Introduction
Several studies have found a significant link between depressive symptomatology and tobacco smoking.1-3 Smokers with a history of depression are more likely to relapse into depression when quitting smoking,4 just as current smokers and former smokers are more likely to have depressive symptoms compared with people who have never smoked.3 The manifestation of these addiction symptoms predicts tobacco smoking relapse.5
Regarding differences by gender, it has been reported that women who smoke are two times more likely to have depressive symptoms compared to non-smoking women, and five times more likely to suffer this symptomatology than men that smoke. In the other hand, men who smoke more than a pack of cigarettes per day are five times more likely to develop symptoms of depression in comparison with men that do not smoke.1
It has been suggested that many people smoke as a way to self-medicate against depressive symptomatology,6,7 due to nicotine properties to reduce both the incidence and the severity of depressive symptoms.8
According to the Mexican Comorbidity Survey, the prevalence of the minor depressive disorder episode was 1.5% in the 12 months prior to the survey.9 It has also been reported that depressive symptoms and dysthymia increase the risk of developing major depression, since both forms of depression share common risk factors (demographic, social and physical), and also increase the risk to use other drugs.10
Transdermal nicotine patches (TNP) and bupropion are among the most effective treatments for tobacco smoking.11-13 Both increase abstinence rates in the long-term, and have the best cost-effectiveness results.14,15 The simultaneous use of TNP and bupropion in smokers with no mood disorder increases the chances of long-term abstinence.16 Likewise, the use of TNP before abstinence in combination with low nicotine cigarettes (LNC) has proven to increase abstinence rates in healthy severe smokers.17,18
Psychological interventions proven to be effective for tobacco smoking cessation include identifying high-risk situations, relapse prevention, self-monitoring, self-control, functional analysis of tobacco smoking pattern, problem solving techniques and action plans, mostly based on the cognitive-behavioral model.19,20
The need to address comorbid disorders simultaneously has been recently noted.21-23 The hypothesis for the present study is that an integral treatment against tobacco smoking for heavy smokers with low-mild depressive symptoms, with three experimental settings using TNP, bupropion or TNP and bupropion, will increase the chances for patients to ease their depressive symptomatology and achieve abstinence.
Method
The present study was a randomized clinical trial with a three-armed parallel design and an allocation ratio of 1:1:1.
Sample
A minimal sample size of 50 patients was determined by the result of the difference expected to be found among the different experimental settings, considering the results of a previous study in which we assessed the depressive symptoms of chronic smoking abstinence.7 A non-response rate of 40% was estimated, distributed in the three settings, with a significance level of 5% and a power of 0.85 to detect a difference of 5 points and a variance of 75 points in the Beck Depression Inventory (BDI).
A total of 89 heavy smokers, who sought treatment for smoking cessation, were evaluated at the Clinic for Addiction Treatment at the Psychology School of the National Autonomous University of Mexico (UNAM) in the summer of 2009. A total of 60 smokers met the inclusion criteria and agreed to participate voluntarily in this study by signing an informed consent. A group of treatment assignment was randomized using a raffle.
Inclusion criteria: Men and women 18 to 65 years old with current minimal-mild depressive symptomatology, who consumed 10 or more cigarettes daily and expressed the desire to quit.
Exclusion criteria: Having high blood pressure, heart disease, history of seizures, skin allergies, another psychiatric disorder, pregnancy, lactation, other substance abuse, drug abuse (last six months) and hypersensitivity to drugs used in the study.
Patients who did not meet the inclusion criteria were referred to other tobacco smoking treatments. No control group was included because of the ethical implications desired from not providing treatment to patients with depressive symptoms. Comparisons were made between the different treatment settings.
Randomization
To generate a random allocation sequence, eligible subjects were interviewed to learn if they met inclusion criteria for the study. Then, they were given a written consent sheet. Those who agreed to continue in the study, entered the raffle (three different color balls in a dark box) to assign a treatment setting, and were evaluated.
To ensure the reliability of the study, initial interviews were performed by a clinical psychologist blind to the study. In turn, evaluations and treatments were conducted by clinical psychologists who were not blind to the study, while the analyst of the retreived data was also blind to the study.
Instruments
The Composite International Diagnostic Interview (CIDI) was used to assess a possible depression diagnosis.24 The level of nicotine dependence was evaluated using the Fagerström Test for Nicotine Dependence (FTND),25 and the 21-item BDI was used to assess the level of depressive symptomatology.26 The latter has been validated for its use in Mexican population, and the Spanish version of the FTND has been extensively used in Mexico.27-29 The FTND has three levels of nicotine dependence obtained according to the raw score (0-3 low, 4-6 medium, and 7-10 high). The BDI has four levels of depressive symptomatology obtained according to the raw score (0-9 minimal, 10-16 mild, 17 - 29 moderate, and 30-63 severe).
