INTRODUCTION
Affective disorders contribute extensively to the worldwide burden of disease in non-developed (third world) countries (Walther et al., 2019a; WHO, 2017). Accumulated data have shown an increasing incidence of stress- and anxiety-related disorders. Likewise, these disorders are associated with an increase in severity comorbidity, and burden of affective disorders. In women, the prevalence of depression and the number of years living with disability are at least two-times higher than men (Li & Graham, 2017; WHO, 2017).
Sexual differences in anxiety-related disorders appear at puberty or in early childhood, and women have an increased risk to develop anxiety disorders and/or exacerbation of anxiety symptoms during the different phases in the reproductive cycle (i.e., menstruation, postpartum, menopause) that are influenced by fluctuations in hormone levels (Li & Graham, 2017; Kaspi et al., 1994; Labad et al., 2005; van Veen et al., 2009; Steiner et al., 2003). The higher prevalence rates for mood-related disorders among women appear to be partly due to the fluctuation of sex steroids, which participate in mood disorders pathophysiology (Li & Graham, 2017).
Interestingly, some authors have proposed two wide and extensive mechanisms regarding fluctuations of sexual hormones that appear to influence the gender imbalance exposed to stressors and anxiety. It is believed that sex hormones could mediate this imbalance through the increase in high-risk factors commonly linked to the appearance of anxiety disorders or by enabling a long-term manifestation of anxiety, with implications in the modulation of the neural systems that regulate stress, and the responses produced by psychological and/or drug-associated treatments, among other responses (Li & Graham, 2017).
Animal studies have extensively shown that estradiol (E2) and progesterone (P4) play critical roles in behavioral and cognitive pathways (Li & Graham, 2017). However, it has been challenging to conclude the precise role of sex hormones based on the findings reported from animals and human studies, in naturally cycling rodents and reproductive women (Li & Graham, 2017).
Stress-related disorders and anxiety are characterized by altered activity in several cell-signaling molecules, usually modulated by steroid hormones, neurotransmitters [i.e., serotonin (5-HT)], and the P4-associated neurosteroid, allopregnanolone (ALLO; Cover et al., 2014; Le Mellédo & Baker, 2004; Rubinow et al., 1998; Schüle et al., 2014).
It has been shown that the downregulation of serotonergic function and ALLO activity in rodents are involved in the increased expression of anxiety-related behaviors, for instance, panic disorders and/or post-traumatic stress disorder (PTSD; Holmes et al., 2003; Maron et al., 2004; Murrough et al., 2011). Earlier studies demonstrated the role of sex steroids in improving depression and anxiety symptoms (Li & Graham, 2017; Fiacco et al., 2019; Walther et al., 2019b). Dehydroepiandrosterone (DHEA), P4, and testosterone (T) administration in rodents promotes an increase in ALLO secretion and facilitates antidepressant-inducing neuronal plasticity in regions of the hippocampus (Walther et al., 2019a; Li & Graham, 2017; Walther et al., 2019c). These data are in line with clinical observations indicating that T and DHEA supplementation produce antidepressive and anxiolytic responses in reproductive women (Walther et al., 2019a; Fiacco et al., 2019).
Sex steroids are neuroactive mediators that directly influence mood and behavior and bind their cognate receptors, which are distributed throughout the brain, particularly in areas involved in depression and anxiety-related behaviors (Schiller et al., 2016; Mahmoud et al., 2016).
However, clinical studies concerning the modulation of sex steroids in affective disorders in reproductive-age women shed conflicting results. Some studies report no associations between sex steroids and these disorders, while others have exhibited positive or negative associations between sex hormones and depressive illness (Asselmann et al., 2019; Giltay et al., 2017). Pregnant women displaying high levels of anxiety are associated with several adverse effects that impact the maternal mental health and birth outcome, and thereby, represent a risk factor for developing post-partum depression (PPD). Prenatal anxiety impacts the fetal heart rate and motor activity, predisposing to obstetric complications, preterm delivery, and altered changes in infant behavior (Alipour et al., 2012; Monk et al., 2003; Mancuso et al., 2004; Davis et al., 2004). The effects of prenatal anxiety responses continue in the offspring during infancy and childhood, negatively affecting their emotions and mental development (Field, 2017).
