Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Ciencias marinas
versão impressa ISSN 0185-3880
Cienc. mar vol.29 no.1 Ensenada Mar. 2003
Artículos
Proteinaceous exotoxins of shrimp-pathogenic isolates of Vibrio penaeicida and Vibrio nigripulchritudo
Exotoxinas proteicas de cepas de Vibrio penaeicida y Vibrio nigripulchritudo, patogénicas para camarón
Gabriel Aguirre-Guzmán1, Yannick Labreuche2, Dominique Ansquer2, Benoît Espiau2, Peva Levy2 Felipe Ascencio1 and Denis Saulnier2*
1 Centro de Investigaciones Biológicas del Noroeste (CIBNOR) Unidad de Patología Marina La Paz, Baja California Sur, México.
2 Laboratoire d'Aquaculture Tropicale Centre Océanologique du Pacifique, IFREMER. *E-mail: Denis.Saulnier@ifremer.fr
Recibido en junio de 2002;
aceptado en octubre de 2002.
Abstract
The pathogenicity of two V. penaeicida strains, AM101 and KH-1, with different geographic origin, and V. nigripulchritudo strain AM102, were investigated in juvenile blue shrimp species Litopenaeus stylirostris. Alive bacteria and protein fractions (PFs) obtained from cell-free supernatants (CFS) were used in experimental challenges. Strains AM102, AM101, and KH-1 produced respectively 60, 54 and 12% mortality at 96 h after infection using 104 cfu mL-1 of bacterial suspension. Exocellular toxin-like factors were evidenced in CFS from the New Caledonian strains (AM102 and AM101) but not in the Japanese strain (KH-1). At 48 h post injection of each CFS, mortality rates were respectively 96, 98 and 5% when these strains were cultivated at 20°C, whereas only 0, 16 and 5% mortality rates were observed when these strains were cultivated at 30°C. Clear differences in pathogenicity between both V. penaeicida strains of distinct geographic origin (AM101 and KH-1) were thus emphasized. Protein fractions were obtained from CFS of all the strains tested in this study and cultivated at 20°C, by ammonium sulfate precipitation. Whatever the used strain, significantly higher mortalities were produced with PFs obtained with 0-l-00/o of ammonium sulfate saturation, in respect to those produced with PFs60 and PFs80. Shrimp injected with PFs40 from strains AM102, AM101 and KH-1, and at a 20-µg org-1 dose produced respectively 100, 90 and 60% mortality 46 h after the challenge. The strain AM101 showed a median lethal dose of approximately 5 µg protein org-1 (1-1.25 µg protein g-1 body weight) 59 h after injection. The PFs40 from New Caledonian strains were found sensitive to heating and proteinase K treatments, reinforcing thus the hypothesis of their proteinaceous nature. Surprisingly, PFs40 from each bacterial strain displayed similar protein bands by SDS-PAGE suggesting that the tested strains share a common exotoxic compound regardless their distinct geographic origin or species.
Key words: Vibrio penaeicida, Vibrio nigripulchritudo, Litopenaeus stylirostris, shrimp, exotoxin, pathogenicity.
Resumen
La patogenicidad de dos cepas de V. penaeicida, AM101 y KH-1, con diferente origen geográfico, y de la cepa AM102 de V. nigripulchritudo fue investigada en camarones azules juveniles de la especie Litopenaeus stylirostris. Las bacterias vivas y las fracciones proteicas (FPs) obtenidas del sobrenadante libre de células (SLC) fueron usadas en un ensayo experimental. Las cepas AM102, AM101 y KH-1 produjeron respectivamente una mortalidad de 60, 54 y 12%, 96 h después de la infección al usar una suspensión bacteriana de 104 ufc mL-1. Factores tipo toxina exocelular fueron evidenciados en los SLC de las cepas de Nueva Caledonia (AM102 y AM101) pero no en la cepa japonesa (KH-1). A 48 h post-inyección de cada SLC, el porcentaje de mortalidad fue respectivamente de 96, 98 y 5% cuando estas cepas fueron cultivadas a 20°C, mientras que sólo 0, 16, y 5% de mortalidad fue observado cuando las cepas fueron cultivadas a 30°C. Se observó una clara diferencia en la patogenicidad entre las dos cepas de V. penaeicida provenientes de diferentes orígenes geográficos (AM101 y KH-1). Las FPs fueron obtenidas a partir del SLC de todas las cepas cultivadas a 20°C, por medio de precipitación con sulfato de amonio. Se obtuvo una mortalidad significativa con las FPs precipitadas con 40% de sulfato de amonio, en comparación con las precipitadas con 60 y 80%. Los camarones inyectados con FPs40 de AM102, AM101 y KH-1 a una dosis de 20 µg de proteína org-1 tuvieron una mortalidad de 100, 90 y 60%, respectivamente, 46 h después del ensayo. La cepa AM101 mostró una dosis letal media de aproximadamente 5 µg proteína org-1 (1-1.25 µg de proteína g-1 de peso del cuerpo), 59 h después de ser inyectada. Las FPs40 de las cepas de Nueva Caledonia fueron sensibles al tratamiento con calor y proteinasa K, reforzando la hipótesis de su naturaleza proteica. Sorpresivamente, las FPs40 de cada cepa bacteriana mostraron bandas proteicas similares cuando se analizaron por SDS-PAGE, sugiriendo que las cepas ensayadas comparten un elemento exotóxico en común, independientemente de su diferente origen geográfico.