Abstinence was confirmed by urinary cotinine assessments. Urine cotinine levels were analyzed using a gas chromatograph connected to a mass spectrometer, using the method by James et al.30 Only levels below 20 ng/ml were considered acceptable to confirm abstinence. An internal record was used to evaluate pharmacological treatment adherence.
Procedure
The initial evaluation was carried out by an M.D., who also monitored the pharmacological treatment through the whole study.
Evaluations and treatments were carried out by four psychologists trained in CBT. The protocol for this study was reviewed and approved by the research ethics committee of the UNAM's addiction research macro-project. The pattern of tobacco consumption was assessed in the initial interview (baseline).
Sixty patients were randomly assigned to three integral pre-abstinence treatment settings. All treatment settings included sessions of cognitive-behavioral psychological therapy (CBPT) and a pre-withdrawal stage using 0.1 mg LNC. Group A: TNP pharmacological therapy; Group B: bupropion pharmacological therapy, and Group C: TNP and bupropion pharmacological therapy.
During the pre-withdrawal stage of the treatment (two weeks), patients of each setting assisted to two individual sessions (one per week) of CBPT. The first one was assess their cognitions regarding their tobacco smoking addiction, and the second one to explore the specific pattern of their addiction, such as number of cigarettes consumed daily, years of tobacco smoking and age of the first cigarette use. At the same time, in this stage, patients of the three settings changed their usual cigarettes for 0.1 mg LNC, making a reduction of 40.0% on the total number of cigarettes consumed per day each week (the reduction was adjusted according to their usual daily intake report). At the beginning of the third week of treatment, total tobacco abstinence was initiated and patients received two more individual sessions of CBPT (one per week) that focused on the implementation of strategies to prevent and/or deal with specific situations related to their addiction and preventing relapse. During the pre-withdrawal stage, patients in group A received two weeks of 21 mg TNP. Once they initiated total abstinence, they received four weeks of 21 mg TNP, followed by two weeks of 14 mg NTP, and two weeks of 7 mg TNP. During the first week of the pre-withdrawal stage, patients in group B received 150 mg bupropion once a day. Then, from the second week on and until completing the three months of pharmacological treatment, they received 150 mg bupropion tablets twice a day. Patients in group C received both TNP and bupropion in the pre-abstinence phase, and continued to receive them for three months, following the same doses and times indicated for groups A and B.
A total of nine evaluations were performed as follows: baseline (day one), at the end of the pre-abstinence phase (day fifteen), one month (final phase of psychological treatment), 2.5 months (end phase of drug treatment), 3.5 months (beginning of follow-up stage), 5.5 months, 7.5 months, 9.5 months and 12.5 months.
Data analysis
The descriptive analysis was conducted with gender, smoking patterns, baseline depressive symptomatology and socio-demographic characteristics of smokers. Subsequently, a linear mixed model, fitted with a random effect for each individual, was used to compare baseline BDI data and subsequent assessments to evaluate the effect of the three different treatment settings. In addition to the treatment settings, the analysis was controlled for socio-demographic variables (sex, education and occupation), variables related to the pattern of consumption (FTND, partial abstinence from smoking, and age of first cigarette use) and study phase (pre-abstinence phase included in the pharmacological phase and psychological treatment phase, pharmacotherapy maintenance phase, and follow-up phase). The interaction of these variables with the type of pharmacological treatment was also analyzed. A value of P≤0.05 was set as a minimal level of statistical significance.
Results
Out of 89 patients assessed for eligibility, 29 were not included due to various reasons, 60 patients were allocated in the three different treatment settings, 20 in each one. One patient in the TNP group did not begin treatment because he did not showed up. The rest were 19 patients (17 men and two women). Five patients in the bupropion treatment group did not start treatment: two women because of personal reasons and three males that were not willing to take the medication, 15 patients (seven men and eight women). In TNP+Bupropion group, 20 patients (nine men and 11 women) received both TNP and bupropion. A total of 54 heavy smokers were included in this study (Figure 1).
