Background
The cannabis sativa plant contains more than 60 phytocannabinoids of which delta-9-tetrahydrocannabinol (THC) is the most abundant ( Potter et al., 2008 ; “van Laar et al., 2015” ). Among the other cannabinoids, the most studied ones are dronabinol (DBN) and nabilone, followed by cannabinol (CBN), cannabidiol (CBD), which seems to possess some anti-inflammatory, analgesic ( Hohmann & Suplita, 2006 ; Rea, Roche & Finn, 2007 ; Jhaveri et al., 2008 ), anti-schemic ( Lamontagne et al., 2006 ), antipsychotic ( Leweke, Koethe & Gerth, 2005 ), ansiolitic ( Crippa et al., 2011 ), and anti-epileptic effects ( Mortati, Dworetzky & Devinsky, 2007 ); and finally, cannabigerol (CBG) and cannabicromeno (CBC) ( Barceloux, 2012 ), which possess some properties which have been studied mainly in preclinical or animal models. Some other properties of the cannabinoid drugs have been tested on type I diabetes ( Di Marzo, Piscitelli & Mechoulam, 2011 ; Horváth et al., 2012 ), the immunological system ( Malek, 2008 ; Bihl et al., 2011 ), and cancer ( Hermanson & Marnett, 2011 ; Sarfaraz et al., 2008 ; McAllister et al., 2007 ; McKallip et al., 2006 ). These so diverse physiological effects of cannabinoids are due to the existence of specific receptors distributed in some body organs and systems. This has drawn the attention of the scientific community for study and research.
Despite a long list of expected potential benefits, these have been difficult to assess because many of them are biphasic, that is to say, they initially present a higher acute response with low doses, which quickly decrease with their repeated administration [tachyphylaxie] ( Fernández-Ruiz et al., 2000 ), making it necessary to gradually consume higher doses to reach the same effects which were originally achieved [tolerance] ( Maldonado, Valverde & Berrendero, 2006 ).
The pertinence and opportunity to scientifically approach such a relevant theme as is the medical justification of the therapeutic use of cannabis is framed in a moment of debate in the public opinion and its media influence. Mexico’s Consejo Nacional de Salud (National Health Council), through the 25/V/CONASAVI/2014 Agreement on the eventual legalization of marijuana, states:
In the face of the debate which has arisen in different forums and organisms about the legalization of marijuana, the opinion of the members of the Consejo Nacional de Salud (National Health Council) is that, before making a decision on the matter, an evidence-based assessment about the harmful effects which its consumption brings about for human health should be made and they consider that legalization is not the matter under discussion. The issue should be focused in warranting the protection of the Mexican population health as established in article 4o of the Constitution of the Mexican United States and that this is a fundamental human right.
In the light of this situation, the questions the researchers made themselves before initiating the study were: 1. Does cannabis have in its different forms any therapeutic effects?, and, being this the case, 2. Which symptoms or diseases are the most benefited?; and 3. Which of the drugs or cannabis plant are the most beneficial?
The objective of this paper was to assess, through a narrative search of the bibliography, the use and therapeutic effects of the cannabis plant and cannabinoid drugs for the treatment of several symptoms or diseases. Their efficacy over the conventional treatment or the prototypical drug was also evaluated.
Method
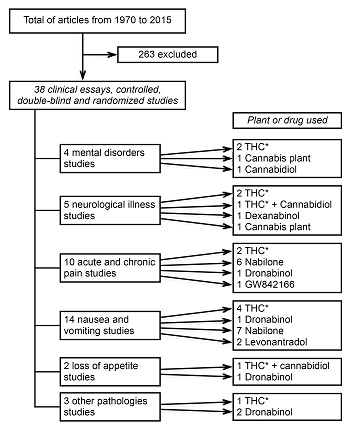
A search of the bibliography on the medicinal use of the cannabis plant and cannabinoid drugs to treat symptoms and diseases was carried out. The search was made in the websites Medline, Cochrane, PubMed, LILACS, PsycINFO, Psychology and Behavioral Sciences Collection, DynaMed, Google academic, Scopus, Embase.com and SciELO from 1970 to 2015. The keywords used both in English and Spanish were: cannabis, marijuana, cannabinoid system, evidence-based medicine, therapeutic and harmful effects, medical uses, cannabinoid drugs, clinical trials, case-control studies and effectiveness. Psychiatry post-graduate students were assigned the search duties. They were asked to look for, as a first inclusion criterion, articles on the cannabis plant and cannabinoid drugs with medical applications from 1979 to 2015. They found 301 articles with these features. The second process was to classify the articles according to their methodological design and to select those which were clinical trials, controlled, double-blind cross-over, and randomized (34 studies) (Table 1). In each one of these, the reported therapeutic effect, its effect on the symptom or disease on which it was used, the type of cannabinoid drug or cannabis plant employed and the administration route were assessed. Other variables, such as the number of participants, doses, time of administration and control group, were not assessed because there was such a high heterogeneity among them that they did not allow any comparison. The remaining articles were considered as uncontrolled clinical studies with methodological inconsistencies, case reports or anecdotic accounts (267 studies) ( CONADIC, 2014 ) because they did not include the doses, administration route or sample randomization, or else they did not clearly specify the study procedure.
