Introduction
Diabetes mellitus (DM) is a metabolic disease that has been associated with a large number of comorbidities, such as neuropathies, cardiovascular diseases, and psychosocial problems, being depression the most frequent (Roy & Lloyd, 2012). It is not surprising that other behaviors could be affected by the homeostatic imbalance caused by DM, because the brain (like the rest of the body) is dependent on glucose and a failure in its control influences its functioning (Andrade, Benton, Brain, Ramirez, & Walmsley, 1988). Many patients with DM report psychiatric symptoms, such as hyperactivity, unpredictable behavior, internalizing symptoms, and solitary tendencies (van Son et al., 2013). Particularly, diabetic women experience more distress, lower selfesteem, and more depressive symptoms than men (Forsander, Bogelund, Haas, & Samuelsson, 2017; Hood et al., 2006; Lašaitė et al., 2016; Lašaitė, Ostrauskas, Zalinkevicius, Jurgeviciene, & Radzeviciene, 2015). Aggressiveness is another important concomitant symptom of DM; however, few studies have systematically analyzed their relationship. In these studies, it has been reported that there is an association between glycosylated hemoglobin and aggression (Leonard, Jang, Savik, Plumbo, & Christensen, 2002). Accordingly, patients with DM1 exhibit higher verbal aggression compared with patients suffering from other chronic diseases (Tilov, Semerdzhieva, Bakova, Tornyova, & Stoyanov, 2016) or with the general population (Efe & Erdem, 2018). Nevertheless, these studies have not analyzed the data according to the sex of the patient.
Natural aggression has been considered as an adaptive behavior commonly displayed with different purposes. Firstly, it can be expressed to obtain a benefit (food, sexual partners, or territory) (Trainor, Sisk, & Nelson, 2009). Secondly, it might be exhibited as a form of defense (a response to a previous stimulation, without inflicting intentional damage) (in rats, this form of aggression is characterized by side kicks and small bites towards the flanks). Finally, it can be executed offensively, i.e., intended to cause injury to the opponent (in the rat offensive aggression consists in boxing posture or bites towards vulnerable body parts) (Adams, 1979; Umukoro, Aladeokin, & Eduviere, 2013). Notably, exaggerated levels of aggression ‒quantitatively characterized by short latencies to the first attack and high frequency and duration of attacks‒ are considered pathological (Takahashi & Miczek, 2013). The study of aggression in preclinical studies has been done primarily in males because females exhibit less aggressiveness (Cordero, Ansermet, & Sandi, 2013). These studies have been intended to determine the motivation underlying each aggressive behavior and the neutrotransmitters and brain areas involved in their control.
Preclinical studies of DM (using streptozotocin [STZ], a cytotoxic of pancreatic β cells, that generates hyperglycemia and hypoinsulinemia) have shown that diabetic animals also exhibit various behavioral changes as observed in humans, such as anxious- and depressive-like behaviors and a decrease in sexual behavior (Can, Ozturk, & Ozkay, 2011; ElBatsh, 2015; Sahin, Gocmez, Eraldemir, & Utkan, 2019; Várkonyi & Kempler, 2014). Few studies have evaluated the relationship between hyperglycemia and aggressiveness in animal models. They have used the intruder-resident paradigm (IRP), and their results have been controversial. Thus, the groups of Meehan (1987) and Leedom (1987) reported that male mice treated with STZ had lower aggression and investigation when placed with another male. Moreover, they presented more static defense postures and escapes (Leedom, Meehan, & Zeidler, 1987; Meehan, Leedom, Nagayama, & Zeidler, 1987). Four years later, in another work, it was reported that males administered with STZ showed a tendency to spend less time interacting socially with the resident and more time expressing aggressive behaviors (Hilakivi-Clarke, 1991).
To the best of our knowledge, in females there is only a single work reporting increased aggressiveness in STZ-treated rats tested in two distinct mating conditions (Hernandez-Munive, Rebolledo-Solleiro, Ventura-Aquino, & Fernandez-Guasti, 2018). In that study, we showed that in the non-paced mating paradigm (NPM), which implies that the timing of copulation is imposed by the male (resulting in some rejection behaviors by the females) (Hardy & DeBold, 1972), the hyperglycemic females displayed high levels of aggression, which were diminished after insulin administration (Hernandez-Munive et al., 2018). When evaluated in the paced mating paradigm (PM), where the number of aggressive behaviors in control animals is minimal because the female controls the timing of copulation by moving away from the male (Paredes & Vazquez, 1999), the aggressiveness of hyperglycemic females also tended to increase (Hernandez-Munive et al., 2018). In that report, however, we failed to analyze the features of the aggressive behaviors. Thus, the present study was aimed at establishing whether aggressiveness towards the male was offensive or defensive.
