Introduction
Autism spectrum disorder (ASD) is characterized by persistent impairments in communication and social interaction across multiple contexts, including deficits in social reciprocity, nonverbal communication behaviors, and developmental skills, and the maintenance and understanding of relationships interpersonal manifestations that manifest themselves in early childhood (American Psychiatric Association, 2013). The DSM-5 makes a distinction according to the severity of the symptoms, placing them at levels 1, 2, or 3. Since the new classification incorporates DSM-IV developmental disorders, the characteristics of the DSM-Asperger’s syndrome IV now correspond to level 1 of the autism spectrum in the DSM-5.
As a theoretical concept, the theory of mind (TM) states that the behavior of other people is predictable on the basis of the ability to understand their feelings, intentions, and beliefs (Tirapu-Ustárroza, 2007). Within the study of ASD, patients with autism do not have a correct functioning of this ability. For this reason, they do not understand the behavior of other people, which causes them confusion, anxiety, and inappropriate behavior when interacting with others. Within this context, researchers have sought a possible neuronal basis related to the ability to understand the mental state of others people by studying the activation of brain regions crucial to the representation of one’s own experience of those states.
The ability to activate these regions is based on the ability to imitate the actions observed in other people (Hauswald, Weisz, Bentin, & Kissler, 2013). The modulation of electroencephalographic (EEG) and neuromagnetic activities on somato-sensorial regions found within the alpha frequency range (8–13 Hz) called the Mu rhythm has been reported (Hauswald, Weisz, Bentin, & Kissler, 2013). This rhythm has been studied during the person’s own motor execution as well as during the observation of actions performed by other people (Hari & Salmelin, 1997). It has been postulated that the Mu rhythm is related to the handling of social abilities, as a result of which its functional deficiency in patients with ASD provides an explanation for some of the difficulties in social interaction reported in this population (Bernier, Dawson, Webb, & Murias, 2007).
Likewise, various authors have suggested a functional relationship between mirror neurons and the Mu rhythm (Braadbaart, Williams, & Waiter, 2013; Hamilton, 2013; Oberman et al., 2005; Oberman, Ramachandran, & Pineda, 2008; Pineda & Hecht, 2009; Pineda, 2005; Ramachandran & Oberman, 2006; Rizzolatti & Craighero, 2004; Williams et al., 2006), where the Mu rhythm reflects a descending modulation from the motor cortex by prefrontal mirror neurons (Pineda, 2005), which are involved in imitation, learning, and the ability to understand the actions of others (Rizzolatti & Craighero, 2004).
At the same time, sleep is crucial to adequate brain maturation since its benefits during childhood impact the physical development and cognitive processes of the individual (Dahl, 1996; Frank, Issa, & Stryker, 2001). In children with ASD, sleep disorders have been described in various studies, of which insomnia is one of the most commonly reported problems (Ayala-Guerrero, Mexicano, & Huicochea-Arredondo, 2014; Cortesi, Giannotti, Ivanenko, & Johnson, 2010; Johnson, Giannotti, & Cortesi, 2009; Malow et al., 2006; Richdale, 1999; Souders et al., 2009; Vriend, Corkum, Moon, & Smith, 2011; Wiggs & Stores, 2004). The prevalence of problems reported in the sleep of children with ASD ranges from 40% to 80% (Cortesi, Giannotti, Ivanenko, & Johnson, 2010). When these sleep alterations have been identified and treated, improvements have been reported in diurnal symptoms; the opposite effect is observed when sleep quality deteriorates (Malow et al., 2006; Schreck, Mulick, & Smith, 2004).
In addition to its importance in development, there are other phenomena that occur during sleep that are relevant to the study of autism. It has been suggested that sensory motor processes may be active during REM sleep. For example, in patients with behavioral disturbance of REM sleep, people act out their dreams and are capable of reacting to stimuli in the environment while they are asleep (Duntley, Kim, Silbergeld, & Miller, 2001). Due to the association between the Mu rhythm and sensorimotor processes and the decreased reactivity during wakefulness of people with ASD, it is important to explore this rhythm during the sleep of this population, in which there are also abnormalities compared to the population with typical development.
According to the rule of excitatory response in the brain, if a brain structure can generate intrinsic activity in a set frequency channel, then this structure is also able to response to sensory stimulation in the same frequency channel (Yordanova & Kolev, 1997). Having established the above, studying this rhythm in the spontaneous sleep EEG allows us to observe the activity without external stimulation, which will provide a better understanding of its response to external stimuli.