Table 1. Studies reported according to methodology and results
| Authors | Objective | Main results and conclusions |
| Frytak et al., 1979. 1 | To evaluate antiemetic activity of THC in comparison with prochlorperazine and placebo. | The antiemetic activity and side-effects of THC and prochlorperazine for gastrointestinal carcinoma were evaluated in 116 patients. The THC had superior antiemetic activity in comparison to placebo, but it showed no advantage over prochlorperazine. |
| Kluin-Neleman, Neleman, Meuwissen & Maes, 1979. 2 | To evaluate the effect of THC as an antiemetic in patients treated with cancer chemotherapy. | THC had antiemetic effects, but side effects were severe. Most patients preferred the nausea and the vomiting after chemotherapy to the use of THC. |
| Neidhart, Gagen, Wilson & Young, 1981. 1 | To evaluate the efficacy and toxicity of THC and haloperidol in patients with chemotherapy. | Fifty-two patients were evaluated. THC and haloperidol were equally effective in controlling nausea and vomiting. |
| Sallan, Cronin, Zelen & Zinberg, 1980. 2 | To compare the antiemetic effect of THC and prochlorperazine. | Twenty-five patients were treated. THC was more effective than prochlorperazine. |
| Meiri et al., 2007. 2 | To compare the efficacy and tolerability of dronabinol and ondansetron for delayed chemotherapy-induced nausea and vomiting. | Sixty-four patients were randomized. Total response was similar with dronabinol (54%), ondansetron (58%), and combination therapy (47%) versus placebo (20%). |
| Crawford & Buckman, 1986. 1 | To compare the efficacy and tolerability of nabilone versus metoclopramide to treat vomiting. | Thirty-two patients were entered into the study. There was no difference between the two treatments in the overall incidence or severity of vomiting; subgroup of patients who received metoclopramide had a substantial reduction in episodes of vomiting. |
| Cunningham et al., 1988. 4 | To compare the efficacy of nabilone and prochlorperazine versus metoclopramide and dexamethasone. | Seventy patients completed the cross-over assessment. It was significantly favoured metoclopramide and dexamethasone. |
| George, Pejovic, Thuaire, Kramar & Wolff, 1982. 1 | To compare nabilone in cancer patients treated with cisplatin. | Twenty patients were included. Nabilone in comparison with chlorpromazine did not significantly reduce vomiting. |
| Jones, Durant, Greco & Robertone, 1982. 2 | To evaluate nabilone versus placebo in chemotherapy-induced nausea and vomiting. | Fifty-four patients were entered. Patients experienced less vomiting and nausea while receiving nabilone compared to placebo. Side effects were common with nabilone but acceptable. |
| Priestman, Priestman & Canney, 1987. 5 | Comparing nabilone versus metoclopramide to control emesis induced by radiation. | Forty patients with emesis were included. There was no difference in the efficacy of the two drugs but the incidence and severity of adverse reactions was significantly greater in those patients who received nabilone. |
| Steele, Gralla, Braun Jr. & Young, 1980. 5 | To compare the effects of nabilone and prochlorperazine on chemotherapy-induced emesis. | Thirty patients receiving cancer chemotherapy were included. Both nabilone and prochlorperazine appeared to produce antiemetic effects. |
| Hutcheon et al., 1983. 3 | Comparison of levonantradol with chlorpromazine in patients receiving their first cytotoxic chemotherapy. | One hundred and eight patients were included. Levonantradol is a more effective antiemetic than chlorpromazine. However, its use generates a high incidence of unacceptable central nervous system side-effects. |
| Lucraft & Palmer, 1982. 1 | Comparison of the antiemetic effect of chlorpromazine with levonantradol. | Both drugs were well tolerated. The frequency of vomiting was similar in all groups. |
| Abrams et al., 2003. 1 | To determine the short-term effects of smoked marijuana on the viral load in HIV-infected patients. | Sixty-two patients were eligible. Smoked and oral cannabinoids did not seem to be unsafe in people with HIV infection with respect to HIV RNA levels, CD4+ and CD8+ cell counts, or protease inhibitor levels over a 21-day treatment. |