Method
Subjects
In this study, we used 46 ovariectomized (OVX) females (250-300g) and 15 sexually experienced male Wistar rats. All animals were housed under standard conditions (22 ± 2 °C, reversed 12-hour light and 12-hour dark cycle, starting the dark phase at 10:00 a.m.) and provided with food and water ad libitum.
Measurements
a) Behavioral test
We registered aggression in two different mating paradigms:
Where the male regulates sexual interaction in the non-paced mating condition (NPM). In this test, the animals were placed in a transparent circular arena (52 cm in diameter X 45 cm high). The test was carried out during the first 10 mounts (approximately 15 minutes).
Where the female controls the rhythm of copulation, a paradigm called “paced mating” (PM). This test was performed in a transparent acrylic box (61 cm long X 30.5 cm wide X 45 cm high) divided in two compartments. The division between compartments had two holes (4.0 cm) in each bottom corner, through which the female, but not the male rat, could freely pass from one compartment to the other. In this test, the evaluation time was longer (1h) because a greater number of mounts and intromissions are necessary to establish the pacing behavior.
To induce sexual receptivity, females were administered with estradiol benzoate (10 micrograms per rat, subcutaneously, 24 hours before the test; Sigma Chemicals, St. Louis, MA.) followed by progesterone (3 mg per rat, subcutaneously, 4 hours before the test; Sigma Chemicals, St. Louis, MA.). Animals were habituated to each paradigm for 5 minutes before introducing the sexually experienced male.
b) Aggressive behavior
For both paradigms, the latency for the first attack (time –in seconds– between the introduction of the male into the experimental arena to the first aggressive behavior), the number of aggressive females (reported as the proportion of females showing aggression), and the number of aggressive events were recorded. The behaviors considered as aggressive were the following:
Boxing: the female stands on her two hind legs and pushes the male with the front legs.
Bites: small bites towards the male from the front or the side, without damaging it.
Lateral offensive posture kicking the male with hind legs.
Twist: the female turns half of her body towards the male (Gonzalez, Vaziri, & Wilson, 1996; Madlafousek & Hliňák, 1977).
Furthermore, we determined if these behaviors were displayed offensively, ‒i.e., if they occurred without a prior stimulus‒, or defensively, ‒if they were the response to a male´s approach‒.
Procedures
a) STZ-Treatment
To generate hyperglycemia, OVX-females (surgery performed one week before administration) received STZ (Sigma Chemicals, St. Louis, MA) on two consecutive days (50 mg/kg/day, ip.) using citrate buffer (sodium citrate dehydrate and citric acid monohydrate .1 mol/L, pH 4.8) as vehicle. Behavioral tests were performed ten days after the last administration. At the end of the behavioral tests, plasma glucose levels were determined using a glucometer and glucose strips (Accu-Chek Performa, Roche, Buenos Aires, Argentina).
b) Insulin treatment
The insulin treatment consisted in the daily subcutaneous administration of insulin, glargine (Cronix, PiSA Laboratories, Guadalajara, Mexico) in a 12-12 h scheme. Two days after the last STZ administration, 2U of insulin were administered in the morning and 4U in the night (for 6 days). To avoid hypoglycemia, the last 2 days of treatment, the dose was lowered to 2U in the morning and 2U in the night. Only the females that at the end of the test showed blood glucose levels between 70-100 mg/dL were included.
Our data are the results of two different experimental trials, in which 5 animals of each group were evaluated. Efforts were taken to have the same experimental conditions in each trial in order to assure reproducibility.
Statiscal analysis
The data were analyzed using the GraphPad 6.0 software; results are presented as the mean ± the standard error of the mean (SEM). Glucose values and aggressiveness data were analyzed using the MannWhitney U-test because the data failed to follow a normal distribution. Proportions of females that exhibited aggressive behaviors were analyzed with the Fisher`s exact test.
Ethical considerations
All procedures were done in accordance with the guidelines of the Laws and Codes of Mexico (Norma Oficial Mexicana NOM-062-ZOO-1999. Especificaciones técnicas para la producción, cuidado y uso de los animales de laboratorio) following the guidelines of the National Institutes of Health for the use of animals. The Institutional Ethics Committee (Cicual-CINVESTAV) approved the protocols.