Palabras claves: Vibrio penaeicida, Vibrio nigripulchritudo, Litopenaeus stylirostris, camarón, exotoxina, patogenicidad.
Introduction
The commercial shrimp aquaculture systems are plagued by different diseases that affect shrimp survival and growth. Vibrio species are opportunist bacteria or true pathogens that take advantages of ecological changes introduced by aquacul-ture, causing a serious loss in the shrimp production (Lightner 1988; Brock and LeaMaster 1992; Costa et al., 1998). The effects of Vibrio and severity of vibriosis are a function of the following factors: Vibrio species and strains, their concentration in shrimp microflora or rearing water, water quality, shrimp species and age, and management practices (Prayitno and Latchford 1995; Lightner 1996; Saulnier et al., 2000a). Vibrio species that have caused high mortality of shrimp include V. penaeicida, V. campbellii, V. harveyi, V. nigripulchritudo, V. parahaemolyticus, and V. alginolyticus (de la Peña et al., 1993; 1995; Jiravanichpaisal et al., 1994; Karunasagar et al., 1994; Hameed 1995; Alapide-Tendencia and Dureza, 1997; Lee et al., 1997; Costa et al., 1998; Robertson et al., 1998).
Vibrio nigripulchritudo and V. penaeicida were reported as the principal bacteria involved in the outbreak of disease found in L. stylirostris shrimp farm production in New Caledonia (Costa et al., 1998). Another V. penaeicida strain was considered a serious problem in the P. japonicus shrimp culture in Japan (Ishimaru et al., 1995). The virulence of some V. penaeicida and V. nigripulchritudo strains, including those used in this study, was detected by experimental infections of juvenile L. stylirostris (Le Groumellec et al., 1996; Saulnier et al., 2000b) or P. japonicus (de la Peña et al., 1993, 1995). Experimentally infected shrimp suffered severe bacterial invasion as revealed by some pathogenesis studies (de la Peña et al., 1995; Saulnier et al., 2000b).
Besides the demonstration of active infections, extracellular products (ECP) obtained by in vitro cultivation of particular Vibrio strains have been implicated as a virulence factor in a wide range of marine organisms (Fukasawa et al., 1988; Liu et al., 1996; Sainz et al., 1998; Montero and Austin 1999; Hõrmansdorfer et al., 2000). In two different shrimp-pathogenic isolates of Vibrio harveyi Liu et al. (1996), Lee et al. (1999) and Montero and Austin (1999) have suggested that a cysteine protease and lipopolysaccharides respectively constituted the major toxins produced by the bacteria. Goarant et al. (2000) showed toxic activities of ECP from the same V. penaeicida and V. nigripulchritudo strains used in this study, when injected into juvenile L. stylirostris and used in vitro assays on haemocyte primary cell cultures. Interestingly, the in vivo toxicity of ECP from V. penaeicida was found only when bacteria were cultivated at 20°C and not at higher temperature (25 or 30°C).
In this study, three Vibrio strains known to be shrimp pathogens were used to experimentally infect juvenile L. stylirostris under environmentally controlled culture conditions. Because the severity of vibriosis is partly related to the Vibrio species, bacterial strains and shrimp species, the pathogenicity of two Vibrio strains (AM101 and AM102) from the same geographic origin (New Caledonian) but different species and two strains (KH-1 and AM101) from the same Vibrio species but from different geographic origin and originally isolated from two different shrimp species, was compared. Emphasis was put upon the pathogenicity of some protein fractions (PFs) obtained by sequential ammonium sulfate precipitation of cell free superna-tants (CFS) from in vitro culture of these strains, in order to further facilitate the purification and identification of the virulence factors involved.