It has been established that the physiological reactivity of Mu during wakefulness differs in samples of patients with ASD (Oberman et al., 2005; 2008; Ramachandran & Oberman, 2006). However, when they are awake, sensory information passes through multiple channels during processing to generate this reactivity. Observing the Mu rhythm during sleep would provide more information about its spontaneous generation in order to determine whether differences are generated intrinsically or whether there is any alteration in the way sensory information is being processed.
Given the importance of sensorimotor processing in imitation and learning (elements in which there are flaws in autism), the role of the Mu rhythm as a reflection of these processes, as well as the impact of sleep on the development and symptomatology of autism, the purpose of this paper is to characterize the Mu rhythm in the sleep of children with level 1 autism spectrum disorder (formerly Asperger’s syndrome), between the ages of six and 10 years and compare it with control children matched by age and sex.
Method
Sample description
The sample consisted of 10 children with ASD and seven children with typical development (TD) within a range of six to 10 years. For the sample size, previous studies on autism and sleep carried out with polysomnography were used as reference (Malow et al., 2006), and the Mu rhythm (Bernier, Aaronson, & McPartland, 2013; Lepage & Théoret, 2006; Oberman et al., 2005).
The inclusion criteria for the groups were as follows:
ASD: Having a multiaxial diagnosis of ASD conducted by psychologists from the Caritas de Amistad association according to DSM-IV and DSM-5 criteria through an interview with parents and children.
TD: Sex- and age-matched with a member of the ASD group.
For all participants, informed consent was obtained from the parent or tutor.
Exclusion criteria for TD group were as follows:
Observation of any sleep disturbance during the habituation night or one that had been previously diagnosed, taking medication, previous diagnosis of a chronic disease, or health problems that will impact sleep at the time of registration. Failure to complete two nights of PSG.
ASD: Failure to complete two nights of PSG.
Location
For the participants of the group with ASD, sampling was performed by voluntary participants in the Caritas de Amistad association. Conversely, the DT group was obtained via network sampling.
Procedure
Data collection was carried out at the Laboratorio de Neurociencias from the Facultad de Psicología at the Universidad Nacional Autónoma de Mexico (UNAM) during the period from September 2013 - December 2014. Two eight-hour polysomnographies were performed for two consecutive nights, in which an electroencephalogram (EEG), an electrooculogram (EOG), and an electromyogram (EMG) were obtained. Respiratory and cardiac activity (ECG) and the percentage of circulating O2 were also obtained. The Easy II 32-Channel Amplifier and software from Cadwell Laboratories were used.
The first night was regarded as habituation and the second as the data collection night. The referrals for night 1 were: C3, C4, O2, O1; and for night 2: F3, F4, C3, C4, T3, T4, P3, P4, O1, O2. During the second night, no sensors were included for respiratory activity. During the physiological calibration, the participant was asked to perform a physical exercise to obtain the basic reactivity of the Mu rhythm.
Measures used
For the EEG on both nights, the assembly was bipolar, with 35 Hz high pass filters and .53Hz low pass filters and a sensitivity of 10μV/mm. The EEG segments identified as the Mu rhythm were exported in EDF format and converted to ASCII format to obtain the power spectrum. In the Fast Fourier Transformation (FFT), the following bands were established: 1-3Hz, 4-7Hz, 8-13Hz, 14-18Hz, 19-24Hz, and 25-30Hz.
Data analysis
Data analysis was performed in two stages: first, a visual examination of the EEG signal was conducted to identify and mark the different sleep phases, as well as the presence of the Mu rhythm. In relation to the Mu rhythm segments, the following criteria were taken into account: 1. that the EEG fragment complied with the morphology, frequency, and topographic distribution characteristics of the Mu rhythm; 2. that the Mu wave train had a duration of at least two seconds; and 3. that it was free of artifices.
Once the above criteria had been complied with and in order to corroborate that the selected segments belonged to the desired frequency band, the power spectrum and the correlation of segments was obtained through a FFT performed using Potencor software (Guevara, Ramos, Hernández-González, Zarabozo, & Corsi-Cabrera, 2003). Only the segments whose activity was greater in the 8-13Hz band for the C3 and C4 electrodes were kept (the area where the Mu rhythm is concentrated) (Bernier, Dawson, Webb, & Murias, 2007; Hari & Salmelin, 1997; Pineda, 2005).