Results
Control animals showed normal glucose levels (103 ± 4 mg/dL) while STZ-treated animals had hyperglycemia (492 ± 19*** mg/dL [U = 0, ***p < .0001]). Figure 1 shows the different aggressive behaviors (boxing posture [panel A], bites [panel B] lateral kicks [panel C] and twists [panel D]) displayed by females treated with buffer (n = 10) or STZ (n = 10) in a mating condition where they cannot regulate the timing of copulation (NPM). The control females displayed some aggressive behavior during mating; however hyperglycemic females showed more aggressions, reflected as a significant increase in the number of lateral kicks (U = 21, p = .026).

Figure 1 Aggressive behaviors: boxing (panel A), bites (panel B), lateral kicks (panel C) and twists (panel D) in NPM (Buffer n = 10, STZ n = 10). *p < .05 by Mann-Whitney U-test. STZ: streptozotocin.
Figure 2 shows the same aggressive behaviors as those shown in figure 1, but in females tested under PM, where they regulate the time of sexual interaction. In this paradigm, the number of aggressive events was also increased in hyperglycemic females; particularly, there was a significant increase in the number of bites (U = 22, p = .042).
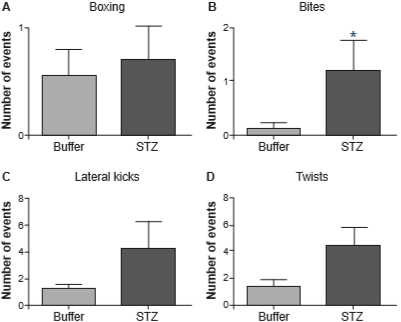
Figure 2 Aggressive behaviors: boxing (panel A), bites (panel B), lateral kicks (panel C) and twists (panel D) in PM (Buffer n = 9, STZ n = 10). *p < .05 by Mann-Whitney U-test. STZ: streptozotocin.
As shown in Table 1, all females treated with STZ presented at least one aggressive response in both paradigms; however, only in PM, where the female can escape to the neutral compartment, the proportion of females showing aggression was significantly increased (p = .02, Fisher’s exact test). Moreover, all aggressive behaviors were performed defensively, i.e., in response to a male approach. There were no significant differences in the latency to the first attack between normoglycemic and hyperglycemic females (p = ns). Finally, the mean number of attacks was increased in hyperglycemic females, such increase reach statistical significance only in the NPM condition and therefore only in this group insulin was administered to explore a possible blockade action.
Table 1 Proportion of females that presented aggressive behaviors, type of each response (defensive or offensive), latency to the first attack and the mean number of attacks presented in NPM and PM by vehicle- or STZ-treated females
| Proportion of females | ||||||||
|---|---|---|---|---|---|---|---|---|
| showing aggression | Type of response | Latency to the first attack (s) | Mean number of attacks | |||||
| NPM | PM | NPM | PM | NPM | PM | NPM | PM | |
| Buffer | 7/10 | 4/9 | Defensive | 56 ± 29 | 236 ± 68 | 3 ± 1 | 3 ± 1 | |
| STZ | 10/10 | 10/10^ | Defensive | 52 ± 9 | 272 ± 106 | 9 ± 2* | 11 ± 2 | |
Note: NPM = non-paced mating paradigm; PM = paced mating paradigm; STZ = streptozotocin.
^p < .05 vs. buffer by Fisher`s exact test,
*p < .02 vs. buffer by Mann-Whitney U-test.
Interestingly, insulin treatment administered to hyperglycemic females tested in NPM reduced the mean number of attacks (4 ± 2) to control values (p = .02, Mann -Whitney U-test) as well as the proportion of animals administered with STZ that showed aggression (4/7) (p = .05, Fisher’s exact test). Furthermore, the aggressive events executed by these females were scarce, defensive and were displayed with a similar latency (92 ± 31) than those of the other groups.
Discussion and conclusion
The main findings of the present study were that the STZ-treated animals exhibited aggressiveness (mostly side kicks and bites) only when the male attempted to mount the female. Remarkably, insulin treatment reverted such increased aggressive behaviors.
As aforementioned, when the female does not regulate the rhythm of sexual interaction (NPM), there is an increased aggression towards the male intended to control the pattern of stimulation that she receives (Paredes & Vazquez, 1999). In STZ-treated females, the number of these behaviors were much greater than the ones exhibited by controls. Additionally, all these responses were defensive, i.e., they were displayed as a reaction to an approach of the male. The detailed analysis of each aggressive behavior allows to infere about the underlying motivation. Adams (1979) pointed out that the lateral bites or kicks (as the ones exhibited by STZ-treated females in both mating paradigms) are deployed to a part of the body (flanks of the opponent) less vulnerable to serious damage. This observation suggests that hyperglycemic females try to reject the male without hurting him.
