Introduction
Borderline personality disorder (BPD) is a severe mental disease with a pervasive pattern of emotional instability, suicidal-attempts, and marked impulsivity that begins in adolescence and early adulthood (American Psychiatric Association, 2013). DSM-5 criteria focus on the principal symptom, although in BPD are oscillatory and heterogeneous because they could result in over 100 different clinical variants, which affect the clinical presentation (Forti & Forti, 2012). Nonetheless, clinic relevant characterization for epidemiologic studies shows a BPD prevalence between 1-6% (Hughes, Crowell, & Coan, 2012). It is the most common personality disorder (Hughes et al., 2012; Tajima & de Anta, 2009; Tajima et al., 2009) and epidemiological studies such as the one developed by Grant et al., (2008) point out that the prevalence of BPD does not differ according to gender. In the other hand, the American Psychiatric Association (2013) mentions that 75% of diagnosed cases are women. In agreement with it, the conclusion about gender prevalence is still controversial.
The clinic course of BPD patients is variable, and almost constantly unstable with acute periods of crisis, auto-mutilation, aggressive behavior, suicide attempts, drug abuse, etc. All of them comes along with an important affective correlate. In this case, the estimated prevalence increased 50% in patients attended in urgencies, and nearly 5% to 10% committed suicide (Paris, 2008). BPD patients usually show high co-morbidity rates for mood disorders, anxiety, and drug abuse (Leichsenring, Leibing, Kruse, New, & Leweke, 2011).
A highlight is that 24% of the patients have hallucinatory and severe psychotic symptoms (Pope, Jonas, Hudson, Cohen, & Tohen, 1985) and 75% also show paranoid ideation and dissociative experiences. Several studies show that psychotic symptoms are temporal, and related to elevated trauma/stress periods (Igarashi et al., 2010; Zanarini, 2000). Recent studies suggest that those symptoms are present in early childhood (Yee, Korner, McSwiggan, Meares, & Stevenson, 2005), being a source of important clinic traits.
The etiology of BDP is complex, and researchers and clinicians do not have an etiopathogenic model that integrates all its characteristics. However, a multidimensional approach (between neurobiology to cognitive and social aspects) is more appropriate in BPD and other neuropsychiatric disorders.
Indeed, some studies have related some neurocognitive profiles in many disorders to social cognition (Andreou et al., 2015), a sum of mental (cognitive and socio-emotional) processes implicated in social interaction, and adaptive behavior.
Perhaps, in BPD patients, data are contradictory as well as unclear upon how these processes are related to clinic features. For example, the mentalizing, a core process in social cognition, refers to cognitive and emotional ability to understand your own mind and that of the others. Data reveals that BPD patients have normal or even better mentalizing abilities (Arntz, Bernstein, Oorschot, & Schobre, 2009; Arntz & Haaf, 2012; Fertuck et al., 2009; Franzen et al., 2011; Lynch et al., 2006). Nonetheless, other studies suggest an impairment in mentalizing (Robin et al., 2012; Roepke, Vater, Preißler, Heekeren, & Dziobek, 2012; Unoka, Fogd, Füzy, & Csukly, 2011).
Although the mentalizing process was initially described in autism spectrum disorder and schizophrenia (Baron-Cohen, Leslie, & Frith, 1985; Baron-Cohen, Wheelwright, Hill, Raste, & Plumb, 2001; Fernández-Abascal, Cabello, Fernández-Berrocal, & Baron-Cohen, 2013; Gavilán-Ibáñez & García-Albea, 2013), is not limited to them. Indeed, in relation BPD, some specific patterns can differ [e.g., under-mentalizing vs. over-mentalizing (Andreou et al., 2015; Vaskinn et al., 2015)].
Another subdomain in social cognition is the decision-making process. Is a cognitive function involved with reflecting on the consequences of a certain choice (Bechara, 2005; Bechara, Damasio, Damasio, Anderson, 1994) based on somatic markers (Damasio, 1994). BPD patients seem to have more difficulties to learn from negative feedback (Svaldi, Philipsen, & Matthies, 2012). Also, these processes have shown some relation with other cognitive abilities, but only a few have shown statistically significant relations (Toplak, Sorge, Benoit, West, & Stanovich, 2010).
