Introduction
The systemic administration of pentylenetetrazole (PTZ) has been widely employed as an animal model of epilepsy (Peterson & Albertson, 1998; Sarkisian, 2001; Meldrum, 2002; Eraković, Župan, Varljen, & Simonić, 2003; Löscher, 2017). Just like patients with epilepsy, animals that are administered PTZ often exhibit behavioral, cognitive and motor abnormalities that generate seizures per se (Ahmadi, Dufour, Seifritz, Mirnajafi-Zadeh, & Saab, 2017). PTZ acts as an antagonist of GABAA receptors in the central nervous system (Macdonald & Barker, 1977; Kulkarni & George, 1995; Huang et al., 2001). Histological changes following PTZ administration are: neuronal death and induction of massive degenerative processes (Franke & Kittner, 2001; Koyuncuoglu et al., 2017). PTZ induces changes in the activity of monoaminergic systems (Becker, Grecksch, Thiemann, & Höllt, 2000), reduction in levels of noradrenaline (NA) and dopamine (DA) and increased levels of serotonin (5-HT) have been associated with the development of seizures (Shouse, Staba, Ko, Saquib, & Farber 2001) and motor disorders (Yonekawa, Kupferberg, & Woodbury, 1980). Currently, the decrease in DA has been related to motor deficit (Felger & Treadway, 2017), and 5-HT, DA and NA have been implicated in the control of neuronal excitability in epileptic episodes in animal models (Weinshenker & Szot, 2002). Electroencephalogram has shown an increase in cortical activity during the postictal stage in patients with epilepsy (Harris, Schevon, & Bateman, 2017). This period of time comprehends a part of the global phenomenon of epilepsy in particular for the recovery of brain activity and to date it has not been clarified how brain levels of 5-HT, DA, and NA influence motor deficit during postictal stage. The aim of the study is to evaluate the concentration of 5-HT, NA, and DA in the hippocampus, cerebellum and cortex on motor deficit during the postictal stage in an animal model of epilepsy induced by PTZ.
Method
Subjects
Eighteen male Wistar rats with an average weight of 290-300 g were used. The animals were housed in a 12:12 h light/dark cycle, with a temperature (22 serotonin a -24 °C) and humidity (49% - 50%), and fed regular diet and water ad libitum (Purina Chow commercial). The animals were treated according to the Guide for the Care and Use of Experimental Animals (Institute of Laboratory Animal Resources, Committee on Care, Use of Laboratory Animals, & National Institutes of Health, 1985). In the same way, we were guided according to the Mexican Official Standard NOM-062-ZOO-1999 (2001).
Procedure
Once used in behavioral tests for the evaluation of motor function (two motor tests such as the impression of the footprint, which evaluates the gait and the equilibrium bar paradigm, which evaluates motor coordination) for five days, subsequently the animals were randomized into two groups: control (n = 9, saline solution 1 ml/kg, subcutaneous administration [s.c.]); experimental (n = 9, PTZ 90 mg/kg s.c.).
Analysis of the motor deficit and gait
After PTZ injection, the number of myoclonic seizures of each rat was recorded for three hours. Twenty-four hours after PTZ administration, global locomotor activity was assessed by means of the equilibrium bar paradigm test, described previously by Brailowsky, Knight, Blood, and Scabini (1986). The records were performed at 0 (Basal), 1, 2, and 3 h. As described previously by González-Piña, Bueno-Nava, and Montes (2005) the wood tunnel test was used to assess the nature of gait behaviors of rats. The impression of the footprint (length [L], width [A] and the angle [An]) was analyzed. Records were performed at 0 (Basal), 1, 2, and 3 h.