It is well known that the main motivation behind the enforcement of aggressive defensive behaviors is the pain or threat posed to another organism (Umukoro et al., 2013). Under this premise, we can suppose that in the case of STZ-treated females, the male represents an aversive stimulus. In support of this idea, it has been evidenced that these animals exhibit fibrosis in the vaginal tissue (Kim et al., 2006; Park et al., 2001) and neuropathy (Banafshe et al., 2014; Gao & Zheng, 2014; Tripathi, Mehta, & Yadav, 2016), which are accompanied by inflammatory reactions characterized by inflitration of macrophages and T-cells (Chakrabarty, Liao, Mu, & Smith, 2018). These factors might produce nociception in the female’s rump when mounted.
Another possible explanation for the execution of aggressive defensive behaviors is that the male provokes anxious-like responses in hyperglycemic females. Accordingly, it has been reported that these females have high levels of experimental anxiety (Aksu et al., 2012), and that in postpartum females the kicks against an intruder male are anxious or fearful responses (da Veiga, Miczek, Lucion, & de Almeida, 2011). This interpretation agrees with what occurs in clinics where women with DM1 have more symptoms of internalization (anxiety, depression) (Kakleas, Kandyla, Karayianni, & Karavanaki, 2009; Naar-King et al., 2005; Tilov et al., 2016).
An interesting finding of this work is that the increased aggression produced by hyperglycemia was counteracted by insulin treatment. Naturally, peripheral insulin crosses the blood-brain barrier via a saturable transport process Kleinridders, Ferris, Cai, & Kahn, 2014) and, after prolonged periods of hypoinsulinemia, this transport is reduced, decreasing the amount of insulin in the brain (Banks, Owen, & Erickson, 2012; Šerbedžija & Ishii, 2012). Binding of insulin to its receptor in the rodent brain is greater in areas related to cognitive and emotional functions such as: hippocampus, hypotalamus, amygdala, cortex, olfactory bulb, and septum (Craft & Stennis Watson, 2004); therefore, a decrease in insulin could interfer with the correct function of these areas. For example, it has been demonstrated that an insulin failure leads to an abnormal aminoacids metabolism, which could cause a decrease in the brain serotonin production (Korczak, Pereira, Koulajian, Matejcek, & Giacca, 2011), a neurotransmitter widely studied in the deployment of aggressiveness (Umukoro et al., 2013). In females treated with STZ, a decrease in the hypothalamic serotonin precursor (tryptophan) has been found since the first week with hyperglycemia (Chaouloff et al., 1989). In addition, it has been shown that insulin has an antidepressant-like effect in mice (Hilakivi-Clarke, 1991), probably due to the increase in serotonin levels (Gupta, Kurhe, & Radhakrishnan, 2014; Manjarrez-Gutierrez, Herrera-Marquez, Bueno-Santoyo, González-Ramírez, & Hernández, 2000), making possible to propose that insulin decreases aggressiveness by increasing the activity of this neurotransmitter system.
It is worth mentioning that our results show acute effects of hyperglycemia on the expression of aggressive behaviors. However, in the clinical setting (where the effects are chronic) there is scarce information about the association between glycemic control and aggression. Uncontrolled blood sugar in patients suffering DM1 leads to substantial psychological distress, negative moods, irritable, or aggressive behavior, and closely associated problems with relationships, self-image, and confidence (Vanstone, Rewegan, Brundisini, Dejean, & Giacomini, 2015). Accordingly, some studies have evidenced a direct relationship between poor glycemic control and a rise in aggressive behaviors (Bryden et al., 2001; Leonard et al., 2002; Zheng & Chen, 2013). Interestingly, it has been reported that for every 1 mmol/L rise in glucose levels, there is a concomitant rise of 1.7 in externalizing behavior score (McDonnell, Northam, Donath, Werther, & Cameron, 2007). Nevertheless, other authors failed to found this association (Akbaş et al., 2009). Remarkably, it has been demonstrated that intranasal administration of insulin reduces anger in healthy subjects (Benedict et al., 2004), without knowing its effects in diabetic patients. Thus, more studies are necessary to clarify the role of insulin in the modulation of aggressive behaviors.
In conclusion, the present work gives evidence that the increased aggressiveness of hyperglycemic females is a form of defense against the proximity of the male and that insulin reverts such aggression. Further studies to clarify the mechanisms underlying this association are warranted.











 nueva página del texto (beta)
nueva página del texto (beta)