On the other hand, executive functions, which are a set of processes such as working memory, attention, shifting, planning, etc., that people use to control and coordinate their cognitive abilities and behavior to achieve specific objectives, have not been studied enough in BPD. Nevertheless, the dorsolateral prefrontal cortex executive function process has shown a normal range performance in BPD (LeGris, Links, van Reekum, Tannock, & Toplak, 2012) or at least memory impairments in subgroups of BPD subjects (Fertuck, Lenzenweger, Clarkin, Hoermann, & Stanley, 2006). So far, certain possible underpinnings of executive functions in BPD have received little attention.
In summary, social cognition studies suggest impairment in BPD but are still unclear or contradictory. As well as decision-making and executive function evidence have not been consistently studied in BPD. Therefore, the aims of this study are to assess the contribution of social cognition and executive function to socio-emotional and cognitive patterns in BPD, as well as to investigate the possible relationships between social cognition tasks and EF measures and clinic features in BPD.
Method
Participants
The clinic group consisted of 20 females with BPD in ambulatory hospitalization, medicated, and right-handed. All of them were diagnosed with DSM-5 criteria (American Psychiatric Association, 2013). Patients had not used drugs or alcohol for at least three months before the assessment and those who had a history of schizophrenia, psychotic disorder, bipolar and affective disorder were excluded. The mean time of disease was 11.95 years (SD ± 6.73). Patients had a mean age of 32.40 years (SD ± 11.82 years), and had high-school diplomas. The control group consisted of 20 healthy women with no history of mental diseases (mean age of 33.50 ± 11.66) with the same educational level.
Regarding the clinical characteristics of the sample, 100% of the patients attempted suicide on at least two occasions in their life, with a maximum of seven attempts. They consumed some psychotropic substances in their life (95%), mostly alcohol (60%), 15% of them used marijuana, 10% cocaine, and 10% had used multiple substances. They also had a history of sexual assault at some point in their life (80%) ( Table 1 ).
Table 1. Characteristics of the sample
| Control Group | Clinic Group | ||
|---|---|---|---|
| (n = 20) | (n = 20) | ||
| Agea | 33.50 ± 11.669 | 32.40 ± 11.829 | |
| Sex | Females | 100% | 100% |
| Schooling | High School | 30% | 40% |
| Technician | 25% | 20% | |
| Professional | 45% | 40% | |
| Suicidal attemptsb | Presence | ------- | 100% |
| Absence | ------- | ------- | |
| Drug abuseb , c | Presence | ------- | 95% |
| Absence | ------- | 5% | |
| Kind of drug abuse | None | 65% | 5% |
| Alcohol | 35% | 60% | |
| Marijuana | ------- | 15% | |
| Cocaine | ------- | 10% | |
| Multiple drugs | ------- | 10% | |
| Sexual mishandlingb | Presence | ------- | 80% |
| Absence | ------- | 20% | |
| Disease timea | ------- | 11.95 ± 6.739 | |
Note:
amean and standard deviation;
bpresence or absence of any suicidal attempt;
cat least 3 months after the assess.
Neuropsychological test
For assessing our domains of interest we used a flexible neuropsychological battery. To assess social cognition, the “Reading the mind in the eyes” (Baron-Cohen et al., 2001) and the “IOWA Gambling task” (Brevers, Bechara, Cleeremans, & Noël, 2013; Bechara, Damasio, Tranel, & Damasio, 2005; Bechara, 2004) was performed.
The IOWA Gambling task was in virtual format (Psychology Experimental Building Language, [Mueller & Piper, 2014]), and evaluated the decision-making emotional character, to determine the risk-benefit following the selection of four possible cards. At all times the assessed subject would know how to make their decisions or not profits, and after 100 attempts the amount of successes/failures that mediated somatic markers (Bechara, 2004) were measured. An emotional learning curve with five principal scores of each 20 responses was designed. The purpose of the game is to obtain the greatest possible reward.