Materials and methods
Vibrio strains and culture condition
Vibrio penaeicida strain AM101 and V. nigripulchritudo strain AM102 were isolated from haemolymph of syndrome 93-diseased L. stylirostris shrimp in a New Caledonian shrimp farm. The species to which these two Vibrio belonged was identified by DNA-DNA hybridization assays and arbitrarily primed PCR fingerprinting (Costa et al., 1998; Goarant et al., 1999). Vibrio penaeicida KH-1 reference strain (Ishimaru et al. , 1995) was isolated from diseased P. japonicus shrimp in Japan. Although strains AM101 and KH-1 belonged both to V. penaeicida, genotypic differences were revealed by ribotyping and arbitrarily primed PCR fingerprinting (Costa et al., 1998; Goarant et al., 1999). Bacteria stored at -80°C were revived in marine agar (Diagnostics Pasteur, Marnes la Coquette, France) petri dishes and incubated at 27°C for 24 h. Before their use, the culture purity and the main physiological and biochemical characteristics of the strains were checked.
Preparation of cell free supernatants and protein fractions
Each Vibrio strain was cultured on 0.5 L nutrient medium containing brain heart infusion (BHI, Diagnostic Pasteur) (Goarant et al., 2000) and artificial seawater (2.3% wt/vol NaCl, 20 mM KCl, 5 mM MgSO4, 2 mM CaCl2) under constant stirring at 20 and 30°C for 48 h and 24 h, respectively. Cell free supernatants (CFS) were prepared by centrifugation at 4000 x g for 10 min at 4°C from each bacterial suspension, followed by filtration through a 0.2-µm pore size filter. A control was prepared using a sterilized and filtered BHI. The sterility of all CFS and nutrient medium were verified by spreading 100 of each preparation onto reconstituted marine agar plates and incubating plates at 27°C at least of 16 h. Only CFS obtained from bacterial strains cultivated at 20°C incubation temperature were further used to prepare consecutive protein fractions (PFs) by sequential ammonium sulfate precipitation. Salt was gently added under slow stirring to 0.5 L of each CFS maintained at 4°C until 40, 60, and 80% saturation (w/v) was reached (Harlow and Lane 1988; Scopes 1998). Between each saturation step, the resulting mixture was stored overnight at 4°C. Protein precipitate fractions (PFs40, PFs60 and PFs80) were serially collected by centrifugation at 4400 x g for 30 min at 4°C (Hernández-Sontoyo et al., 1998). Each PF was then suspended in 10 mL of sterilized and distil-lated water and dialyzed against distilled water at 4°C for 72 h using semi-permeable dialysis bags (SIGMA, USA) at a 12,000 kDa cut off.
Protein determination
Soluble protein concentrations in each dialyzed PFs40, PFs60 and PFs80 were evaluated using a microprotein determination test (SIGMA), based on a modified procedure of the standard method by Biuret and Lowry (Ohnishi and Barr, 1978) at a 750-nm lengthweight. Bovine serum albumin was used to determine a standard curve.
Experimental animals
In this study, different batches of juvenile shrimp (L. stylirostris) were reared in captivity at Aquapac, Co. shrimp farm in Tahiti and transported to the Centre Océanologique du Pacifique (COP) experimental facilities. Shrimps belonging to the infectious hypodermal and haematopoietic necrosis virus-specific pathogen-resistant strain (SPR43) were acclimated to laboratory conditions in tanks supplied with filtered and aerated seawater pore-size filter), aeration and no substrate bed for at least two days. The environmental variables of the filtered seawater measured during the study were: pH 7.8-8.2, temperature 24-26°C and salinity 35%o. Juvenile shrimp were fed ad libitum with extruded feed for shrimp containing 40% crude protein and produced in a local factory (Huilerie de Tahiti) according to the COP's formulation. Shrimp mortality was monitored and the results were expressed in mortality rate (%) according to the formula:

Shrimp infection with living Vibrio strains
All Vibrio strains were cultured in Zobell's liquid nutrient medium at 30°C under vigorous stirring by 16 h. Afterwards, bacterial suspensions were ten-fold serially diluted with artificial seawater (Saulnier et al., 2000a). Colony Forming units (cfu) of each bacterial suspension sample (100 µL) were spread and cultured at 27°C for 16 h in Zobell's nutrient agar plates to check a posteriori the number of bacteria used in the experimental challenges. All experimental challenges were conducted in a controlled area since V. penaeicida and V. nigripulchritudo have not been reported in Tahiti. Shrimp (3.5 to 5.0 g weight) were infected by immersion for 2 h in 10 L of filtered and aerated seawater containing 1-3.5 x 104 cfu mL-1 of bacterial strain. After the challenge, batches of 25 shrimps were carefully transferred to cylindrical-conical plastic tanks with 100 L of filtrated seawater and aeration. Control shrimp were treated as above except for the addition of the pathogenic strain. Two culture tanks were used for each treatment as replicates.