HPLC-EC analysis of 5-HT, DA, and NA concentration
Rats (n = 18) were decapitated and the hippocampus, cerebellum, and cortex were dissected from 9:00 h am to 13:00 h pm to eliminate circadian variations. Extraction of brain structures and analysis of 5-HT, DA, and NA were performed according to González-Piña and Paz (1997). Tissue was collected in Teflon tubes, and a solution of .5 ml of perchloric acid .4 N containing .1% w/v of sodium metabisulfite was added. The tissue was then homogenized and centrifuged at 15,000 rpm for 20 min in 4°C. The supernatant was filtered and stored at -70°C for High Performance Liquid Chromatography (HPLC). 5-HT, DA and NA content was analyzed by HPLC (Alltech, HPLC Pump, Model: 626) using a coulometric electrochemical detector (ESA, Model: Coulochem III). Detection conditions were as follows: analytical cell (ESA 5011A) with the potentials = +350 mV, E1= +200 mV and E2 = -200 mV. The peaks were sent to a computer with an EZCrom SI (version 3.2.1) program. To determine the concentrations of 5-HT, DA, and NA the chromatograms of the samples were interpolated using chromatograms from five standard samples with known 5-HT, DA, and NA concentrations. An analytical column for catecholamines was used (Alltech, Adsorbosphere Catecholamine, 100×4.6 mm, 3 µm of particle size). The mobile phase consisted of a phosphate buffer solution (.1 mM, pH 3.2) containing (in mM) sodium octyl sulfate (.2), EDTA (.1) and methanol (15% v/v). The flow rate was 1.6 ml/min.
Statistical analysis
Statistical analysis of 5-HT, DA, and NA measurements was performed with a one-way analysis of variance (ANOVA) and a post hoc Tukey test, and the data of the motor function tests was analyzed using a non-parametric Mann-Whitney’s U. In all cases, the differences were considered significant at p < .05. The results in the text and figures are expressed as the means ± S.E.M.
Results
Effect of PTZ on myoclonic shakes
Systemic administration of PTZ (90 mg/kg) provoked myoclonic shakes in all animals in the experimental group, which were quantified for three hours. A maximum in the number of shakes was reached in the first hour and then it began to decline reaching almost zero at the end of the recording. When comparing the hours of recording, a significant decrease was found at hour 1 (p = .001), hour 2 (p = .001), and hour 3 (p = .003) (Figure 1).
Assessment of gait
After the PTZ (90 mg/kg) administration, a significant increase in the percentage of stride length was found when comparing hour 1 (p = .0005), hour 2 (p = .0005), and hour 3 (p = .001) between control and experimental groups a tendency of the data to return to basal conditions was observed. Regarding stride width, significant differences were present between the two groups at hour 1 (p = .026), hour 2 (p = .0019), and hour 3 (p = .001). The highest percentage of increase in stride width was found in hour 2 (Figure 2).
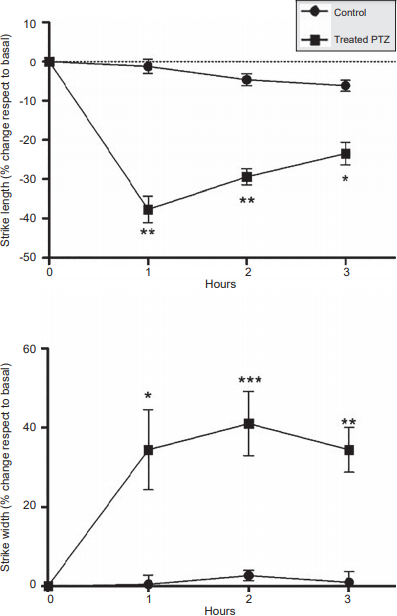
Figure 2. Percentage of change in the stride length and the width of animals in experimental group (PTZ 90 mg/kg) and control group, recorded during three hours. Data are expressed as mean ± S.E.M. *p = .001, **p = .0005.
Likewise, there was an increase in the percentage of stride angle when comparing between control and experimental groups at hour 1 (p = .0027), hour 2 (p = .0027), and hour 3 (p = .0005). A tendency to recover the basal values was also present as time elapsed (Figure 3).
Evaluation of motor coordination
There was a 456% increase in motor deficit at hour 1 (p = .0003); 284% at hour 2 (p = .0003), and 137% at hour 3 (p = .024) when comparing control and experimental groups (Figure 4).