Theory of mind or advance meta-representational skills were evaluated with the “Reading the mind in the eyes” (Baron-Cohen et al., 2001). We showed the subjects 36 images of human faces expressing a complex mental state whether thought, intention, or emotion, through facial gestures and particularly the eyes. Around the image there were adjectives that described the mental state and thus allowed four possible choices by the subject. This allowed the detection and discrimination of a social stimulus in the immediate environment (Sabbagh, 2004). Following the presentation of each image, we also requested the sexual identity of the people in the pictures as a control measure of perceptual skills.
Finally, to assess the executive function, we performed the letters and numbers sequence test, digit-spam test -direct and reversible, from the WAISS-III (Wechsler, 1977), the trail making test, form A and B, as the verbal fluency, semantic and phonologic (Jaichenco, Wilson, & Ruiz, 2007). These tests evaluated executive functions, a “global” concept that includes processes such as concentration, attention, working memory, sequencing, planning, manipulation, and display of stimuli, change control and inhibition, lexical access, and semantic information retrieval (Villodre et al., 2006). Additionally, we used the Wisconsin Card Sorting Test ‒a particularly sensitive test for frontal dysfunction‒, which is a measure of executive function that requires planning strategies, organized environmental investigations and use of “feedback” to change schemes (De la Cruz, 2001).
This was an ex post fact to retrospective study (Montero & León, 2007) with two groups. We sought to measure the performance in neuropsychological tasks at a given point of time after the event of interest comparing both groups. A non-probabilistic sampling was used (Hernández, Fernández, & Baptista, 2010).
Testing procedure
Subjects were tested individually in a quiet room at the hospital. The sequence in which tests were administered was identical for all subjects. The procedure took two sessions (almost two hours). Patients were tested in the morning at 10 a.m., and had 15 minutes to relax between sessions.
Ethical statement
The study was approved by the Ethics Board of a mental health hospital in Arequipa, in accordance with the declaration of Helsinki. All participants were informed about the aims of the study and gave written informed consent. All data were collected in an anonymous database.
Data analysis
Statistical analysis was performed with SPSS version 20 (SPSS, Inc., USA). Contrast tests of the parametric and nonparametric type were used, depending on the Kolmogorov-Smirnov normality test and the variance homogeneity test (Levene). The analyses of participants’ learning curves in the IOWA gambling Task were carried out via a repeated measures ANOVA and Bonferroni post hoc analysis. We also used the Spearman correlation between variables. Results were significant with *p < .05, and **p < .01.
Results
The core cognitive processes in social cognition are theory of mind and decision-making mediated by somatic markers. In theory of mind evaluation, the “Reading the mind in the eyes” test does not find any difference for recognition of sex in gaze (u: 181, p < .620); in relation with gaze recognition of intentions, thoughts and emotion, statistically significant differences between BPD patients and the control group (u: 96, p < .004*) were found ( Table 2 ).