| Strasser et al., 2006. 1 | To compare the effects of the cannabis extract, THC and placebo on appetite and quality of life in patients with cancer-related anorexia-cachexia syndrome. | Two-hundred and forty three patients were randomly assigned. No differences in patients’ appetite or quality of life were found between three groups at the dosages investigated. |
| Volicer, Stelly, Morris, McLaughlin & Volicer, 1997. 2 | To compare the effects of dronabinol versus placebo in patients with Alzheimer’s disease who were refusing food. | Fifteen patients were included. Dronabinol treatment decreased the severity of anorexia versus placebo, but adverse reactions were more common. |
| Maurer, Henn, Dittrich & Hofmann, 1990. 1 | To evaluate THC in antispastic and analgesic effects in a single case. | A patient with spasticity and pain due to spinal cord injury was included. THC and codeine both had an analgesic effect in comparison with placebo. Only THC showed a significant beneficial effect on spasticity. |
| Pooyania, Ethans, Szturm, Casey & Perry, 2010. 2 | To determine whether nabilone alleviates spasticity in people with spinal cord injury. | Twelve patients were recruited. There was a significant decrease on spasiticity with nabilone. |
| Svendsen, Jensen & Bach, 2004. 1 | To evaluate the effect of dronabinol on central neuropathic pain in patients with multiple sclerosis. | Twenty four patients were icluded. Dronabinol has a modest but clinically relevant analgesic effect on central pain. Adverse events were more frequent than placebo. |
| Wissel et al., 2006. 2 | To evaluate the safety and efficacy of low dose treatment with nabilone on spasticity. | Eleven of the 13 patients included completed the study. Nabilone showed a significant decrease of pain. |
| Frank, Serpell, Hughes, Matthews & Kapur, 2008. 2 | To compare the analgesic efficacy and side effects of nabilone with dihydrocodeine for chronic neuropathic pain. | Ninety-six patients with chronic neuropathic pain were included. Dihydrocodeine provided better pain relief than nabilone and had slightly fewer side effects, although no major adverse events occurred for either drug. |
| Bestard & Toth, 2011. 6 | To compare the efficacy of nabilone and gabapentin in patients with peripheral neuropathy. | The benefits of monotherapy or adjuvant therapy with nabilone appear comparable to gabapentin for the management of neuropathic pain. |
| Noyes, Brunk, Avery & Canter, 1975. 6 | To estimate the potency of the analgesic effects of THC and codeine and to compare their side effects. | Thirty-six patients were selected for this study. THC induced side effects but at low doses was well tolerated. |
| Ware, Fitzcharles, Joseph & Shir, 2010. 5 | To determine the effects, quality of life, and global satisfaction of nabilone versus amitriptyline in patients with fibromyalgia. | Thirty-two were recruited. Both nabilone and amitriptyline had a favorable effect; nabilone showed superiority for sleep quality. Adverse effects were more common with nabilone. |
| Pinsger et al., 2006. 2 | To investigate the efficiency of nabilone on patients with chronic pain. | Thirty patients were included. Nabilone treatment was superior to placebo. |
| Ostenfeld et al., 2011. 1 | To evaluate the efficacy of GW842166 versus ibuprofen in acute pain. | Ibuprofen and GW842166 demonstrated clinically meaningful analgesia in the setting of acute dental pain. |
| Weber, Goldman Truniger, 2010. 2 | To determine the effect of THC on cramps in amyotrophic lateral sclerosis patients. | Twenty-two patients participated in the study. THC was well tolerated. |
| Freeman et al., 2006. 1 | To test whether cannabinoids reduce urge incontinence episodes without affecting voiding in patients with multiple sclerosis. | The multicentric study randomized 630 patients to receive oral administration of cannabis extract, THC or placebo. Cannabis extract and THC showed significant effects over placebo. |
| Brady et al., 2004. 6 | To evaluate the safety, tolerability, and efficacy of THC and cannabidiol in bladder dysfunction. | Twenty-one patients were recruited. There were few side effects, and cannabis-based medicinal extracts were safe and effective treatment for urinary dysfunction in patients with multiple sclerosis. |