Concentration of 5-HT, DA, and NA
Table 1 shows the total concentration of 5-HT, DA, and NA in the cortex, cerebellum, and hippocampus of the control and experimental groups. A significant increase of 5-HT (p < .05) and a highly significant increase of DA (p < .001) were present in the cortex comparing control and experimental groups. No significant differences were found in NA.
Table 1 Effect of PTZ (90mg/kg), on total levels of serotonin (5-HT), dopamine (DA) and noradrenaline (NA) in the brain of the rat
| 5-HT (nmol/g) | DA (nmol/g) | NA (nmol/g) | |
|---|---|---|---|
| Cortex | |||
| Control | 3.63 ± .90 | 64.68 ± 21.99 | 207.74 ± 19.89 |
| Experimental | 7.32 ± 1.08* | 245.85 ± 91** | 215.57 ± 23.75 |
| Cerebellum | |||
| Control | 9.32 ± 1.42 | 2.38 ± .34 | 94.88 ± 2.67 |
| Experimental | 15.82 ± 1.76* | 1.58 ± .22 | 99.63 ± 1.68* |
| Hippocampus | |||
| Control | 5.55 ± 1.21 | 38.72 ± 7.80 | 255.72 ± 39.03 |
| Experimental | 23.95 ± 7.75* | 94.05 ± 37.11* | 277.55 ± 34.07 |
Note: Values were expressed as the M ± S.E.M. During the statistical analysis a one- way ANOVA was used. The significant differences compared to the control group were
*p < .05,
**p < .001.
The concentration of 5-HT in the cerebellum exhibits a statistically significant increase in the experimental group compared to the control (p < .05). The same was observed regarding NA concentration (p < .05); no significant differences in DA concentration were observed between the two groups. In the hippocampus, the concentration of 5-HT and DA significantly increased (p < .05) in the experimental group three hours after subcutaneous administration of PTZ (90 mg/kg); there were none significant differences between groups in NA concentration.
Discussion and conclusion
In animals under an epilepsy model induced by PTZ (90 mg/kg), a significant increase was present in total 5-HT levels in hippocampus, cerebellum, and cortex, whereas DA displayed a significant increase in both hippocampus and cortex. As for NA, a significant increase was present in the cerebellum.
On the other hand, there was a decrease in the motor function in the adult rat during the postictal stage. 5-HT projects to almost all brain areas and participates in sensory, behavioral, and motor activity processes (Pérez-García, Liy-Salmerón, & Meneses, 2006). Acute administration of PTZ (90 mg/kg) induced an increase of 5-HT at the hippocampus, cerebellum, and cortex levels, which caused an imbalance of the serotonergic system resulting in an aberrant behavior as described by Adamec, Burton, Blundell, Murphy, and Holmes (2006). Our results are consistent with studies conducted by Gholipour, Ghasemi, Riazi, Ghaffarpour, and Dehpour (2010), which describe an increase in serotonergic neurotransmission in the hippocampus after the induction of seizures. Increased levels of 5-HT in the hippocampus, cerebellum, and cortex of PTZ-treated rats could be a compensatory response to neuronal excitability following PTZ-induced brain damage. During the convulsive process produced by the PTZ, not only are glutamatergic and GABAergic systems involved, but also monoaminergic systems (Kalynchuk, 2000). PTZ is a GABA antagonist, which apart from inhibiting excitation in the brain, also modulates the activity of monoaminergic systems (Becker et al., 2000). It has been previously shown that direct lesions on serotonergic neurons can produce a compensatory response such as serotonergic reorganization in the area of the dentate gyrus after damage to the median raphe nucleus (Haring, 1991). On the other hand, the levels of DA in our study were elevated in the hippocampus and the cortex, these results agree with what was proposed by Lee et al. (2015), whose aim was to evaluate the relationship between the dopaminergic and serotoninergic systems in an animal model of Parkinson’s disease. They concluded that there was a positive correlation between the dopaminergic deficit and the serotonergic deterioration in terms of potential binding. Although different pathologies are evaluated, some relations can be inferred in the particular case of the postictal stage as we can observe an increase in the concentration in both neurotransmitters in the hippocampus and cortex, i.e., a positive correlation to regain homeostasis within the postictal stage. In this study, NA displayed a statistical increase in the cerebellum. This particular area, in contrast to the cerebral cortex and hippocampus, exhibits fewer metabolic changes during epileptic status (Folbergrová, Ingvar, & Siesjö, 1981); however, high levels of noradrenaline in the brain activate the alpha-1 autoreceptor in the serotonergic neuron, increasing the rate of triggering and releasing serotonin (Bhagya, Srikumar, Raju, & Shankaranarayana, 2015).