Table 2. Sample characteristics and clinical variables of women with borderline personality disorder and healthy comparison subjects
| Control group | Clinic group | u | p | |
|---|---|---|---|---|
| (n = 20) | (n = 20) | |||
| M ± SD | M ± SD | |||
| Social cognition | ||||
| ToM–Sex recognition | 34.15 ± 1.268 | 33.95 ± 1.099 | 181.000 | .620 |
| ToM–Gaze recognition | 24.0 ± 3.325 | 20.70 ± 3.164 | 96.000 | .004* |
| IOWA 1a | -.85 ± 1.387 | -.20 ± 3.548 | .750 | >.999 |
| IOWA 2a | 1.35 ± .813 | 1.95 ± 4.136 | .693 | >.999 |
| IOWA 3a | 2.80 ± .696 | 3.70 ± 5.110 | 1.040 | >.999 |
| IOWA 4a | 4.60 ± .940 | 1.60 ± 5.175 | 3.465 | .004** |
| IOWA 5a | 5.15 ± .988 | 2.00 ± 4.267 | 3.638 | .002** |
| Executive function | ||||
| WCST 1 | 80.50 ± 5.346 | 63.55 ± 16.321 | 93.500 | .003* |
| WCST 2 | 19.25 ± 5.447 | 36.15 ± 16.246 | 96.500 | .004* |
| WCST 3 | 10.65 ± 3.897 | 19.15 ± 12.550 | 113.500 | .018* |
| WCST 4 | 8.15 ± 3.543 | 16.70 ± 10.413 | 93.500 | .003* |
| WCST 5 | 10.55 ± 3.137 | 19.35 ± 9.499 | 92.000 | .003* |
| WCST 6 | 71.80 ± 8.224 | 47.95 ± 18.483 | 64.000 | .001** |
| WCST 7 | 6.00 ± 0.00 | 4.05 ± 1.669 | 70.000 | .001** |
| WCST 8 | 11.15 ± 0.813 | 18.00 ± 15.583 | 125.000 | .043* |
| WCST 9 | 0.15 ± 0.366 | 0.70 ± 0.979 | 135.500 | .081 |
| WCST 10 | .400 ± 1.930 | -5.75 ± 10.254 | 95.000 | .004* |
| Total time | 16.15 ± 3.675 | 17.85 ± 5.905 | 171.500 | .445 |
| Letters and numbersb | 5.65 ± 0.671 | 5.60 ± 1.847 | 192.500 | .841 |
| Direct digitsa | 5.95 ± 1.050 | 7.55 ± 1.432 | 75.000 | .001** |
| Reverse digitsa | 4.40 ± 0.995 | 4.30 ± 1.689 | 186.500 | .718 |
| TMT A | 33.55 ± 6.168 | 51.50 ± 22.758 | 67.500 | .001** |
| Time - 1° error | .00 ± .00 | 2.50 ± 6.932 | 170.000 | .429 |
| TMT B | 74.65 ± 11.421 | 135.90 ± 84.298 | 109.000 | .013* |
| Time - 1° error | 1.90 ± 6.164 | 21.95 ± 30.045 | 80.000 | .001** |
| Phonological fluency | 16.30 ± 3.097 | 12.70 ± 3.435 | 93.000 | .003* |
| Semantic fluency | 22.20 ± 2.949 | 18.50 ± 4.310 | 100.000 | .006* |
Note: WCST 1: percentage of success; WCST 2: error percentage; WCST 3: perseverative response percentage; WCST 4: error perseverative percentage; WCST 5: error non-perseverative percentage; WCST 6: conceptual response; WCST 7: number of categories completed; WCST 8: attempts in the first category; WCST 9: failure of attentional set; WCST 10: learn to learn score;
aPost hoc Bonferroni analysis after Repeated measures ANOVA;
bb two subjects in control group make a perseverative mistakes; u: Mann-Whitney U test;
*p < .05;
**p < .001.
In Figure 1 , the performance of these groups is presented. The theory of mind in BPD is clearly reduced compared with a healthy control group. The absence of differences in sex recognition displays a preserve perception process in BPD.
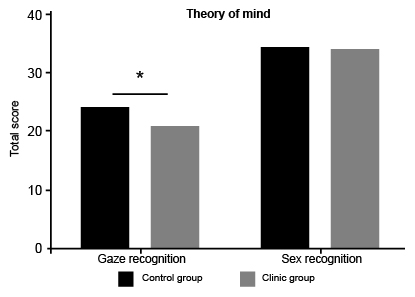
Figure 1. Reading the mind in the eyes. Comparison between clinic and control group. BPD patients display worse recognition of gaze than controls.
Regarding the next process of social cognition, the decision-making mediated by somatic markers, we used a mixed-design Repeated Measures ANOVA analysis to compared control versus clinical learning´s curves (IOWA scores – and groups factor) on IOWA gambling task performance across blocks. The main effect of IOWA across time scores was found (F = 15.51; p = .0001**). But, the effect of groups (total scores in all trials) was not found (F = 1.277; p = .2726). Nonetheless, a main interaction effect (Block per Group) was observed (F = 5.775; p = .0004**) between blocks 4 (p = .0044) and 5 (p = .0025). These results suggest that learning curves were significantly different between the BPD and the control group. Showing the last group as a better performance after 60 trials.