Shrimp injection with CFS and PFs
Shrimp (2.5 to 4.0 g weight) were injected with 60 µL of each CFS obtained at 20 or 30°C incubation temperature. Similarly, shrimps were intramuscularly injected with 20 µg protein org-1 (20 µL) of dialyzed PFs40, PFs60, and PFs80 obtained from each pathogenic CFS or the control (BHI). Each organism was injected intra-muscularly between the third and fourth abdominal segments. Two control groups were used (not injected and injected with 60 µL of BHI). Each shrimp treatment, in duplicate, was transferred to cylindrical-conical plastic tanks with 100 L of filtrated seawater and aerated, at a density of 20 to 25 shrimps per tank.
In order to calculate a median lethal dose (LD50) of PFs40 for strain AM101, shrimp (4 to 5 g weight) were injected at different doses (1, 5, 10 and 20 µg protein org-1) using the same protocol as above.
Thermal and chemical treatments on PFs40
The PFs40 from strain AM101 and strain AM102 were subjected to the following thermal and chemical treatments before being injected intra-muscularly (20 µg protein org-1, quantified before treatment) as described by Montero and Austin (1999): (1) heat at 60 and 100°C for 10 min, (2) digestion with proteinase K at a 100-µg mL-1 final concentration, for 1 h at 37°C, (3) exposure to a soybean trypsin inhibitor at a 200-µg mL-1 final concentration of ; or (4) exposure to EDTA at a 100-uM final concentration. Control shrimp groups were injected with one of the three chemical products at the same concentrations that were previously used. All injected shrimps were transferred to cylindrical-conical plastic tanks with 100 L of filtered and aerated sea water, at a density of 20 shrimp per tank.
SDS-polyacrylamide gel electrophoresis of PFs40
The protein band profile from the PFs40 from strains AM101, KH-1 and AM102 was obtained by SDS-PAGE. The PFs40 were denatured in sample buffer for 10 min at 96°C (Laemmli, 1970), and then loaded on a SDS-polyacrylamide (Sambrook et al., 1989) at 110 V for 1.4 h. Protein bands were stained with 0.2% AgNO3 solution (200 µg of AgNO3, 75 mL of 30% formaldehyde and 100 mL of distilled water; Harlow and Lane, 1988). A commercial molecular weight marker (Bio-Rad, USA) was used in order to evaluate the size of the protein bands.
Statistical analysis
The mortality data were analyzed by Chi-square tests using the StatView software. Protein quantifications were compared by analysis of variance, multiple range test (Duncan) and correlation analysis using a Statistica software.
Results
Pathogenicity of living Vibrio strains
Significant differences in the strain pathogenicity were noticed when shrimp were infected by immersion with each of the three strains used at a similar infection dose (fig. 1). At day 4 post-infection, 60 and 54% cumulative mortalities were observed for the AM101 and AM102 strains respectively, whereas the cumulative mortality for KH-1 strain was only 12% (x2 = 25 and 19.95, respectively, P < 0.0001). Shrimp survival in the KH-1-infected group was not significantly affected by the V. penaeicida infection compared to the control group (x2 = 2.17, P = 0.13).

Toxicity of CFS
Even though Vibrio strains cultured at 30°C grew faster than those cultured at 20°C, cultures incubated for 24 and 48 h showed similar turbidity and cell density at 490-nm wavelength. The sterility of each CFS preparation was confirmed by the plate spreading method indicating that both bacterial centrifugation and filtration processes were successfully achieved. Only the CFS originating from AM101 and AM102 cultures incubated at 20°C exhibited 98 and 96% mortalities, compared to 16% and 0% of strains cultured at 30°C. Control treatment (not injected and BHI-injected shrimp) and shrimp injected with KH-1 CFS showed mortalities ranging from 2 to 5%, at both culture temperatures.
Toxicity of dialyzed PFs produced from CFS
To study the in vivo toxicity of PFs for shrimp, protein concentrations of PFs40, PFs60, or PFs80 obtained from each bacterial strain were first established. Protein fractions from strains AM101, KH-1 and AM102 revealed no significant differences (P > 0.05) for the same level of protein precipitation, whereas significant differences (P < 0.05) were observed between different fraction levels. The protein concentrations ranged from 0.5 to 0.6 mg mL-1 for PFs40, 0.7 to 0.9 mg mL-1 for PFs60, and 1.1 to 1.2 mg mL-1 for PFs80. For each bacterial strain cultivated at 20°C, a significant toxic activity was detected in PFs40 (fig. 2) compared to the toxicity obtained with either PFs60 or PFs80 (x2 > 6.4, P < 0.013). When injected to shrimp, PFs40 from AM101 induced a higher mortality rate than PFs40 from KH-1 (90 and 60%, respectively), 40 h after the injection. PFs40 from AM102 showed the highest toxic effect since all the shrimp died after only 22 hours post-injection. The mortality rates of shrimp in other experimental groups including PF60s and PF80s-treated groups ranged from 0 to 20% and were not significantly different from the one obtained in the control group injected with dialyzed BHI (x2 < 2.06, P > 0.15). The approximate LD50 of PFs40 from AM101 was determined to be around 5 µg protein org-1 or 1-1.25 µg protein g-1 body weight at 59 h post injection (fig. 3).