| Esfandyari et al., 2007. 1 | To compare the effects of dronabinol and placebo on colonic motility and sensation in healthy volunteers. | Fifty-two volunteers were randomized. Dronabinol relaxes the colon and reduces postprandial colonic motility and tone. Increase in sensation ratings to distension suggest central modulation of perception. |
| Müller-Vahl et al., 2002. 2 | To evaluate the treatment of Tourette’s syndrome with THC. | Twelve patients were included. There was a significant improvement of tics and obsessive-compulsive behavior after treatment with THC compared to placebo. |
| Müller-Vahl et al., 2003. 1 | To evaluate THC effectiveness in the treatment of tics in Tourette’s syndrome. | Twenty-four patients with Tourette’s syndrome were included. No serious adverse effects occurred. The THC is effective and safe in the treatment of tics. It can be hypothesized that the central cannabinoid receptor system might play a role in this pathology. |
| Carroll et al., 2004. 2 | To examine the hypothesis of the beneficial effect of cannabis on dyskinesia in Parkinson disease. | Nineteen patients were randomized. Cannabis was well tolerated, and had no pro- or antiparkinsonian action. |
| Maas et al., 2006. 1 | To study the efficacy and safety of dexanabinol in severe traumatic brain injury. | Eighty hundred and forty-six patients were included. Patients in the dexanabinol and in the placebo group had an unfavourable outcome. Dexanabinol is safe, but is not efficacious in the treatment of traumatic brain injury. |
Note: THC=Delta-9-tetrahydrocannabinol;
1Randomized and Double-blind study;
2Double-blind, placebo-controlled cross-over study;
3A randomized multicentre single blind;
4Open cross-over study;
5Randomized double-blind cross-over study;
6Open-label study.
To consider all the studies conducted and their reported results was the reason to include all the articles where the cannabis therapeutic use from 1970 to 2015 was mentioned. Nevertheless, only the results from the studies which met the inclusion criteria will be presented. Articles where only the abstract could be accessed or whose results were not published in any online access journal were excluded.
Results
In total, 301 articles were found. There were 34 clinical trials, double-blind, cross-over, and randomized studies. The cannabinoid drugs used were: delta-9-tetrahydrocannabinol (THC) (11 studies), THC + cannabidiol (two studies), cannabidiol (one study), dronabinol (five studies), nabilone (11 studies), levonantradol (two studies), GW842166 (one study), and dexabinol (one study).
From the 34 studies, one was carried out in gastrointestinal disorders, four in neurological, nine in different kinds of pain, 14 in nauseas and vomiting secondary to medical treatment, two in appetite loss due to some medical condition and two studies in urologic diseases, while only two articles reported the use of these substances in treatment of Gilles de la Tourette’s syndrome (Figure 1).
Regarding the administration route, the most common was the oral or sublingual one (90%).
Discussion and conclusion
Most studies reached the conclusion that cannabinoid drugs are not more effective than most common or conventional medications. This was also the case with the use of the cannabis plant where the two studies that used it did not report more effectiveness than conventional medications. However, the type of cannabis plant used in these two studies was not also specified because the THC concentration may vary considerably from 10% to 30%, not to speak of the new varieties which may even contain up to 40% of THC ( Potter et al., 2008 ; van Laar et al., 2015 ). This influences both their therapeutic efficacy and their side effects.
A noteworthy difference between the cannabis plant and cannabinoid drugs is that the former presents a higher acute response with a low dose, and consequently there is the risk that this effect decreases with its repeated administration. This may result in the need to increase the dose to reach the same effects which could in turn provoke its chronic use and an increase in the secondary effects. Cannabinoid drugs have not reported this same effect ( Potter et al., 2008 ; van Laar et al., 2015 ).