Figure 2 displays an emotional-learning curve in this task. The healthy control group took more advantageous choices over time agreeable with feedback across elections. In contrast, the BPD group took disadvantageous choices and their feedback did not show an effect in better selection. Over time patients grew more impulsive, took risky elections, and, this is related with severe BPD symptomatology. For the executive function ( Table 3 ), the Wisconsin card sorting test display significant differences in its distinct scores, percentage of success WCST1, (u: 93.5, p < .003**), error percentage WCST 2 (u: 96.5, p < .004**) and perseverative response percentage WCST 3 (u: 113.500, p < .018*). BPD patients presented also differences in error perseverative percentage (u: 93.5, p < .003**), error non-perseverative percentage WCST5 (u: 92, p < .003**), as well as conceptual response WCST6 (u: 64, p < .001**).
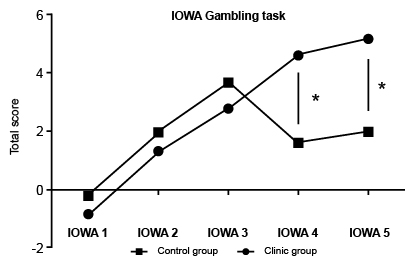
Figure 2. IOWA Gambling task. BPD patient shows |significant differences in IOWA scores 4 and 5 compared with controls. They take more disadvantage decisions showing more impulsivity than controls.
Table 3. Spearman correlation
| S. A. | D. T. | ToM S | ToM G | IOWA 3 | IOWA 4 | IOWA 5 | WCST 1 | WCST 2 | WCST 3 | WECH 2 | TMT A | TMT B | PVF | SVF | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S.A.a,b | 1 | .891** | .000 | -.502** | .128 | -.369* | -.378* | -.446** | .435** | .383* | .457** | .583** | .391* | -.398* | -.390* |
| . | .000 | .998 | .001 | .431 | .019 | .016 | .004 | .005 | .015 | .003 | .000 | .013 | .011 | .013 | |
| D. T. | .891** | 1 | -.130 | -.432** | .172 | -.287 | -.287 | -.486** | .483** | .374* | .429** | .602** | .486** | -.434** | -.395* |
| .000 | . | .422 | .005 | .290 | .073 | .073 | .001 | .002 | .017 | .006 | .000 | .001 | .005 | .012 | |
| ToM S | .000 | -.130 | 1 | .215 | -.259 | -.248 | -.045 | .193 | -.179 | -.256 | - .062 | -.222 | -.124 | -.066 | .125 |
| .998 | .422 | . | .182 | .107 | .123 | .781 | .234 | .269 | .111 | .702 | .170 | .445 | .687 | .443 | |
| ToM G | -.502** | -.432** | .215 | 1 | -.093 | .129 | .434** | .160 | -.186 | -.159 | .051 | -.558** | -.293 | .447** | .339* |
| .001 | .005 | .182 | . | .567 | .426 | .005 | .324 | .250 | .326 | .754 | .000 | .067 | .004 | .032 | |
| IOWA 3 | .128 | .172 | -.259 | -.093 | 1 | .294 | .214 | -.354* | .365* | .256 | .240 | .107 | .077 | .200 | .161 |
| .431 | .290 | .107 | .567 | . | .066 | .186 | .025 | .021 | .111 | .135 | .510 | .636 | .216 | .321 | |
| IOWA 4 | -.369* | -.287 | -.248 | .129 | .294 | 1 | .508** | .066 | -.045 | -.059 | -.265 | - .04 | .162 | .277 | .337* |
| .019 | .073 | .123 | .426 | .066 | . | .001 | .688 | .782 | .718 | .098 | .805 | .318 | .084 | .033 | |
| IOWA 5 | -.378 | -.287 | -.045 | .434** | .214 | .508** | 1 | .022 | .002 | -.058 | -.048 | -.321* | .060 | .455** | .184 |
| .016 | .073 | .781 | .005 | .186 | .001 | . | .895 | .991 | .723 | .768 | .043 | .711 | .003 | .255 | |
| WCST 1 | -.446** | -.486** | .193 | .160 | -.354* | .066 | .022 | 1 | -.996** | -.889** | -.188 | -.469** | -.294 | .279 | .339* |