Effects of the thermal and chemical treatments on the toxicity of PFs40 from strains AM101 and AM102
Heat treatment for 10 min at 100°C completely inactivated the toxicity of PFs40 from strains AM102 and AM101 since any shrimp died during the survey period (data not shown) compared to the total mortality induced in the groups injected with non denatured raw PFs40 (fig. 4) . At moderate heating (60°C for 10 min) the toxicity of PFs40 from both strains was reduced, with 15 and 16% mortality rates, respectively, 40 h after injection. These extracts showed a delayed toxicity with 55 and 64% mortality rates, 70 h after their injection. The lethal toxicity of PFs40 from AM101 and AM102 was also affected by the proteinase K treatment showing mortality rates of 14 and 23%, respectively, 32 h after the injection. Similarly to the heat treatment, a bimodal curve was observed after the proteinase K digestion of the extracts (fig. 4), with final mortality rates of 77 and 64%, respectively, 70 h after injection. The EDTA and trypsin inhibitor treatments did not induce any effects on the in vivo toxicity of PFs40, showing mortality rates of 100% in both tests (data not shown).

SDS-PAGE of PFs40
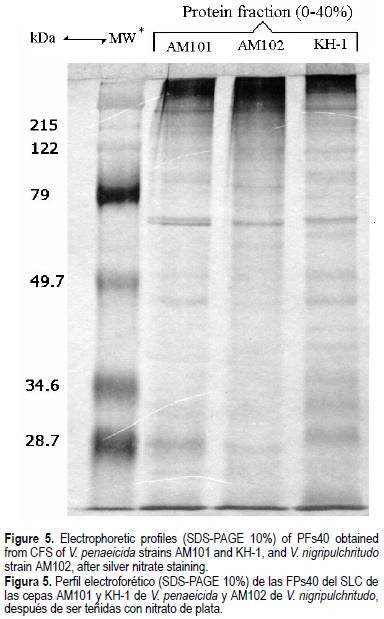
Each PFs40 corresponding to V. nigripulchritudo strain AM102 and V. penaeicida strains AM101 and KH-1 were analyzed by SDS-PAGE (fig. 5). Unexpectedly, PFs40 obtained from these three strains displayed similar protein profiles, despite belonging to different Vibrio species or their distinct geographic origin (AM101 versus KH-1). The PFs40 sample from BHI medium alone was also analyzed by SDS-PAGE as control, but no band was observed revealing the specificity of the detected protein bands (data not shown).
Discussion
Vibriosis is known to affect a wide range of fish and shellfish organisms (Lightner 1988; Brock and LeaMaster 1992). Vibrio penaeicida strains KH-1 and AM101 were involved in a major decline of the shrimp production in Japan (Takahashi et al., 1998) and New Caledonia (Costa et al., 1998), respectively. Our results showed that living strain KH-1 exhibited a very weak mortality rate (12%) compared to strain AM101 (54%) 4 days after infection, at a dose of 104 cfu mL-1. Vibrio nigripulchritudo produced higher mortality (60%) to shrimp. Saulnier et al. (2000a) reported similar results for strain AM101. Costa et al. (1998) showed a LD50 of 1.3 x 104 cfu mL-1 for shrimp (L. stylirostris) by the bath challenge. De la Peña (1993) and Takahashi et al. (1998) reported that Vibrio sp PJ, later named V. penaeicida (Ishimaru et al., 1995), showed a LD50 of 102 and 103 cfu org-1 in juvenile P. japonicus when injected intramuscularly. Prayitno and Latchford (1995), Hameed (1995), Harris and Owens (1999), and Saulnier et al. (2000b) showed that the virulence of Vibrio species is related to the Vibrio strains, method of infection and host factors (species, age, physiological state).
This report shows the different pathogenicity effect and toxicity of the corresponding CFS and PFs40 of both reported V. penaeicida strains (KH-1 and AM101) for the same shrimp specie (L. stylirostris) using the same infection doses and methods. This shows that both Vibrio strains may still have a specific difference which is demonstrated by the variation in their pathogenicity and toxicity (CFS and PFs40) effects on shrimp. Harris and Owens (1999) suggested the possible transmission of virulent factors to shrimp pathogen V. harveyi through the infection by a bacteriophage. Oakey and Owens (2000) reported isolating bacteriophages from a toxin-producing V. harveyi (strain 47666-1) in a moribund shrimp. Since bacteriophages are known to confer virulence to bacteria upon infection (Ruangpan et al., 1999), this may explain the common toxins found in this study. Complementary research needs to be carried out to explore the hypothesis of a toxin-producing bacteriophage. This may facilitate the understanding of their role in pathogenicity and mode of action.