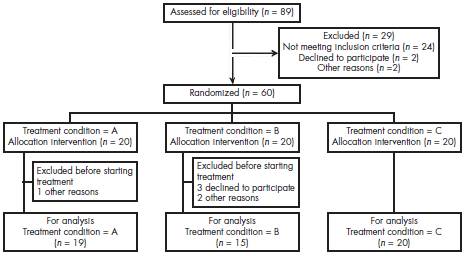
Figure 1 Flow of study participants and allocation. Treatment condition A = TNP; B = Bupropion; C = TNP + Bupropion.
In order to determine whether there were differences between patients who dropped out and those who continued in treatment, a mean differences test for continuous variables and a Pearson χ2 for categorical variables were carried out. Significant differences were thus found regarding gender, with females more frequently dropping out from treatment ( p = 0.017), and treatment setting, with the TNP group being the treatment with more dropouts ( p = 0.36). The same analysis was done for the drop out during the different stages, both by gender and treatment setting, and no significant differences were found. The mean BDI score of the patients who abandoned the study was 9.6 points higher than the average of patients who continued treatment (8.79 points), although this difference was not statistically significant. The analysis of BDI baseline scores in relation to the treatment setting and gender showed no statistically significant differences for any of these variables, both on their own or in interaction. The dropout rate of patients throughout the study is described in Table 1.
In the TNP group 16 patients finished the pharmacological phase of the treatment (14 men and 2 women), and 15 finished the follow-up phase (14 men and 1 women), while in the B group 12 patients finished both the pharmacological phase and the follow up phase of the treatment (7 men and 5 women). In the TNP+B group 15 patients finished the pharmacological phase of the treatment (6 men and 9 women), and 7 finished the follow-up phase (3 men and 4 women).
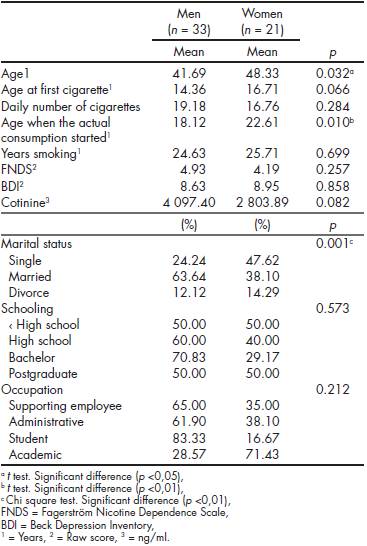
Participants reported a daily average consumption of 18.2 cigarettes ( SD = 8.03), a mean age of tobacco first consumption of 15.27 years ( SD = 4.59), an average of 25 years smoking ( SD = 9.88) and an average level of nicotine dependence of 4.65 points ( SD = 2.34) (moderate level). Mean depressive symptomatology was 8.76 points ( SD = 6.27) (minimum-mild level). No significant differences were found in the level of education, employment status, age of first cigarette consumption, number of years of smoking, level of nicotine dependence, depressive symptoms, and pattern of smoking behavior. To evaluate differences between men ( n = 33) and women ( n = 21), categorical variables were analyzed using the Pearson χ2 test, and continuous variables were analyzed using the t test for mean differences. Significant differences were found in age, marital status and number of years smoking regularly. In women, age at initiation of treatment was higher, as well as the age of beginning to smoke with the current number of cigarettes. Single women showed a higher risk to continue smoking than single men. Table 2 shows, in relation to gender, socio-demographic characteristics of the 54 heavy smokers included in this study, their consumption pattern and level of depressive symptomatology at baseline.
The total rate of abstinence was 46.29% considering all patients included in this study. It is important to clarify that this percentage is reflecting that the urine cotinine level of the last measurement in each patient was below 20 ng/ml. Due to the differences among patients who continued throughout the study and those who dropped out, both in gender and in the pattern of tobacco consumption, the analysis of the effect of the three experimental settings was divided according to gender.
Groups in the mixed models were the patients and the variable of interest was the treatment setting with three levels and its interaction with time. The covariables of the mixed model for women were: occupation, schooling, Fagerström's score and age of first cigarette, whereas for men marital status was significant. Both models were corrected by the same variables, which are all the ones mentioned before.
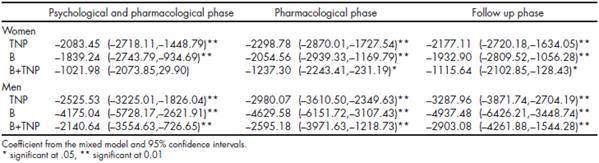
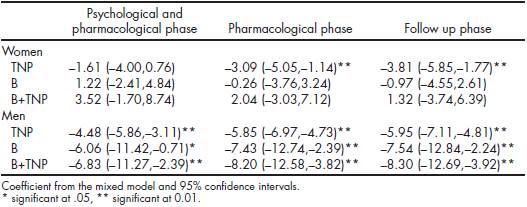
For women, the nicotine patch showed to be effective to reduce depressive symptoms, while cotinine levels decreased in almost all of the evaluations on the three treatment settings. In the case of men, all the evaluations in all of the phases of treatment, for the three treatment settings, showed a positive effect on depressive symptomatology, as well as a continued reduction on cotinine levels.