During pregnancy, the fetoplacental unit becomes the primary source of sex steroids, among other active mediators (i.e., growth factors, neuroactive hormones), which enhance significant modifications in maternal physiology (Costa, 2016; Edey et al., 2018; Napso et al., 2018). For instance, E2 and P4 are crucial for the mother’s insulin sensitivity and glucose metabolism (Napso et al., 2018). Furthermore, a reduction of E2 and P4 blood levels appear to deprive the organism of its natural defense against postpartum depression (Trifu et al., 2019).
Regarding testosterone, high T levels are believed to be associated with an increase in anxiety symptoms in humans, as shown by Walther and collaborators who found an association between a higher K6 scale punctuation and T levels after adjusting for confounders (Walther et al., 2019a). Similarly, studies in female rats with induced polycystic ovary syndrome (PCOS), have demonstrated a correlation between prenatal androgens exposure and anxiety-like behavior (ALB) in the offspring of PCOS females. Besides, an association between T microinjections into the amygdala of female mice and ALB was observed (Hu et al., 2015; Risal et al., 2021).
Interestingly, another recent study found a significant association between anxiety and circulatory T levels in subjects exhibiting PCOS, but not in women without PCOS (Glowinska et al., 2020). In spite of the vast number of reports showing the functional role of sex steroids in depression in non-pregnant women (Walther et al., 2019a; Matsuzaka et al., 2013; Weber et al., 2000) or in healthy pregnant women during mid and late gestation and the peripartum (Trifu et al., 2019; Martínez-Paredes & Jácome-Pérez, 2019); there is scarce information concerning the role of sex hormones in women with severe anxiety in late pregnancy. Thus, in the present paper, we quantified P4, E2, and T serum levels in women with high levels of anxiety during late pregnancy.
METHOD
Subjects / description of the sample
A non-probabilistic sample of pregnant woman between 18 to 30 years old, who attended the outpatient department, was invited to participate in the present study.
Places
Pregnant women were interviewed and evaluated at the OB-Gyn Department at General Hospital of Mexico (Hospital General de Mexico, Dr. Eduardo Liceaga of Mexico, Mexico City).
Measurements
Anxiety Scale: Properties
The Hamilton Anxiety Rating Scale (HARS) is a reliable and adequate psychometric instrument, use to assess the severity of anxiety in a global context, in patients who meet the criteria for anxiety or even depression. This scale exhibits similar properties reported in the original scales (Hamilton, 1959; Maier et al., 1988) rendering it a highly useful instrument in healthcare practice and clinical research in our country.
The psychometric properties of the scale reported by Lobo et al. (2002); consist of the following: 1. Discriminant validity (MADRS/HARS-Clinical Global Impression of Severity: p < .001). 2. Convergent validity (MADRS-Hamilton Depression Rating Scale: p < .05 and .01, respectively; MADRS/HARS-EuroQoL 5D: p < .05; HARS-State Trait Anxiety Inventory: p < .05). 3. Internal consistency (Cronbach’s α: MADRS = .88; HARS = .89). 4. Test-retest and inter-observer reliability (intraclass correlation coefficient: MADRS = .94 and .98, respectively; HARS = .92 and .92). 5. Sensitivity to change (effect size: MADRS = 2.05; HARS = 1.36).
Anxiety scale: Characteristics
The anxiety scale consists of 14 items designed to assess the severity of a patient’s anxiety. Each of the 14 items contains a number of signs and symptoms. Each group of signs/symptoms is rated on a scale of zero to four, with four being the most severe (i.e., each item measures the frequency and intensity of anxiety symptoms on a 0–4 Likert-type scale) (Hamilton, 1959; Maier et al., 1988). Although the symptoms enlisted within each item facilitate the assessment of anxiety, no specific anchor points are considered in this tool, when compared to other rating-scale measuring anxiety symptoms. One point to consider is that this instrument takes no more than 30 minutes to complete in the interviewer’s presence. Moreover, 13 out of the 14 items, refer to the assessment of anxiety symptoms, and the last item (14), evaluates the patient’s behavior after the interview. The rating score ranges from 0 to 56 points; thus, the final score results from the sum of the points considered from each item. Worth noting is that two main points are merely measured throughout the scale; psychic anxiety (items 1-6, and item 14) and somatic anxiety (items 7-12, and item 13) respectively (Hamilton, 1959; Maier et al., 1988). Interestingly, this scale is highly useful to quantify the symptomatic variations overtime during or after exposure to drug treatment.
This instrument has been shown to be a highly reliable, specific, and sensitive tool (Maier et al., 1988) and has been validated in the local language (Lobo et al., 2002).