Of the files that met the previous requirement, the absolute band (ABS), which indicates the amount of EEG activity in a given band without relating it to the other bands, was analyzed. This method makes it possible to interpret the variations in a specific frequency band (Pivik et al., 1993).
Statistical analyses
In order to determine the differences between the power spectrum of the Mu rhythm (8-13Hz) of the groups, two t tests were carried out for independent groups on the data obtained from the FFT in the absolute band of the segments with the highest activity in the 8-13Hz band. In other words, a t test for C3 data comparing the ASD and TD group, and for C4 data. In addition, Levene’s test was performed in both cases to determine whether there was homogeneity of variance. All data analyses were performed using the IBM SPSS STATISTICS Version 20 statistical package.
Results
The final sample comprised 10 male participants for the ASD group and seven for the TD group, the mean age being 8.2, (SD = 1.23) and 8.3 (SD = 1.59), respectively. Figure 1 shows the flow of participants.
Note: n = number of participants; ASD = Autistic Spectrum Disorders; TD = Typical Development; PSG = Polysomnography.
Figure 1 Participant flow.
The results of the t tests for independent groups in C3 and C4 were t (1, 144) = 3.038, p = .003 and t (1, 144) = -2.301, p = .023, respectively. The Levene’s tests were not significant, since they showed homogeneity of variances (Figure 2).
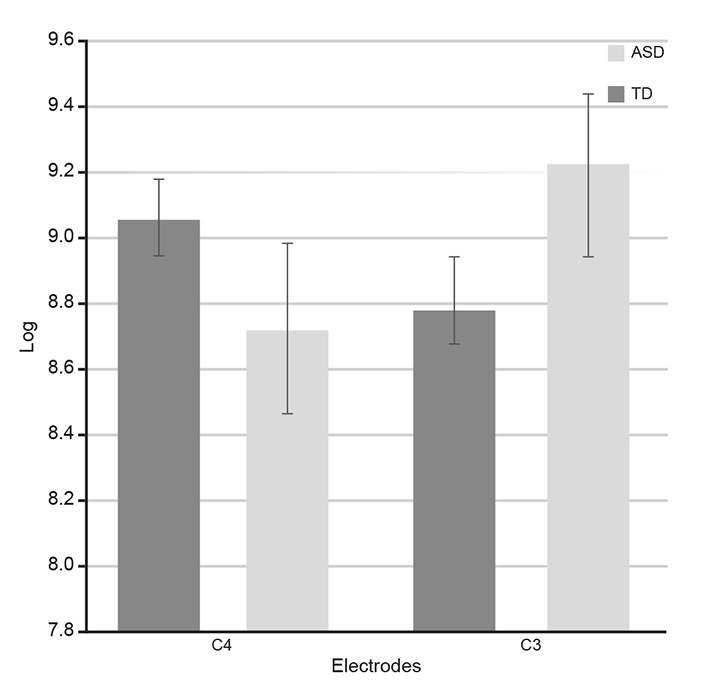
Note: The comparison of means between the ASD group and the control group for C3 (left hemisphere), and the left side, for C4 (right hemisphere) is shown on the right side. In both cases there is a significant difference between the means.
The * represent significant differences. Standard error is marked on each bar
Figure 2 Differences in power spectrum of 8-13Hz band according to t tests.
Figure 2 also shows the differences in hemispheric dominance between the groups; whereas in the TD group there was a peak in the power spectrum in the C4 derivation, in the ASD group, the peak in the power spectrum was in C3.
The frequency of segments with Mu activity identified throughout the nocturnal polysomnographic record is given in Figure 3. It shows that the number of Mu segments detected was larger in the TD group, both overall and in each of the phases of sleep throughout the night. This difference is particularly clear during REM sleep, in the N1 and N2 phases (Figure 3).