| .004 | .001 | .234 | .324 | .025 | .688 | .895 | . | .000 | .000 | .244 | .002 | .065 | .081 | .033 | |
| WCST 2 | .435** | .483** | -.179 | -.186 | .365* | -.045 | .002 | -.996** | 1 | .883** | .171 | .478** | .306 | -.276 | -.323* |
| .005 | .002 | .269 | .250 | .021 | .782 | .991 | .000 | . | .000 | .293 | .002 | .055 | .085 | .042 | |
| WCST 3 | .383* | .374* | -.256 | -.159 | .256 | -.059 | -.058 | -.889** | .883** | 1 | .062 | .427** | .135 | -.185 | -.280 |
| .015 | .017 | .111 | .326 | .111 | .718 | .723 | .000 | .000 | . | .703 | .006 | .407 | .254 | .080 | |
| DDS | .457** | .429** | -.062 | .051 | .24 | -.265 | -.048 | -.188 | .171 | .062 | 1 | .111 | .240 | .023 | -.209 |
| .003 | .006 | .702 | .754 | .135 | .098 | .768 | .244 | .293 | .703 | . | .497 | .136 | .888 | .196 | |
| TMT A | .583** | .602** | -.222 | -.558** | .107 | - .040 | -.321* | -.469** | .478** | .427** | .111 | 1 | .550** | -.608** | -.318* |
| .000 | .000 | .170 | .000 | .510 | .805 | .043 | .002 | .002 | .006 | .497 | . | .000 | .000 | .046 | |
| TMT B | .391* | .486** | -.124 | -.293 | .077 | .162 | .060 | -.294 | .306 | .135 | .240 | .550** | 1 | -.443** | -.294 |
| .013 | .001 | .445 | .067 | .636 | .318 | .711 | .065 | .055 | .407 | .136 | .000 | . | .004 | .066 | |
| PVF | -.398* | -.434** | - .066 | .447** | .200 | .277 | .455** | .279 | -.276 | -.185 | .023 | -.608** | -.443** | 1 | .678** |
| .011 | .005 | .687 | .004 | .216 | .084 | .003 | .081 | .085 | .254 | .888 | .000 | .004 | . | .000 | |
| SVF | -.390* | -.395* | .125 | .339* | .161 | .337* | .184 | .339* | -.323* | -.280 | -.209 | -.318* | -.294 | .678** | 1 |
| .013 | .012 | .443 | .032 | .321 | .033 | .255 | .033 | .042 | .080 | .196 | .046 | .066 | .000 | . |
Note: S.A.: Suicidal attempts; D.T.: Disease time; D.D.S.: Direct digit spam test; P.V.F.: Phonological verbal fluency; S.V.F.: Semantic verbal fluency;
aCoefficient correlation;
bSignificance level;
*p < .05;
**p ≤ .0.
In relation to the number of categories completed, WCST 7 significant differences (u: 70, p < .000 **) were also obtained, as well as attempts in the first category WCST8 (u: 125, p < .043 *). Failure of attentional set WCST 9 score showed no difference (u: 135, p < .081). The WCST 10 score, learn to learn, showed significant differences (or: 95, p < .004 **) between subjects. Run time showed no difference between both groups (or: 171.5, p < .445). Number and letter sequence from WAIS-III displayed no significant difference (u: 192, p < .841), as digit-span reverse test (u: 103.5, p < .109). Digit-span direct test showed (u: 117, p < .255) a significant difference for both groups.
Likewise, trail making test form A displayed significant differences between groups (u: 15, p < .001**), but not in the occurrence for the first error (u: 170, p < .429). Trail making test form B showed difference in time (u: 80, p < .001**) and execution (u: 109, p < .013*), respectively. Verbal fluency also showed statistically difference in semantic (u: 100, p < .006*) and phonologic (u: 93, p < .003*) task.