Participants during late pregnancy showing higher scores on the HARS scale (≥ 25) were considered as subjects, exhibiting high levels of anxiety symptoms; while pregnant women showing lower scores in the HARS questionnaire (≤ 7) were treated as healthy control subjects.
Moreover, pregnant women included in the study were evaluated by psychiatric interviews based on the Diagnostic and Statistical Manual of Mental Disorders (DSM-5; American Psychiatric Association, 2013). Patients with high levels of anxiety were referred to the Psychiatry Department for evaluation and management of their symptomology. All clinical and psychometric evaluations were registered in a constructed clinical database.
Procedure
Pregnant women underwent a complete clinical evaluation, both psychiatric and OB-GYN assessment, including sociodemographic and anthropometric measurements. Patients who voluntarily agreed to participate in the study were requested to sign a written informed consent before beginning the study. At entry, pregnant women required second and third-trimester lab tests (blood count, biochemical testing, urinalysis, thyroid function, 2D fetal ultrasound, and Doppler monitoring; data not shown). Participants were either inhabitants of Mexico City and/or surrounding state areas.
During the initial clinical intervention, women (18-30 years old) coursing a normo-evolutive pregnancy, during the third trimester (28-40 gwk), were assessed for OB-GYN status and previous illnesses and acute and chronic pathologies.
At entry, the anxiety-rating scale questionnaire (HARS or HAM-A) was applied to the participants by the clinician, in order to rate the severity or intensity of anxiety symptoms (Hamilton, 1959). This instrument has been widely used in different clinical and research settings (Maier et al., 1988).
Women recruited into the study had no history of smoking, alcohol consumption, drug use, or abuse, including other mental disorders, such as schizophrenia, psychosis, bipolar disorder, and/or neuropsychiatric pathologies [e.g., seizures, attention-deficit/hyperactivity disorder (ADHD), obsessive-compulsive disorder] except for anxiety without depression. Twin or multi-pregnancies were excluded. Pregnant women exhibiting anxiety plus comorbid depression were discarded from the study, according to Psychiatric evaluation and Hamilton depression rating scale scores higher than seven points. Similarly, subjects included in the protocol were not medicated before the study began. However, women requiring psychotropic medication before or during pregnancy were eliminated from the study.
Exclusion criteria included participants receiving neuropsychiatric medication and/or medications interfering with their anxious-associated symptomology. Patients with a history of illegal substance use or abuse, previous psychiatric disorders, obstetric pathologies (i.e., diabetes, preeclampsia), acute or chronic infections, and other medical illnesses (i.e., metabolic, cardiovascular, autoimmune, neuroendocrine, and/or rheumatic diseases) in addition of assisted conception, were excluded from the study. Furthermore, subjects showing incomplete questionnaires, absence or incomplete laboratory tests, and/or inconsistencies in the evaluation of their mood and emotional status were similarly eliminated from the protocol.
Thus, as mentioned earlier, the study comprised 141 pregnant subjects. Depending on the intensity of anxiety symptoms, patients were clustered into the anxiety group (ANX, n = 101) or control group (CTRL, n = 40). Once patients completed the HARS questionnaire; participants (with 8-12 h fasting conditions) were remitted to the HGM/central lab for collection of blood samples, used to determine sex steroid hormones. Important to mention, clinical and psychometric data collected during the interview from control and anxious (experimental) groups, were used to make all mathematical and statistical calculations.
Blood sampling
Briefly, blood sampling was as carried out at daylight hours (7:00-9:00 am) and 5.0 mL of venous blood was collected in sterile 13 x 100/Vacutainer BD Hemogard Tubes (Becton & Dickinson, USA) used for serum separation. Tubes were allowed to clot at room temperature for 1 h and centrifuged at 1600 x G for 15 min at 4ºC. The prepared serum samples were then aliquoted into 1.5 mL Eppendorff vials. Vials were further stored at -70ºC until use.