While some cannabinoid drugs have been approved for medical use, they have nevertheless restrictions as the use of these medications must be carefully monitored and they must not be administered to individuals under 18 years old or with a psychiatric record (especially schizophrenia) ( Leweke, Koethe & Gerth, 2005 ).
Likewise, a case-control study reported that the daily use of cannabis increased five fold the risk of suffering a psychotic disorder among cannabis users compared with non-users ( di Forti et al., 2015 ). This points out to the need to be more careful with individuals prone to psychotic disorders as is the case with subjects with schizophrenia.
Another important aspect found in the 301 articles under scrutiny were the serious ethical implications, such as the use of the marijuana plant to treat nauseas or vomit secondary to pregnancy or to arouse appetite in geriatric patients. All these have their limitations given their risk of provoking theratogenic harms or worsening old people’s health.
Results from studies carried out with patients with mild or moderate pathologies ‒such as acute pain for different causes, nausea and vomiting, or loss of appetite in geriatric patients‒ do not justify submitting patients to such a potential risk as there are other approved drugs without major side effects ( CONADIC, 2014 ). Being this the case, marijuana is only approved to reduce the symptoms of some diseases or the secondary/undesirable/side effects of some other medical or chirurgical handlings, but occupying only a secondary position as a line of treatment.
It is also necessary to say that the studies reviewed here have a short-term basis and so no follow-up has been given as to their long-term effect in individuals who received such a treatment once the research ‒mainly in the studies concerning the cannabis plant‒ was finished.
In short, according to the evidence-based medicine model, the medical indications of commercial cannabinoid drugs are minimal and all are replaceable with other medications whose efficacy and side effects are perfectly well-known.
In the light of their heterogeneity, the reviewed articles do not show a solid scientific support of the effectiveness of the medical use of the cannabis plant or its superiority versus conventional treatments and so its usefulness for therapeutic ends is limited. Other studies reach the same conclusions ( del Bosque et al., 2013 ).
Despite which has been exposed so far, it is worth pointing out that multi-centric and open label studies have been reported recently, especially about the Dravet syndrome and the Lennox-Gastaut syndrome, both of which report the tolerability and efficacy of cannabidiol as an alternative therapeutic option for cases with resistant epilepsy. These studies were mainly carried out in pediatric population, and they yielded apparently promising results in their preliminary phases ( Devinsky et al., 2016 ; Press, Knupp & Chapman, 2015 ; Hussain et al., 2015 ).
The strengths and limitations of this paper are presented next.
Strengths
This is one of the few articles to carry out a search in the last 40 years about cannabinoid drugs and the marijuana plant, their medical use and the ethical dilemmas surrounding the issue. A wide review was conducted to reach conclusions which are closer to the possible benefits of cannabinoid drugs. This review tries to guide decision-makers on health policies about what is known about the medical use of cannabinoid drugs, their benefits and consequences. Likewise, this review tries to shed light on the research level they are regarding therapeutic ends so that they are used as a phytocannabinoid extracted from the plant and not from the use of the cannabis plant in itself.
Limitations
To contrast the information, this article tried to include most of the articles reported about cannabinoid drugs and their medical use. However, this was not possible given the extent of the bibliography. Likewise, there is inaccessible medical literature because it is made up of internal documents or articles published in low-impact journals. Another limitation is that the review was carried out only online and no hard-copy journals were taken into account. So, articles written before the 90’s which were not uploaded to Internet may have been excluded.
Articles do not comment either on the cross-over tolerance by association of the cannabinoids with other medications or psychoactive substances. This effect may affect the short- and medium-term result. Another important aspect is the heterogeneity of the studies, which does not allow for a better analysis of the results or reaching statistically significant or more weighty conclusions, and so it is only possible to make a description of them. These and various other biases were observed in several studies and thus they were deleted from the beginning. Nevertheless, it is worth noting that biases were also observed in the studies included and these were not mentioned by the authors. All these limitations are an invitation to delve into the matter under a strict methodology.
Finally, unlike what was the case in the articles published mainly in the 70’s and 80’s, lately journals ask for a higher specification of the methodology, the statistical analysis, and the ethical considerations. In the light of this, it is not difficult to see that journals are now more exigent and vigilant about ethical and methodological aspects before accepting an article for publication.











 nueva página del texto (beta)
nueva página del texto (beta)