Table 3 displayed a Spearman Rho correlation for two clinical variables (suicidal attempts and time disease), and neuropsychological test scores. Suicidal attempts correlated positively with time disease (p ≤ .001**) and negatively with gaze recognition of the “Reading the mind in the eyes” test (p ≤ .001**), as well as emotional-learning scores 4th. (p ≤ .019*) and 5th. (p ≤ .016*), respectively. Correlates negatively with success percentage WCST 1 (p ≤ .004*) and positively with error percentage WCST 2 (p ≤ .004*), perseverative percentage response WCST3 (p ≤ .005*). Indicating a negatively correlation besides with semantic (p ≤ .012*) and phonologic (p ≤ .005*) verbal fluency.
Gaze recognition of theory of mind correlated positively with IOWA 5 score (p ≤ .005*), as well as phonologic (p ≤ .004*) and semantic (p ≤ .032*) fluency. Besides gaze recognition correlated negatively with trail making test A (p ≤ .001**). IOWA 5 score correlated negatively with trail making test A (p ≤ .043*), and positively with phonologic fluency (p ≤ .003*). All variables of executive function exhibit a high correlation between them.
Discussion and conclusion
The aim of this study was to evaluate the neuropsychological profile of BPD patients and compare it with a healthy control group. Appraising the social cognition and executive function cognitive process, and analyzing how they are related. The main result is that women with BPD are characterized by worse social cognition abilities and executive function when compared to the control group.
Regarding social cognition, theory of mind and decision-making were assessed. For the first one, BPD showed a normal recognition of sex as a control perceptual task. In contrast, recognition of intentions, thoughts, and emotions in BPD patients are less proficient. Perceptual emotional recognition in these patients are related to higher vigilance of social stimuli and worse stability in their relationships. Dysfunctional social processing is conditioned to mentalizing, while BPD patients are not only more vigilant for negative emotions, intentions, and thoughts but also reduce skills to integrate social stimulation with suitable interpretations of social context. Our results support the idea of deteriorating mentalizing processes in BPD (Domes, Schulze, & Herpertz, 2009; Roepke et al., 2012; Unoka et al., 2011).
Nonetheless, these findings contradict the Krohn paradox, which assumes that BPD patients have normal or even better mentalizing abilities (Arntz & Haaf, 2012; Fertuck et al., 2009). This paradox could be interpreted from different points of view. For example, differences in those studies might be associated with assessing different specific sub process in theory of mind, such as the cognitive theory of mind versus recognition of basic emotions. Methodological (e.g., test deployed, different stimulus, the social context of tasks) or even cultural, ethnic, and educational differences. It is also possible that clinic impairment characterizes for increased effects of child abuse or trauma, psychological severity, disease time or co-morbidity.
In our study, BPD patients were medically stable and controlled in ambulatory hospitalization. Of them, 80% mentioned a history of trauma such as sexual abuse, domestic violence, or neglect. Also 95% had a history of drug abuse. This psychopathological severity is related to a less socio-cognitive process.
BPD patients have high clinical heterogeneity inter-individual, and intra-individual regard psychopathology display. Therefore, these patients may have a fluctuation in their cognitive process conditioning its psychopathology. Nonetheless, the impairment of the theory of mind could be considered as an endophenotype of this disorder. This process represents a complex control of socio-emotional and monitoring, and this handicap would be less stable and worse than in other disorders such as schizophrenia or autism spectrum disorder. It also explains dissociative states and paranoid symptoms, because BPD patients do not interpret correctly some mental or emotional states, or even they could not recognize and interpret their own mental states.
Traumatic events disturb socio-emotional and cognitive monitoring contributing to depersonalization or derealization symptoms, but also to emotional instability and disrupted social relationships. BPD patients do not recognize the mental states correctly and give those the wrong “cognitive and emotional tag” to the others and themselves (Fonagy & Bateman, 2008). This shifting depends on context, promoting idealization-devaluating relations. It could make more vulnerable to emotional changes a wicked experience; this hypothesis need more support.