Quantification of sex hormones
Quantification of sex hormones was performed according to standard procedures depicted in BMC Psychiatry (2020). Briefly, Estradiol-17β (E2), progesterone (P4), and total testosterone (T) were measured using a two-step chemiluminescent enzyme immunoassay (IMMULITE 2000 Analyzer System, Siemens USA) following manufacturer instructions. Serum samples (.5 mL) were incubated with their specific polyclonal steroid antibody followed by colorimetric detection using the enzyme-labeled chemiluminescent substrate (IMMULITE 2000, USA). Previous studies showed that triglyceride concentrations (≥ 3000 mg/dL) had no effect on immunoassays measuring steroid hormones in either serum, heparinized or EDTA-treated plasma (Lee et al., 1991). Nonetheless, serum lipemic samples were centrifuged (1600 x g/15 min at 4ºC) in order to collect free-lipemic serum fractions, avoiding thus, any interference with steroid readings. E2 was assayed using the L2KE22 analytical kit (sensitivity, 55 pmol/L). P4 was assayed through the L2KPW2 kit (Analytical sensitivity, .3 nmol/L). T was tested using the LKTW1 kit (sensitivity, .5 nmol/L). Both intra- and inter-assay covariances was < 4.0% and < 7.5%, respectively.
Statistical analyses
The mean ± SEM of each sex hormone serum concentration was calculated and used to create the plots depicted herein. Data collected and calculated either as, the mean ± SEM obtained from the estimated steroids’ serum concentrations, and/or the mean ± SD obtained from clinical and psychometric (HARS) measures described in the text, were used to detect significant differences in the clinical measures between the control and anxious groups, using the parametric t-test analysis with Welch correction. Moreover, the demographic parameters were analyzed by calculating the percentage (%) from the total population recruited in each tested group. In addition, bivariate Person correlation analysis was applied to detect plausible associations between steroids and clinical measures among the study groups. In the same line, partial Pearson correlation analysis was used to estimate the significant associations between serum steroids and clinical measures in each of the tested groups after controlling parameters by clinical confounders. Both GraphPad Prism 7 (GraphPad Softwares Inc. USA) and SPSS software v.24.0 (Armonk, NY: IBM Corp) were used for the statistical analysis. The statistical significance was established at a p-value < .05.
Ethical considerations
Both Ethics and Research Committees from the Institution Ethical Committee approved the human study and gave their permission to involve human participants. All participants provided a signed written informed consent before their recruitment into the study. The human study was performed according to the Declaration of Helsinki. Approval from the Institution Ethical Committee was obtained prior to the beginning of the study (HGM, D1/14/112/04/072, 2014-2016; INPer Grant. No. 212250-07311, 2013-2015).
RESULTS
Demographic and clinical measures
The sociodemographic characteristics of non-white Latin pregnant women (n = 141) is depicted in Table 1. This table shows third trimester pregnant women displaying an average age of 25.7 ± 6.1 years-old and a mean gwk of 34.2 ± 4.1 among the study groups. Healthy subjects (CTRL) were older than women with anxiety. No significant differences in the gwk period were observed among the study groups. T-test analysis shed significant differences between age (p = .001) among groups.
Table 1 Sociodemographic and clinical characteristics
|
Participants
n = 141 |
CTRL
n = 40 |
ANX
n = 101 |
|---|---|---|
| Parameters | mean (SD) | mean (SD) |
| Age (year) | 28.2 (6.6) | 24.2 (5.0)** |
| BMI (kg/m2) | 28.4 (3.5) | 28.1 (3.7) |
| Weight (kg) | 68.3 (10.2) | 67.4 (8.9) |
| HARS (Score) | 3.1 (0.6) | 27.1 (4.2)** |
| Gestation weeks | 33.9 (3.4) | 34.7 (4.4) |
| gwk intervals | n (%) | n (%) |
| 27-29 | 6 (15) | 20 (19.8) |
| 30-32 | 8 (22.5) | 16 (15.8) |
| 33-35 | 11 (27.5) | 13 (12.9) |
| 36-38 | 12 (30) | 24 (23.7) |
| 39-41 | 3 (7.5) | 28 (27.8) |
| Marital status | n (%) | n (%) |
| Never married | 9 (23) | 19 (28.8) |
| Married | 4 (10) | 24 (23.7) |
| Divorced | 12 (30) | 16 (15.8) |
| Cohabiting | 15 (38) | 42 (41.6) |
| Education level | n (%) | n (%) |
| Elementary school | 0 (0) | 8 (7.9) |
| Middle school | 22 (55) | 28 (27.7) |
| High school | 11 (28) | 35 (34.7) |
| Bachelor's degree | 7 (18) | 18 (17.9) |
| Postgraduate | 0 (0) | 5 (4.9) |
| Technician degree | 0 (0) | 7 (6.9) |
| Working status | n (%) | n (%) |
| Employee | 9 (23) | 26 (23.7) |
| Unemployed | 0 (0) | 0 (0) |
| Home labor | 24 (60) | 49 (48.5) |
| Commerce | 7 (18) | 13 (12.9) |
| Profession | 0 (0) | 5 (4.9) |
| Other | 0 (0) | 8 (7.9) |
Notes: Both clinical and psychometric data collected from both control and anxious (experimental) groups, were used to make all mathematical and statistical calculations. As such, the non-parametric, t-test with Welch’s correction was used to detect statistical differences between clinical measures in the tested groups. Data are expressed as the mean ± SD.