The toxicity of CFS of pathogenic Vibrio strains on marine organism was demonstrated in vivo and in vitro, but the exact role of Vibrionaceae toxins in the pathogenesis of aquatic organisms is not well known yet (Liu et al., 1996; Lee et al., 1997; Sainz et al., 1998; Harris and Owens 1999; Montero and Austin 1999; Goarant et al., 2000). Vibrio exotoxin production is controlled by regulatory elements that are sensitive to environmental stress factors, such as high or low temperature (Pelczar et al., 1982). In this study, only the CFS produced at 20°C with V. penaeicida strain AM101 and V. nigripulchritudo strain AM102 showed high lethal effect on L. stylirostris shrimps, suggesting that CFS virulence is related to temperature-dependent conditions. Temperature-induced production of some virulence factors was suggested by Finlay and Falkow (1997). Our results confirm those obtained by Goarant et al. (2000) and also show that production of CFS toxicity is not cell density-dependent because, in both temperature treatments, Vibrio strains had similar cell densities.
To allow further characterization of the compounds involved in virulence, protein from each CFS were precipitated by adding increasing amounts of ammonium sulfate. Only the purified PFs40 from cultures at 20°C showed a strong toxic effect on juvenile shrimp (compared to PFs60 and PFs80). Strains AM101 and AM102 PFs40 were lethal to shrimp (> 90% mortality) and KH-1 PFs40 showed a toxic activity (at the same 20-µg org-1 dose) causing 60% mortality. Strain KH-1 may produce a low quantity of the exotoxic factor, however, the purification method used in this study resulted in concentrated protein fractions. This was probably the main cause of the mortality exhibited by KH-1, which showed higher pathogenicity in juvenile shrimp L. vannamei (2.5 to 3.5 g weight) (Aguirre et al., data not published).
Interestingly, heat and proteinase K treatments on PFs40 of strains AM101 and AM102 produced a similar toxicity inhibition. The loss of toxicity after proteinase K digestion and the fact that CFS could be precipitated by ammonium sulfate strongly suggest a proteinaceous nature of the putative virulence factor. The evidence of residual toxic activity could correspond to insufficient time for the proteinase K digestion or to the presence of other non-proteinaceous exotoxins like lipopolysaccharides in CFS (Montero and Austin 1999). The results of the heat treatment point to the relative thermolability of this exotoxin factor. Other treatments at PFs40, such as EDTA and trypsin inhibitor, had no effects on the in vivo toxic-ity, suggesting that metalloprotease or trypsine-like protease are not the toxic factors involved in the virulence of these strains.
The in vivo toxicity for the PFs40 of AM101 was estimated at 5 µg protein org-1 or 1-1.25 µg protein g-1 body weight. These values can be compared to the LD50 reported in studies about CFS prepared from V. parahaemolyticus and V. harveyi: 8 and 4.4 µg protein org-1 (Sudheesh and Xu, 2001; Montero and Austin, 1999), 1.2-1.5 and 1.8-2.2 µg protein g-1 body weight (Liu et al., 1996; Harris and Owens, 1999). All this LD50 were obtained by intra-muscular injection of CFS in juvenile P. monodon and L. vannamei. This is the first report of the toxicity of a proteinaceous exocellular toxin-like factor on two different V. penaeicida strains and a V. nigripulchritudo strain with similar protein profiles in their corresponding PFs40, as revealed by the SDS-PAGE and silver staining methods. This observation and the fact that CFS of V. penaeicida strain AM101 and V. nigripulchritudo strain AM102 show the same susceptibility level to proteinase K, heat and inhibitor treatments suggest a common putative exotoxinic component.
Acknowledgments
Thanks for the funding from the Mexican Council of Science and Technology (CONACyT) project 132908, the Cortez Sea Research System (SIMAC) project 980106033, the Northwest Center for Biological Research (CIBNOR) project ABM-11. F.A. holds a graduate scholarship from CONACyT. This study was facilitated by an academic exchange program CIBNOR-Centre Océanologique du Pacifique (COP). Vibrio penaeicida KH-1T was donated by Leonardo Lizarraga (Centro de Investigación Científica y de Educación Superior de Ensenada), V. penaeicida AM101 and V. nigripulchritudo AM102 were provided by D. Saulnier (COP-IFREMER, Tahiti, French Polynesia). Thanks to Aquapac, Co. in Tahiti for supplying shrimp and to Ira Fogel at CIBNOR for the editorial advice.