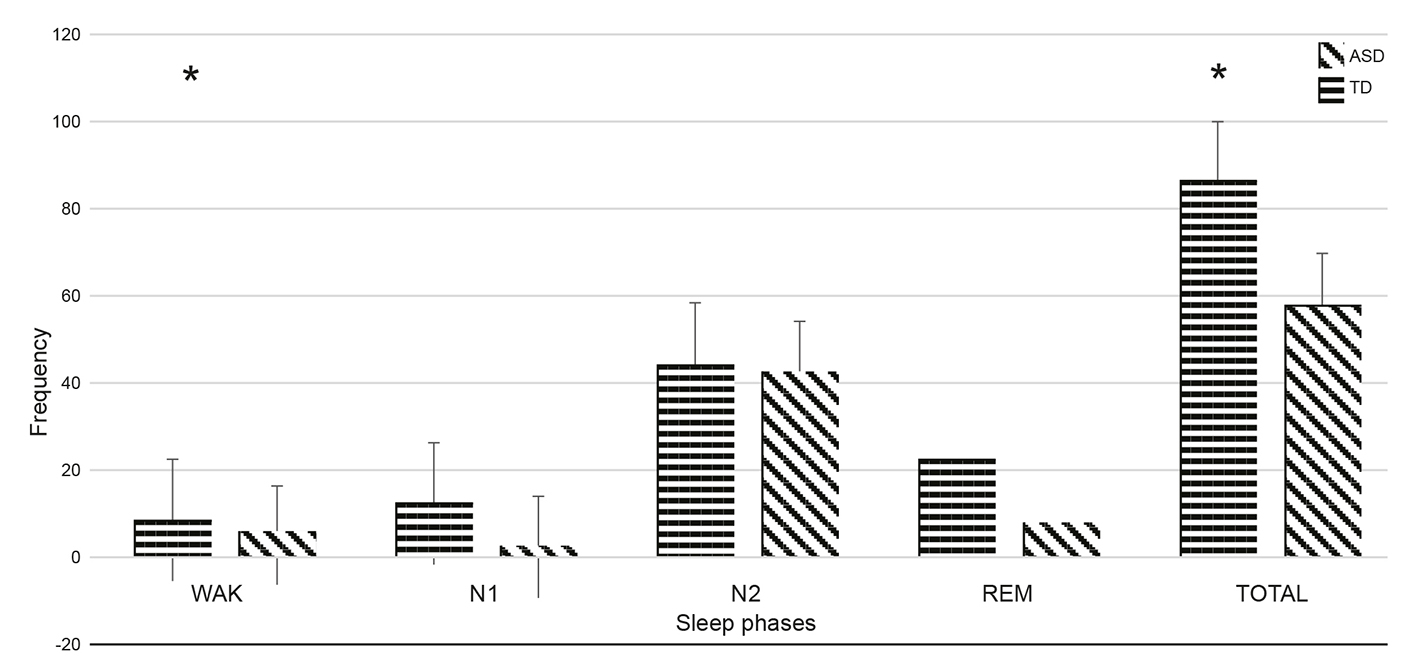
Note: Distribution of Mu segments identified in the various sleep phases. The frequency of segments is plotted on the vertical axis shows while the sleep phases in which they were identified are plotted on the horizontal axis. N3 is not shown because no Mu segments were identified during this phase. The horizontal stripes represent the control group and the diagonal ones represent the ASD group. Standard error is marked on each bar. WAK = Wakefulness; N1, N2 = Phase N1 and N2 of sleep; REM = Rapid eye movement sleep phase.
Figure 3 Frequency of the Mu segment during the night.
Discussion and conclusion
Although very few studies have characterized the Mu rhythm during the sleep of healthy subjects, the results of this paper coincide with those described by other authors during the REM sleep phase (Duntley, Kim, Silbergeld, & Miller, 2001; Marini, Ceccarelli, & Mancia, 2008; Quan et al., 2003) and the N2 phase (Quan et al., 2003). However, in this study, it was observed that the Mu rhythm was more abundant in N2 than in REM, in comparison with the papers cited. These differences could be due to the methodology used, since in previous studies, electroencephalographic analyses were performed visually, whereas in this study, a quantitative analysis (FFT) was carried out in addition to a visual inspection to identify the Mu rhythm during sleep. Likewise, all the segments were analyzed according to the power spectrum. In this section, special care was taken to distinguish the 8-13Hz band from the sigma (14-18Hz), characteristic of the sleep spindles of the N2 phase. It is also important to mention that the sample size of the present study was a limitation since, due to the exclusion criteria, it was not possible to expand it.
Topographically, the Mu rhythm observed complied with the characteristics described in the literature: an arc-shaped rhythm, with an acute negative and rounded positive component, within the frequency of 8-13Hz, expressed most intensely in somatosensory regions, specifically C3 and C4 (Niedermeyer & Silva, 2004). This characteristic was fulfilled in both groups and in both brain hemispheres, meaning that it coincided with what has been described in the literature on the topographical distribution of the Mu rhythm during wakefulness and sleep (Duntley, Kim, Silbergeld, & Miller, 2001; Gastaut, Naquet, & Gastaut, 1965; Gélisse & Crespel, 2014; Hari & Salmelin, 1997; Oberman, Ramachandran, & Pineda, 2008; Pineda, 2005; Yamada & Kooi, 1975) (As shown in Figure 4).