** p < .001, indicates the differences found in age and HARS scores in the tested groups. (%) Percentages obtained from total subjects in each group. Abbreviations: CTRL, control; ANX, severe anxiety; HARS, Hamilton Anxiety Rating Scale; BMI, Body Mass Index; gwk, gestational weeks. Significant differences were established at a p < .05. Data were calculated using GraphPad v.7 and SSPS software v.25.
Serum steroids
Figure 1 shows the serum levels of E2, P4, and T between anxious patients and control subjects. As depicted, the ANX group showed significant higher levels of E2 and T (Figures 1A and 1C), than those detected in the CTRL group (t-test, p = .001). As opposed, the anxiety group displayed lower serum levels of P4 (t-test, p = .001; Figure 1B). The estimated P4:E2 ratio estimated in the anxiety group was 2.0 times lower than that shown in the CTRL group (t-test, p = .001; Figure 1D).
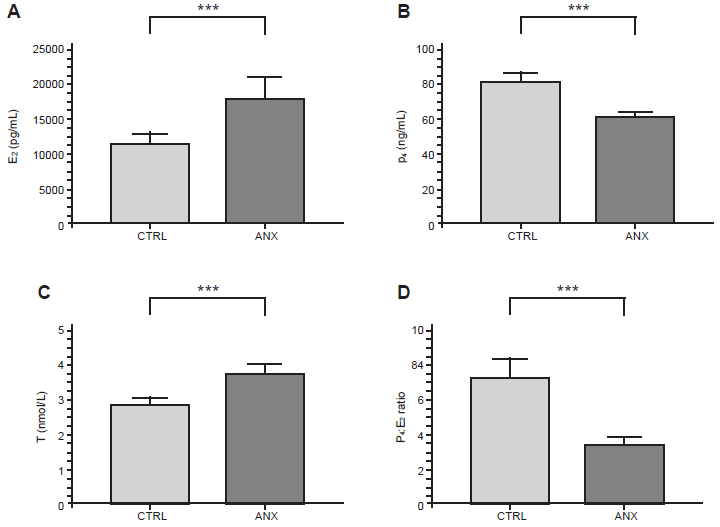
Figure 1 Serum concentration of sex hormones and the estimated P4 and E2 ratios in pregnant women.The figure depicts the serum concentration of E2 (A), P4 (B) and T (C) in addition of the estimated ratios between P4 and E2 serum levels detected among the study groups (D) in the 3rd trimester of pregnancy. Hormones were quantified by two step chemiluminescent enzyme immunoassay (see methods). The concentration values of E2 are expressed as the mean ± SEM: CTRL (11751 ± 1483 pg/mL), ANX (18291 ± 2893 pg/mL). The concentration values of P4 are expressed as the mean ± SEM; CTRL (80.8 ± 5.4 ng/mL), ANX (56.5 ± 3.04 ng/mL). The concentration values of T are expressed as the mean ± SEM, as described: CTRL (2.8 ± .2 nmol/L), ANX (3.8 ± .3 nmol/L). The ratio values estimated between P4 and E2 serum levels are expressed as the mean ± SEM. The mean ratio values calculated in both CTRL and ANX groups were 7.2 ± .04 and 3.6 ± .02, respectively. T-test analysis with Welch’s correction was used to estimate the p values of the steroid serum concentrations and the related P4:E2 ratios estimated among the studied groups. (***) p < .0001. Significant differences were established at p < .05. Data was calculated using GraphPad Prism v.7 and SPSS v.25.
Correlations between steroids and intensity of anxiety adjusted by clinical parameters
The partial correlations observed between serum steroids and the anxiety-rated scale in the tested groups are shown in Table 2. As shown, T serum levels showed a positive correlation with the estimated mean (HARS) scores for anxiety (Pearson, p = .001) in the symptomatic (ANX) group. Conversely, P4 levels displayed a significant negative association with the estimated HARS scores in the anxious group (Pearson, p = .02). In the same line, the calculated P4:E2 ratio in the symptomatic group showed a similar negative correlation with the estimated scores for anxiety (Pearson, p = .04).
