roleda, M.Y., Montaño, N.E., Ganzón-Fortes, E.T. and Villanueva, R.D. (1997). Acetic acid pretreatment in agar extraction of Philippine Gelidiella acerosa (Forsskaal) Feldmann et Hamel (Rhodophyta, Gelidiales). Bot. Mar., 40: 63-69. [ Links ]
Sasikumar, C., Rao, V.N.R. and Rengasamy, R. (1997). Effect of alkali treatment of red algae Gracilaria blodgettii and Gracilaria verrucosa (Rhodophyta) on agar quality. Ind. J. Mar. Sci., 26: 191-194. [ Links ]
Shibata, M. and Nakashizuka, T. (1995). Seed and seedling demography of four co-occurring Carpinus species in a temperate deciduous forest. Ecology, 76: 1099-1108. [ Links ]
Tabatabai, M.A. (1974). Determination of sulphate in water samples. Sulphur Inst. J., 10: 11-13. [ Links ]
Taylor, W.R. (1960). Marine Algae of the Eastern Tropical and Subtropical Coast of the Americas. Univ. Michigan Press, Ann Arbor, 870 pp. [ Links ]
Whyte, J.N.C., Englar, J.R., Saunders, R.G. and Lindsay, J.C. (1981). Seasonal variations in the biomass, quantity and quality of agar, from the reproductive and vegetative stages of Gracilaria (verrucosa type). Bot. Mar., 24: 493-501. [ Links ]
Yao, S.S., Xia, Z.Y., En, L.Z. and Qing, L.W. (1984). The yield and properties of agar extracted from different life stages of Gracilaria verrucosa. Hydrobiologia, 116/117: 551-553. [ Links ]
Yaphe, W. and Arsenault, G.P. (1965). Improved resorcinol reagent for the determination of fructuose, and of 3,6-anhydrogalactose in polysaccharides. Anal. Biochem., 13: 143-148. [ Links ]
Yaphe, W. and Duckworth, M. (1972). The relationship between structures and biological properties of agars. Int. Seaweed Symp., 7: 15-22. [ Links ]