References
Alapide-Tendencia, E.V. and Dureza, L.A. (1997). Isolation of Vibrio spp. from Penaeus monodon (Fabricius) with red disease syndrome. Aquaculture, 154: 107-114. [ Links ]
Brock, J.A. and LeaMaster, B. (1992). A look at the principal bacterial, fungal and parasitic diseases of farmed shrimp. In: J. Wyban (ed.), Proceedings of the special session on shrimp farming. World Aquaculture Society, Baton Rouge, LA. pp. 212-226. [ Links ]
Costa, R., Mermoud, I., Koblavi, S., Morlet, B., Haffner, P., Berthe, F., Legroumellec, M., and Grimont, P. (1998). Isolation and characterization of bacteria associated with a Penaeus stylirostris disease (Syndrome 93) in New Caledonia. Aquaculture, 164: 297-309. [ Links ]
de la Peña, L.D., Tamaki, K.T., Momoyama, T.N. and Muroga, K. (1993). Characteristics of causative bacterium of vibriosis in kuruma prawn, Penaeus japonicus. Aquaculture, 115:1-12. [ Links ]
de la Peña, L.D., Nakai, T. and Muroga, K. (1995). Dynamics of Vibrio sp. PJ in organs of orally infected kuruma prawn, Penaeus japonicus. Fish Pathol., 30: 39-45. [ Links ]
Finlay, B.B. and Falkow, S. (1997). Common terms in microbial pathogenicity revised. Microbiol. Mol. Biol. Rev., 61:136-169. [ Links ]
Fukasawa, S., Nakamura, K., Miyahira, M. and Kurata, M. (1988). Some properties of two proteinases from a luminous bacterium, Vibrio harveyi strain FLN-108. Agric. Biol. Chem., 52: 3009-3014. [ Links ]
Goarant, C., Régnier, F., Brizard, R. and Marteau, A.L. (1998). Acquisition of susceptibility to Vibrio penaeicida in Penaeus stylirostris postlarvae and juveniles. Aquaculture, 169: 291-296. [ Links ]
Goarant, C., Merien, F., Berthe, F., Mermoud, I. and Perolat, P. (1999). Arbitrarily Primed PCR To Type Vibrio spp. Pathogenic for Shrimp. Appl. Environ. Microbiol., 65: 1145-1151. [ Links ]
Goarant, C., Herlin, J., Brizard, R., Marteau, A.L., Martin, C. and Martin, B. (2000). Toxic factors of Vibrio stains pathogenic to shrimp. Dis. Aquat. Org., 40: 101-107. [ Links ]
Hameed, A.S.S. (1995). Susceptibility of three Penaeus species to a Vibrio campbellii-like Bacterium. J. World Aquaculture Soc., 26: 315-318. [ Links ]
Harlow, E. and Lane, D. (1988). Antibodies, A laboratory manual. Ed. Cold Spring Harbor Laboratory. Second edition. New York. pp. 156. [ Links ]
Harris, L.J. and Owens, L. (1999). Production of exotoxins by two luminous Vibrio harveyi strains known to be primary pathogens of Penaeus monodon larvae. Dis. Aquat. Org., 38: 11-22. [ Links ]
Hernández-Sontoyo, A., Hernández-Arana, A., Arreguín-Espinosa, R. and Rodríguez-Romero, A. (1998). Purification and characterization of several digestive proteases from the blue abalone Haliotis fulgens. Aquaculture, 159: 203-216. [ Links ]
Hörmansdorfer, S., Wentges, H., Neugebaur-Büchler, K. and Bauer, J. (2000). Isolation of Vibrio alginolyticus from seawater aquaria. Int. J. Hyg. Environ. Health, 203: 169-175. [ Links ]
Ishimaru, K.M., Akagawa, M. and Muroga, K. (1995). Vibrio penaeicida sp nov. A pathogen of kuruma shrimp (Penaeus japonicus). Int. J. Syst. Bacteriol., 1: 134-138. [ Links ]
Jiravanichpaisal, P., Miyazaki, T. and Limsuwan, C. (1994). Histopathology, biochemistry, and pathogenicity of Vibrio harveyi infecting black tiger prawn Penaeus monodon. J. Aquat. Anim. Health, 61: 27-35. [ Links ]
Karunasagar, I., Pai, R., Malathi, G.R. and Karunasagar, I. (1994). Mass mortality of Penaeus monodon larvae due to antibiotic-resistant Vibrio harveyi infection. Aquaculture, 128: 203-209. [ Links ]
Laemmli, U.K. (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227: 680-685. [ Links ]
Le Groumellec, M., Goarant, C., Haffner, P., Mermoud, I., and Costa, R. (1996). Study of episodes of mortality observed in reared Penaeus stylirostris since 1993 in New Caledonia: IV Investigation of the bacterial hypothesis by experimental infections, with reference to stress-induced mortality. Annual Meeting of the World Aquaculture Society. January 29-February 2, 1996. Bangkok, Thailand, pp. 144. [ Links ]