Table 2 Pearson correlations between serum steroids and HARS scores adjusted by gwk
| ANX | HARS | E2 | P4 | T | P4:E2 |
|---|---|---|---|---|---|
| HARS | |||||
| Corr. | 1 | .06 | -.26 | .39** | -.22* |
| Sig. | .32 | .02 | .001 | .04 | |
| E2 | |||||
| Corr. | .06 | 1 | .06 | .13 | -.64** |
| Sig. | .32 | .34 | .15 | .001 | |
| P4 | |||||
| Corr. | -.26 | .06 | 1 | .29* | .60** |
| Sig. | .02 | .34 | .01 | .001 | |
| T | |||||
| Corr. | .39** | .13 | .29* | 1 | .14 |
| Sig. | .001 | .15 | .01 | .16 | |
| P4:E2 | |||||
| Corr. | -.22* | -.64** | .60** | .14 | 1 |
| Sig. | .04 | .001 | .001 | .16 |
Notes: SSPS software v.25.0 was used to determine the Pearson partial correlations between the anxiety-rated scale and sex steroids in the ANX group, adjusted by gwk.
*Significant differences at p < .01 (bilateral).
**Significant differences at p < .05 (bilateral). Abbreviations: ANX, severe anxiety; HARS, Hamilton Anxiety Rating Scale; E2, estradiol; P4, progesterone; T, testosterone; P4:E2, progesterone: estradiol ratio; Corr., correlation; Sig., significance. Significant differences were established at a p < .05.
Furthermore, partial correlations were found between E2 and gwk (Pearson, p = .000), E2 and T (Pearson, p = .015), and between T and gwk (Pearson, p = .003) in the anxiety group, after controlling clinical parameters by age. However, no significant correlations were observed between serum steroids and HARS or between the clinical measures used in the study and HARS, as well (data not shown).
DISCUSSION AND CONCLUSION
Pregnancy is a crucial biological process that comprises physiological and emotional changes (Costa, 2016; Napso et al., 2018; Soma-Pillay et al., 2016). Signs and symptoms appearing during pregnancy (i.e., nausea, vomiting, facial pigmentation, body shape transformation) represent acute stressors that impinge on women during pregnancy (Soma-Pillay et al., 2016).
Social stress increases during pregnancy due to individual risk factors (i.e., unexpected pregnancy, young age, poor education and low socioeconomic condition, sexual abuse, among others; Hall et al., 2019) which creates an adverse impact on the psychological responses in vulnerable women (Zou et al., 2009).
Several studies showed that emotional disorders are common among pregnant women, mainly anxiety and depression reported as usual causes of mental distress in such populations (Baker et al., 2008).
Using the 14-item Hamilton anxiety questionnaire (HARS) to evaluate psychological and somatic anxiety symptoms in 308 pregnant women, Zou and collaborators showed a prevalence of severe anxiety in 5.1% of women in the first trimester of pregnancy, and a global prevalence of low to moderate anxiety in 31.2% of women exhibiting anxiety and comorbid depression from mid-to-late pregnancy (Zou et al., 2009).
However, Nakić Radoš and collaborators reported a prevalence of anxiety symptoms in 17.3% from late pregnancy to early postpartum (Radoš et al., 2018).
During pregnancy, steroid hormones such as progestogens, estrogens, androgens, glucocorticoids, and cholesterol, are needed to accomplish a full-term pregnancy, including a successful delivery, and a healthy neonate (Costa, 2016). The human placenta is a crucial endocrine tissue involved in the synthesis and metabolic process of steroid hormones, including the exchange of steroids between the fetal and maternal compartments (Chatuphonprasert et al., 2018). According to Zou and collaborators circulatory levels of E2 and P4 increase in 1st trimester of pregnancy, remain stable in the 2nd and 3rd trimesters and rapidly decrease after delivery (Zou et al., 2009). They observed that E2 and P4 blood levels were associated with depressive symptoms, but not with anxiety during pregnancy and postpartum (Zou et al., 2009).
Stress and general anxiety have been hypothesized to promote changes in the bioactivity of the HPA axis and the fetoplacental unit (Van den Bergh et al., 2005; Kane et al., 2014).