On the other hand, BPD patients display disadvantageous decision-making when compared to the control group. Patients persevere more and take more impulsive elections, presenting a “future myopia” (Damasio, 1994) for long-term rewards, looking not only for extensive stimulation and short-term rewards, but also for their ability to recognize and “tag” emotionally negative in the situations would be reduced, refusing “greater” adaptive advantage elections (Maurex et al., 2009; Svaldi et al., 2012). Inability to value negative feedback from context would support these results (Svaldi et al., 2012) and also the absence of cognitive strategies in their decision-making.
Other studies suggest the emotional-learning impairment in BPD and make evident signs of pre-frontal pathology. Poor decision-making is related to executive dysfunction such as working memory, attention, and planning may support mayor incidence of worse decisions, risk behavior, impulsivity, and symptoms severity (Svaldi et al., 2012). Our data suggest this lower processing in behavioral regulation, mental flexibility, and reverse learning as being related to high impulsivity. The BPD group displayed less cognitive monitoring, planning, working memory, attention, concentration, and behavioral regulation, which coincides with the findings other studies (Hagenhoff et al., 2013; Gvirts et al., 2012; Arza et al., 2009; Grosjean & Tsai, 2007; Silvio, 2005; Stevens, Burkhardt, Hautzinger, Schwarz, & Unckel, 2004).
Environmental stress or trauma experiences (Zanarini, 2000) affect behavioral regulation and patients are more susceptible to re-experiment trauma, increasing emotional instability, and worse integration of social context as a symptomatic buckle, promoting epigenetic changes (Klengel, Pape, Binder, & Mehta, 2014; McGowan & Szyf, 2010). “Top-down” cognitive and socio-emotional control regulates complex behavior in normal life; without it, cortical-subcortical processes are inflexible, simple, and stimulus-dependent (Miller, 2010).
Our domains of interest might involve in a neural system related with consciousness as cognitive and socio-emotional monitoring explaining cardinal symptoms in BPD. Those affecting processes have a neurobiological correlate in different cortical and subcortical systems (Jacob et al., 2013; Mier et al., 2013). Especially amygdala (Cullen et al., 2011), anterior cingulate, prefrontal-striatum-limbic (Wolf et al., 2011; Tebartz et al., 2003), prefrontal-temporal-parietal (O’Neill et al., 2014), and prefrontal-thalamic-cerebellar systems (Mier et al., 2013). Involving serotoninergic, glutamatergic, and dopaminergic neurotransmitter systems (Maurex, Zaboli, Ohmanm, Asberg, & Leopardi, 2010; Homberg, 2012; Grosjean & Tsai, 2007).
As discussed earlier, BPD is a serious disorder with a wide clinic heterogeneity and therapeutic difficulty. Neuropsychological profiles delimit functional and structural process implicate as well as help to elucidate specific endophenotype with genetic vulnerability of affected domains (Gottesman & Gould, 2003; McCloskeya et al., 2009).
Reliable endophenotype for BPD are working memory, planning, and mental flexibility as executive processes (Arza et al., 2009). On the other hand, emotional dysregulation, theory of mind, and decision-making as social cognition process (Frick et al., 2012; Hughes et al., 2012; Roepke et al., 2012; Preißler, Dziobek, Ritter, Heekeren, & Roepke, 2010; Domes et al., 2009). Specific subdomains of social cognition and executive function would be good endophenotypes as well as specific cognitive processes for research, and should be objective of specific rehabilitation for BPD patients (Arza et al., 2009). In conclusion, patients stabilized with borderline personality disorder display differences in social cognition and executive function compared with healthy controls. Cognitive and socio-emotional domains are of special interest in clinical practice to address them as endophenotype or predictors of disease course and directing therapeutic intervention.
Limitations
One limitation observed in this study concerns the small sample size of the study and the potential sample bias of the screening phase. Additionally, the use of more ecological tests, for example, in more complex scenes of social interactions, as well as studies that consider sex differences, may advance understanding of borderline phenotypes.











 nueva página del texto (beta)
nueva página del texto (beta)