Note: Average of the absolute power spectrum of Mu segments and the activity of the different bands analyzed, organized according to the area where the record was taken. The horizontal axis shows the frequencies and the vertical axis the power spectrum in logarithm analyzed by the Potencor program. The distribution of Mu activity can be seen topographically: higher in Frontal, Central and Parietal areas and lower in Occipital areas. Standard error is marked on each bar.
Figure 4 Average of absolute power spectrum of Mu segments organized by area.
Graphologically, the Mu rhythm observed complied with the characteristics described in the literature: an arc-shaped rhythm, with an acute negative and rounded positive component, within the frequency of 8-13Hz, expressed most intensely in somatosensory regions, specifically C3 and C4 (Hari & Salmelin, 1997). The features observed resemble those found by Duntley et al. (2001), who visually identified the Mu rhythm during the REM sleep phase of epileptic patients. Although there are differences between the samples, it is possible to observe the characteristic graphological similarities of the rhythm. The features found were topographically distinguished from the alpha rhythm, in other words, they were only observed above the somatosensory region (Figure 5).

Note: Comparison of Mu rhythm traces observed and their comparison with previous literature.
A. = The feature of the Mu rhythm observed during wakefulness in an ASD participant is observed in C4.
B. = Feature of Mu rhythm observed during phase 2 of sleep in an ASD participant in C3.
C. = Feature of Mu rhythm observed during the REM sleep phase in C3 adapted from Duntley et al., 2001.
In all cases, the Mu rhythm is identified by horizontal lines.
Figure 5 Comparison of Mu rhythm features observed
As for the interhemispheric differences found between the groups, a possible explanation could be posited on the basis of the characteristics attributed to each brain hemisphere. Self-motivated or learned behaviors and probabilistic reasoning are attributed to the left hemisphere (Parsons & Osherson, 2001) selective attention, characterization of stimuli from the environment on the basis of one or a few details and selective routines (MacNeilage, Rogers, & Vallortigara, 2009). Conversely, the right hemisphere is the site of the organism’s detection and response to novel or unexpected stimuli, visospatial processing, behavior motivated by the environment, synthesis of global patterns, face recognition, and the interpretation of facial expressions linked to emotions (MacNeilage, Rogers, & Vallortigara, 2009). Another study found alterations in the blood perfusion of the medial temporal lobe in the right hemisphere of individuals with ASD, which they associated with the obsessive characteristics in the repetitive behavior characteristic of this population (Ohnishi et al., 2000). Accordingly, in the case of ASD, it makes sense that the power spectrum increases in the left hemisphere (C3) and decreases in the right hemisphere (C4), since it corresponds to the characteristics described in the population: repetitive, restricted behavior, good attention span, especially for details, affinity for carrying out routine activities, little ability to interpret facial expressions associated with emotions, aversion to changes or novel stimuli in already structured routines, etc. (American Psychiatric Association, 2013). Thus, the results obtained not only tally with previous literature on the Mu rhythm in this population during wakefulness, but also coincide with the other features described in the population with ASD.
This paper found significant differences in the spontaneous generation of Mu between a group of children with level 1 ASD and children with TD. This means that since this rhythm is produced intrinsically, it may be produced in response to external sensory stimulation (Yordanova & Kolev, 1997). Within the context of various theories on ASD, the findings of this paper could contribute to the literature on possible neural foundations associated with understanding the mental state of others, in the case of TM. At the same time, within the theory of mirror neurons and ASD, the spontaneous generation of the Mu rhythm in the sleep of children with ASD could lead to therapeutic applications, if we consider that the Mu rhythm has also been regarded as an electroencephalographic index of the mirror neuron system in humans. These applications could expand the various existing therapeutic alternatives, involving behavioral neuroregulation and neuronal metabolic function techniques (Coben, Linden, & Myers, 2010; Pineda et al., 2008). However, it is clear that more studies are required in this field to corroborate and expand existing information.











 nueva página del texto (beta)
nueva página del texto (beta)