Previously, it has been shown that women with high levels of anxiety during late pregnancy, exhibit an increase in cortisol (CORT) serum levels and a higher CORT: DHEA-S ratio (Leff-Gelman et al., 2020). In the present study, we show that pregnant women with severe anxiety display high levels of E2 and T and lower levels of P4. These findings posit that sex hormones and the P4:E2 ratio might represent an indicator of altered mood disorders and affective symptoms in pregnant women.
Moreover, our findings could result in the hormonal-induced regulatory effects on brain neurotransmitters (Rubinow et al., 1998; Schüle et al., 2014) and cell-signaling cascades in brain areas implicated in mood disorders (Kalin, 2020; Cover et al., 2014; Le Mellédo & Baker, 2004) in pregnant women exhibiting anxiety (Li & Graham, 2017; Holmes et al., 2003; Murrough et al., 2011).
Sex steroid hormones exert pleiotropic effects in the central nervous system (Diotel et al., 2018) and exert diverse physiological functions in the body (Pillerová, et al., 2021) by activating cell-signaling pathways, via binding their cognate intracellular and transmembrane receptors (Pillerová et al., 2021). Both E2 and P4 are indispensable for reproductive function and sexual behaviors. However, fluctuations in hormone levels in reproductive women, impact the female brain morphology functionality (Marrocco & McEwen, 2016) neurochemistry (Barth et al., 2015), and are contributors among other mediators (i.e., cortisol, cytokines,) to female-specific risks for neuropsychiatric conditions such as, depression and anxiety disorders (Pillerová et al., 2021; Altemus et al., 2014).
Pathological anxiety is characterized by an excessive, inadequate anxiety response occurring in situations that normally would not occur in healthy people (Kalin, 2020). Pathological anxiety is defined by different features, including excessive worry, physiological arousal, and avoidance behavior (Pillerová et al., 2021; Kalin, 2020). Patients suffering from pathological anxiety display affective symptoms, which includes nervousness, frustration, impatience, and fearfulness (Pillerová et al., 2021). At the cognitive level, patients with cognitive dysfunction show hypervigilance for threats, poor concentration, and impaired memory (Pillerová et al., 2021; Kalin, 2020). Regarding behaviors, patients with pathological anxiety display higher readiness to respond to danger, restlessness, and agitation (Pillerová et al., 2021; Kalin, 2020).
Interestingly, animal studies revealed that sex-dependent effect of high prenatal T exposure, can increase the risk for anxiety disorders, particularly in adult women. In the same line, the effects of prenatal dysregulation of estrogens and progesterone and/or their metabolites on the brain, on either cognitive or behavioral consequences in adulthood, are still lacking (Pillerová et al., 2021).
Our findings partially match the results obtained in a previous cohort study, which concluded that high levels of depressive and anxiety symptoms were associated with high serum levels of T and P4 in pregnant women living in rural areas (Walther et al., 2019a).
Reduced 5-HT levels and decreased neuroplasticity have been hypothesized to represent a major central mechanism that predisposes individuals to depression and anxiety (Walther et al., 2019a). In this line, sex steroids appear to work as endogenous mood stabilizers, facilitating both anxiolytic and anti-depressive effects by increasing the release of 5-HT and promoting neuroplasticity in the cortex and hippocampal formation (Walther et al., 2019a; Maron et al., 2004).
Regarding androgens and anxiety, several reports have shown controversial results. A report on a male cohort study found a negative correlation between testosterone levels in blood and anxiety symptomology, showing that men exhibiting a T deficiency, are highly vulnerable to anxiety (Berglund et al., 2011; Santoro et al., 2005).
On the other hand, animal and human studies conducted on pregnant females or females with PCOS found a significant positive association between testosterone (T) levels and increased levels of anxiety (Risal et al., 2021; Glowinska et al., 2020; Matsuzaka et al., 2013).
Thus, our findings about the increase in T serum levels in the ANX group, suggest that T may exert an important role in modulating the intensity of anxiety symptoms.
In addition, several studies have demonstrated that both 5-HT and ALLO (synthesized from P4 and positively modulated by E2 and P4, exert a central anxiolytic activity by inducing adaptive stress responses (Andréen et al., 2009). Thus, estradiol (E2) and progesterone (P4) strongly contribute to the regulation of 5-HT and ALLO (Genazzani et al., 1998; Frye et al., 2000).