Table 3 shows the improvement in depressive symptoms, according to gender, with the different treatment settings in relation to baseline data.
Table 3 Effect on depression symptoms of the different treatments at the different treatment phases, base group is TNP and base phase is basal
In turn, Table 4 shows the progression of cotinine in the urine level of patients during the treatment and its follow-up stage according to treatment setting. No significant abstinence differences were found regarding treatment setting or gender.
Discussion and conclusion
The main finding of this study is that the three settings of integral pre-abstinence treatment were successful in maintaining tobacco smoking abstinence and reducing depressive symptomatology, particularly in men. All patients showed a significant improvement in their evaluations of these two variables throughout one year of evaluations, compared to baseline data.
It is important to note that the patients of this study manifested minimal-mild depressive symptoms at the time of the treatment start. It is known that TNP and bupropion are both effective in maintaining abstinence and treating depressive symptomatology.8,14,15,31-33 This might suggest that results obtained in this study have a strong relation with the pre-treatment abstinence from the tobacco phase of the treatment, where patients received simultaneously low nicotine cigarettes and transdermal nicotine patches and/or bupropion, which have individually proven in the past to be effective in the treatment of smoking. The psychological treatment used was aimed to aid patients to modify their behavior and cognitions regarding their tobacco consumption pattern, while the gradual reduction in cigarette intake with LNC helped with both physiological and behavioral desensitization. The results of this study imply that the simultaneous implementation of these components, prior to the initiation of abstinence, may have exerted a protective effect for not developing greater depressive symptomatology and favored the maintenance of tobacco abstinence in patients.
According to prior studies, when only TNP was used in the rehabilitation of healthy smokers, the reported rate of one year abstinence was 23.4%.34 When bupropion was used in the rehabilitation of chronic smokers, abstinence reached a rate of only 30.0%,35 while progressive cigarette reduction with LNC,17,18,36,37 reached only an abstinence rate of 11.8%.34 On the other hand, when CBPT is used as the sole treatment for smoking, it only reaches success rates ranging from 16.2 to 20.9% after one year of abstinence.34,38
In the case of women, from the baseline, only a small number of women participated, and along the study the sample size decreased as a result of desertion of treatment, probably due to tobacco smoking relapse. A trend toward improving depressive symptoms and maintaining the abstinence rate was observed in the three treatment settings. It is known that depressive symptoms in women are related to relapses in tobacco smoking,39 as well as that women who smoke tobacco and have depressive symptomatology are less likely to remain abstinent than men.40
Since this was a randomized clinical trial, and due to the fact that the population of heavy smokers with mild depressive symptomatology was unknown, the sample was obtained by convenience sampling and there was no control of gender assignment to treatment settings. Results show a significant difference in treatment response between men and women, and for future studies we recommend control of gender assignment to treatment settings, as well as a detailed evaluation of patients who quit treatment. The sample size of this study is small, but being it a longitudinal study, its power increases, in particular, to estimate the parameters of interest, since all the longitudinal evaluations were used for each individual.
The drop-out pattern according to gender and treatment setting points to the need to perform separate analyses by gender.
Additionally, in order to develop more accurate treatments against tobacco smoking, it is important to take into account the particular characteristics of smokers such as number of cigarettes smoked per day, degree of nicotine dependence, level of education, degree of depressive symptomatology and their history of depressive symptoms, since these variables may be related to the rate of treatment abandonment.
Acknowledgments
This study was funded by the macro-project: Development of new models for prevention and treatment of addictive behavior of the National Autonomous University of Mexico. UNAM had no role in the study design, data collection, statistical analysis or the writing of this manuscript.
We want to acknowledge: Adán Fuentes Suárez, M.D., Marco Antonio Hernández Delgado, M. Psych., Denyzett Díaz Ayala, B. Psych., Celene Apanco Peña, Neftalí Palomar Villalpando, and Luis Villalobos-Gallegos for their contributions in the development of the study. We also thank Graciela Y. Sánchez Hernández, B. Psych., for her support in editing this manuscript.











 text new page (beta)
text new page (beta)