The acute systemic administration of PTZ caused a marked transient cerebral dysfunction reflected in the motor deficit. The hippocampus has cellular elements that receive inputs and outputs to important areas of pons and cerebral cortex. Newman and Reza (1979) hypothesized that there are relationships between the hippocampus and the cerebellum. The pattern of electrical activity developed by the hippocampus is closely related to the realization of movements that occur during excitation and other behavioral changes. None of the proposals that have been made state that the hippocampus participates in a feedback of muscle activity but emphasizes the possibility of a direct influence on motor control systems (Bueno-Nava et al., 2010). Assuming that the output of the hippocampus can modulate the motor control circuits, it is reasonable to suggest that the hippocampus itself guides the signals of the cerebellum for its normal operations. In turn, the hippocampus is involved in providing the information required for the cerebellum, just as proprioceptive information is used to correct movements in the locomotor system. There are fast and slow connections that operate in both directions. In some morphological studies, it has been shown that PTZ causes loss of neurons in many brain structures. Neuronal necrosis is found in the hippocampus areas CA1, CA3, and in the dentate gyrus regions (Franke & Kittner 2001). Therefore, it is possible that the alteration in the motor activity is due to a deterioration of the acquisition and/or retention of contextual signals, caused by damage to the hippocampus.
Our results indicate that both the long width and angle of stride increased considerably after administration of PTZ. Presumably, these results were induced by loss of neurons and/or damage in the brain-cerebellum connectivity, in addition to monoaminergic imbalance. Similarly, the balance bar is a test that can qualitatively measure the motor coordination of the rat (Hruska, Kennedy, & Silbergeld 1979). It has been reported that focal cortical damage in rats induces a temporary motor deficit (Bueno-Nava et al., 2008: Goldstein, 2006; González-Piña et al., 2005). Together, the reciprocal connections that link the hippocampus to the cerebellum and the cortex derive from the participation of neurotransmitters involved in cognitive and motor aspects, such as 5-HT, NA, and DA (Dempesy et al., 1983; Marcinkiewicz, Morcos, & Chretien, 1989).
The results of the present study revealed that the administration of PTZ significantly increased the motor deficit during the three hours of recording. Data in this study could indicate that the changes in motor behavior observed may be due to the modification in the synthesis and release of the monoamines, caused by altered synaptic regulatory processes, which may occur as a result of neuronal loss, gliosis, or neuronal outbreaks as described by Szyndler et al. (2002). However, the return to basal motor conditions is evident both in motor behavior and in myoclonic shakes after PTZ administration after the second hour, so it can be speculated that mechanisms that lead to a spontaneous recovery of the levels of monoamines may be present, thus reducing the degree of motor deficit. More studies are needed to elucidate the underlying mechanisms of brain recovery after an epileptic seizure. In particular, the specific role of structures such as the rafe dorsal nucleus [5-HT], Locus Coeruleus [NA], and striatum [DA] contributions during the postictal process, in order to develop pharmacological strategies to reduce damage after of the ictal stage in humans. Acute administration of PTZ at a dose of 90 mg/kg generates a motor deficit and increases the concentration of 5-HT in the hippocampus, cerebellum, and cortex due to the convulsive activity. Presumably, there is an indirect participation between serotonin and motor changes, when acting as a neuromodulator, since 5-HT modifies the concentration of other monoamines directly involved in motor aspects such as NA and DA during the postictal stage.











 nueva página del texto (beta)
nueva página del texto (beta)