Lee, K.K., Yu S.R. and Liu, P.C. (1997). Alkaline serine protease is an exotoxin of Vibrio alginolyticus in kuruma prawn, Penaeus japonicus. Curr. Microbiol., 34: 110-117. [ Links ]
Lee, K.K., Chen, Y.L. and Liu, P.C. (1999). Hemostasis of tiger prawn Penaeus monodon affected by Vibrio harveyi, extracellular products and a toxic cysteine protease. Blood Cell. Mol. Dis., 25: 180-192. [ Links ]
Lightner, D.V. (1988). Vibrio disease of Penaeid shrimp. Disease Diagnosis and control in North American marine aquaculture. In: C.J. Sindermann and D. Lightner (eds.), Devlopments in aquaculture and fisheries science. Elsevier, Amsterdam. pp. 4247. [ Links ]
Lightner, D.V. (1996). Disease of culture penaeid shrimp. In: J. P. McVey (ed.), Handbook of Mariculture. Crustacean Aquaculture. 2nd Ed. CRC Press. Boca Raton, FL. [ Links ]
Liu, P.C., Lee, K.K. and Chen, S.N. (1996). Pathogenicity of different isolates of Vibrio harveyi in tiger prawn, Penaeus monodon. Let. Appl. Microbiol., 22: 413-416. [ Links ]
Montero, A.B. and Austin, B. (1999). Characterization of extracellular products from an isolate of Vibrio harveyi recovered from diseased post-larval Penaeus vannamei (Bonne). J. Fish Dis., 22: 377-386. [ Links ]
Oakey, H.J. and Owens, L. (2000). A new bacteriophage, VHML, isolated from a toxin-producing strain Vibrio harveyi in tropical Australia. J. Appl. Micriobiol., 89: 702-709. [ Links ]
Ohnishi, S.T. and Barr, J.K. (1978). A simplified method of quantitating proteins using the biuret and phenol reagents. Anal. Biochem., 86: 193. [ Links ]
Pelczar, M.J., Reid, R.D. and Chan, E.C.S. (1982). Microbiología. McGraw-Hill., México. pp. 1-120. [ Links ]
Prayitno, S.B. and Latchford, J.W. (1995). Experimental infections of crustaceans with luminous bacteria related to Photobacterium and Vibrio. Effect of salinity and pH on infectiosity. Aquaculture, 132: 105-112. [ Links ]
Robertson, P.A.W., Calderon, J., Carrera, L., Stark, J.R., Zherdmant, M. and Austin, B. (1998). Experimental Vibrio harveyi infections in Penaeus vannamei larvae. Dis. Aquat. Org., 32: 151-155. [ Links ]
Ruangpan, L., Danayadol, Y., Direkbusarakom, S., Siurairatana, S. and Flegel, T.W. (1999). Lethal toxicity of Vibrio harveyi to cultivated Penaeus monodon induced by a bacteriophage. Dis. Aquat. Org., 35: 195-201. [ Links ]
Sainz, J.C., Maeda-Martínez, A.N. and Ascencio, F. (1998). Experimental vibriosis induction with Vibrio alginolyticus of larvae of the catarina scallop (Argopecten ventricosus = circularis)(Sowerby II, 1842). Microb. Ecol., 35: 188-192. [ Links ]
Sambrook, J., Fritsch, E.F. and Maniatis, T. (1989). Molecular cloning, A laboratory manual. Ed. Cold Spring Harbor Laboratory. Second edition. New York, pp. 1-18. [ Links ]
Saulnier, D., Haffner, P., Goarant, C., Levy, P. and Ansquer, D. (2000a). Experimental infection models for shrimp vibriosis studies: a review. Aquaculture, 191: 133-144. [ Links ]
Saulnier, D., Avarre, J.C., Le Moullac, G., Ansquer, D., Levy, P. and Vonau, V. (2000b). Rapid and sensitive PCR detection of Vibrio penaeicida, the putative etiological agent of Syndrome 93 in New Caledonia. Dis. Aquat. Org., 40: 109-115. [ Links ]
Scopes, R. (1998). Protein purification, principles and practice. Springer-Verlag. Second Edition. New York, pp. 301-310. [ Links ]
Sudheesh, P.S. and Xu, H.S. (2001). Pathogenicity of Vibrio parahaemolyticus in tiger prawn Penaeus monodon Fabricius: possible role of extracellular proteases. Aquaculture, 196: 37-46. [ Links ]
Takahashi, Y., Itami, T., Maeda, M. and Kondo, M. (1998). Bacterial and viral diseases of kuruma shrimp (Penaeus japonicus) in Japan. Fish Pathol., 33: 357-364. [ Links ]