However, a decrease in sex hormones caused by stress exposure, causing hypothalamic amenorrhea, increases women’s vulnerability to enhance the development of anxiety disorders, or the exacerbation of pre-existing anxious symptoms (Genazzani et al., 2006; Berga & Loucks, 2006). This leads to a pathological downregulation of 5-HT and ALLO bioactivities (Schüle et al., 2014).
Menstruation, hormonal contraceptive use, postpartum, and menopause are associated with low E2 and P4 levels (Walther et al., 2019a). This impairs the hypothalamic-pituitary-gonadal (HPG) axis functioning, causing a reduced effective stress response regulation (Walther et al., 2019a; Andréen et al., 2009).
Thus, it might be feasible to argue that the high serum levels of E2 and T found in women with severe anxiety, could work as a counterbalance mechanism to ameliorate the deleterious effects of the lower P4 levels found in women with high anxiety (Walther et al., 2019a). Furthermore, the significant reduction in the P4:E2 ratios in women with severe anxiety in the 3rd trimester of pregnancy supports the hypothesis that the reduction in progesterone (P4) levels may be linked with high anxiety levels, as detected by the negative associations found between P4, P4:E2 ratio and high scores for anxiety in the symptomatic group. These observations may result from a decreased activity of the allosteric modulation induced by ALLO on the GABA-A receptor, in addition to the negative modulation on the HPA axis (Schüle et al., 2014). This ultimately translates into a decrease in the ALLO-homeostatic function in the presence of acute stressors and distress in vulnerable people (Schüle et al., 2014).
Finally, the reduced activity of ALLO on the HPA axis may result in its functional dysregulation, leading to altered levels of circulating adrenal steroids (Bali & Jaggi, 2014). Thus, the altered response on the HPA axis could be linked to changes in cortisol levels (hypercortisolemia) in pregnant women exhibiting major depression or severe anxiety (Gelman et al., 2015).
Our study shows that pregnant women displaying high levels of anxiety show significant increases in circulating estradiol and testosterone and an important reduction in serum progesterone, in contrast to the healthy controls. Based on the fact that anxiety and depression are commonly comorbid, it’s necessary to carry out complementary investigations that may help to find significant associations between endocrine hormones and the high levels of anxiety symptoms in pregnant women, who are prone to develop mood-related disorders and altered behaviors.
Perspectives
Despite our findings showing the circulating levels of sex steroids in pregnant women with severe anxiety, further studies are needed to explore the relations between serum concentrations of sex hormones and their bioactive metabolites (i.e., ALLO, DHEA-S) in women exhibiting high levels of anxiety symptoms during pregnancy (second and third trimester) and early postpartum (PP, 2-6 weeks). As stress and anxiety symptoms are highly linked, other standardized and structured psychometric-scale instruments are needed to explore the presence or absence of anxiety symptoms, generalized anxiety disorders (GAD), perception of stressors or perceived stress (PS), depressive symptoms (merely due that anxiety is commonly associated with depression) and cognitive functions in vulnerable pregnant women. Furthermore, biological and molecular approaches should be explored and used when investigating anxiety and comorbid mental disabilities, in order to comprehend the cellular mechanism implicated in triggering or promoting affective disorders and their related symptomology.
Limitations
There are some limitations that need to be mentioned herein. We used the anxiety-related questionnaire to measure the intensity of anxiety symptoms in a pregnant population. Albeit, this instrument has not been applied frequently on pregnant women, because they are commonly evaluated with other instruments in this population (e.g., the CES-D, EPDS, and GAD-7); and anxious symptoms have been less evaluated in pregnant women using the State and Trait Anxiety Inventory (Chinchilla-Ochoa et al., 2019). Moreover, the study used a single blood sample to measure serum steroids. However, two-time blood-sampling points should have been taken into consideration to estimate the serum concentration of the steroids in our pregnant population. In addition, cigarette smoking was not considered as a clinical parameter in our study groups. Previous reports showed that cigarette smoking reduced maternal serum levels of E2, P4, and hCG levels in early pregnancy (Bernstein et al., 1989). Although these data could explain some of the adverse effects that smoking produces in pregnant women, our study showed no history of smoking in our recruited population. Finally, the pre-gestational weight could have contributed to the clinical data, regarding the relation that weight gain and BMI have circulating sex hormones (Wuu et al., 2002).











 nueva página del texto (beta)
nueva página del texto (beta)



